Abstract
Although patients with chronic kidney disease (CKD) are at increased risk for end‐stage renal disease and cardiovascular events, adequate drug therapies for preventing the deterioration of these conditions are still not established. This study was undertaken to evaluate a preventive effect of an angiotensin receptor‐neprilysin inhibitor sacubitril/valsartan (LCZ696), which is converted to sacubitril and valsartan in the body, against the progression of renal disease in rats with subtotal nephrectomy, an animal model of human CKD. Mean survival time after subtotal nephrectomy was about 100 days in Wistar rats with vehicle. LCZ696‐(30 mg/kg) and valsartan‐(15 mg/kg) prolonged the survival of these animals, and the effect of LCZ696 on survival was significantly greater than that of valsartan. Renoprotective effects of LCZ696 judged by serum creatinine and urinary protein excretions were larger than those of valsartan. Cardioprotective effects judged by cardiac left ventricular mass, fractional shortening, and fibrosis of LCZ696 and valsartan were not detected under the present condition. Thus, the renoprotective effect of LCZ696 was stronger than that of valsartan in rats with subtotal nephrectomy. This study provides the idea that, compared to valsartan, LCZ696 is more effective for the treatment of human CKD.
Keywords: Chronic kidney disease, LCZ696, renoprotection
Abbreviations
- ACEI
angiotensin‐converting enzyme inhibitor
- ANP
atrial natriuretic peptide,
- ARB
angiotensin II receptor blocker
- CKD
chronic kidney disease
- ET‐1
endothelin‐1
- LCZ696
sacubitril/valsartan
- LV
left ventricle
- NEP
neutral endopeptidase
- STNx
subtotal nephrectomy
Introduction
Chronic kidney disease (CKD) is a life‐threatening condition by progressive and irreversible loss of renal function, which subsequently leads to end‐stage renal disease and causes premature mortality from cardiovascular disease (Lopez‐Novoa et al. 2010; Judge et al. 2015). Angiotensin‐converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs) are used for the treatment of patients with early CKD and provide better outcomes compared to those of other antihypertensive drugs (Ahmed et al. 2016). However, ACEI and ARB are reported to be not superior to other antihypertensive drugs in patients with advanced CKD (Ahmed et al. 2016), and, therefore, new therapeutic strategies are needed for these patients.
Regardless of etiology, the number of nephrons decreases during the progression of CKD (Lopez‐Novoa et al. 2010). The remaining nephrons elevate the filtration rate to maintain the excretory need of the organism at an early stage of CKD. Thereafter, the remaining nephrons cannot cope with the sustained extra load at an advanced stage of CKD. This situation is mimicked in experimental animals by surgically dissecting a large part of the renal mass (subtotal nephrectomy) in order to accelerate the progression of nephron loss, which culminates in renal failure and death (Lopez‐Novoa et al. 2010). The following profiles are reported after subtotal nephrectomy in rats (Koleganova et al. 2009); (1) Blood pressure elevated, and serum creatinine and urinary protein increased. (2) Wall thickness of cardiac left ventricle (LV) and cardiac fibrosis increased, whereas the fractional shortening of cardiac LV decreased.
Neutral endopeptidase (NEP) is a membrane‐bound metallopeptidase with a widespread distribution in the vascular endothelium, smooth muscle cells, and the brush border of tubular cells (Benigni et al. 2004). NEP degrades a number of peptides including atrial natriuretic peptide (ANP) and bradykinin, which stimulate nitric oxide production (Benigni et al. 2004). These data led us to speculate that a combination of ACEI+NEP inhibitor or ARB+NEP inhibitor provides better outcomes in patients with advanced CKD through glomerular hemodynamic alteration. Previous findings that AVE7688 and omapatrilat, which inhibit both ACE and NEP, provided greater renoprotection in rats with 5/6 nephrectomy (Taal et al. 2001; Benigni et al. 2004) support the idea.
Angiotensin receptor‐neprilysin inhibitor, sacubitril [4‐{[(2S,4R)‐1‐(4‐Biphenylyl)‐5‐ ethoxy‐4‐methyl‐5‐oxo‐2‐pentanyl]amino}‐4‐oxobutanoic acid]/valsartan [(2S)‐3‐Methyl‐2‐ (N‐{[2′‐(1H‐tetrazol‐5‐yl)biphenyl‐4‐yl]methyl}pentanamido)butanoic acid] (LCZ696) is a new drug for the treatment of heart failure (McMurray et al. 2014) and probably of hypertension (Ruilope et al. 2010). After ingestion, LCZ696 is converted to sacubitril and valsartan (Gu et al. 2010). To our knowledge, renoprotective effect of LCZ696 in patients with advanced CKD remains to be determined. To explore this question, the renoprotective effect of LCZ696 was compared to that of valsartan in rats with 5/6 nephrectomy.
Materials and Methods
Drugs
LCZ696 and valsartan, an ARB, were kindly provided by Novartis Phama AG (Basel, Switzerland).
Animals
Six‐week‐old male Wistar rats were obtained from Japan SLC Co. (Shizuoka, Japan). They were maintained for more than 2 weeks under free access to standard chow (CE‐2, Japan Clea, Tokyo, Japan) and water before the experiments. Study protocol was approved by the Institutional Review Committee of Jichi Medical University. The experiments were performed in accordance with the Use and Care of Experimental Animals Committee of Jichi Medical University, and Guide for the Care and Use of Laboratory Animals. Under pentobarbital anesthesia (50 mg/kg, ip), subtotal nephrectomy was performed by the ligation of renal artery branches supplying two‐thirds of the left kidney, followed by right unilateral nephrectomy 14 days later. After 8 weeks, the animals with subtotal nephrectomy were randomly divided into five groups in Experiment 1 and three groups in Experiment 2 according to the following criteria; (1) there were no statistical significant differences in body weight, blood pressure, serum creatinine, and urinary protein excretion among the groups, (2) mean values of these variables in each group were within 0.5‐fold of standard deviation from the means in all groups, and (3) all four variables met these conditions.
Study designs
Experiment 1
It is reported that oral dosing of LCZ696 (2–60 mg/kg) induces a dose‐dependent reduction in BP in hypertensive rats (Gu et al. 2010). In a preliminary study, relatively high dose of LCZ696‐(50 mg/kg) was given orally, once daily to four rats with subtotal nephrectomy. However, as two rats died within 2 weeks (on day 12 and on day 13), middle dose of LCZ696 (30 mg/kg) was selected in further studies. The valsartan doses (5 or 15 mg/kg) are approximately the molar equivalent doses of LCZ696 (10 or 30 mg/kg).
Rats with subtotal nephrectomy were orally given valsartan‐(5 or 15 mg/kg), LCZ696‐(10 or 30 mg/kg) or vehicle (corn coil) by a gastric gavage once daily for 12 weeks. Sham‐operated animals were given vehicle alone. After the initiation of the drug treatment, survival was checked once daily for 12 weeks. Before, and at 4, 8, and 12 weeks after dosing of the drug, blood samples were obtained from tail vein, and systolic BP was measured by a standard tail‐cuff sphygmomanometer (KN‐201, Natsume, Tokyo, Japan) in an awake and restrained state. Urine samples were also collected for 24 h in a metabolic cage at each observation point.
In the first trial, the following numbers of rats were alive at 12 weeks; 1 of 6 in the vehicle alone, 3 of 6 in the valsartan‐(15 mg/kg), 4 of 5 in the LCZ696‐(30 mg/kg), and 6 of 6 in the sham‐operated groups. To obtain finally more survived animals at 12 weeks; more than three rats in the vehicle group and more than six rats in the drug‐treated {valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg)} groups, additional trial was done. In the second trial, the numbers of rats with subtotal nephrectomy were used as follows; 12 rats in the vehicle, seven rats in the valsartan‐(15 mg/kg) and five rats in the LCZ696‐(30 mg/kg) groups. In addition, two sham‐operated rats, and 15 rats with subtotal nephrectomy {9 in the valsartan‐(5 mg/kg) and six in the LCZ696‐(10 mg/kg) groups} were also used.
All data obtained at each observation point (before, and at week 4, 8, and 12) were used to evaluate the time‐course of blood pressure, serum creatinine, and urinary protein excretion.
Experiment 2
Rats with subtotal nephrectomy orally received valsartan‐(15 mg/kg), LCZ696‐(30 mg/kg) or vehicle alone by a gastric gavage once daily for 8 weeks. Sham‐operated rats were given vehicle. Echocardiographic evaluation was performed before and at 4 and 8 weeks after treatment. Two or three days after the end of echocardiographic evaluation at 8 weeks, rat was placed into a metabolic cage for 24 h to collect urine, and thereafter, blood and kidney samples were obtained under pentobarbital anesthesia (50 mg/kg, ip).
The initial numbers of rats were as follows; six in the vehicle, valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg) groups, and five in the sham‐operated group. All echocardiographic data obtained at each observation point (before, and at week 4 and 8) were used. Samples for laboratory assays, mRNA expressions and histological analysis were obtained in rats survived at 8 weeks.
Assay methods
Serum creatinine was measured by a Jaffe's method (LaboAssay™ Creatinin, Wako Pure Chemicals, Osaka, Japan), and urinary protein was determined colorimetrically using pyrogallol red method (MICRO TP‐AR, Wako). Concentrations of urinary endothelin‐1 (ET‐1) and nitric oxide {nitrite (NO2 −) and nitrate (NO3 −)} were measured using the enzyme‐linked immunosorbent assay kits (DET100 and KGE001, R&D systems, Minneapolis, MN). Urinary guanosine 3′,5′‐cyclic monophosphate (cGMP) (581022, Cayman Chemical, Ann Arbor, MI) and serum and urinary ANP (EIAR‐ANP, RayBiotech, Norcross, GA) were measured using the enzyme immuno assay kits.
RNA extraction and real‐time PCR
Total RNA in renal cortex was extracted using the PureLink® RNA Mini Kit (Thermo Fisher Scientific, CA), and reverse transcription was done using PrimeScript RT reagent Kit (Takara Bio, Otsu, Japan). Real‐time PCR was performed with SYBR Premix Ex Taq (Takara Bio) using the Stratagene Mx3005P QPCR System (Agilent Technologies, Santa Clara, CA). Sequences of specific primers for each gene were described in Table S1. Each mRNA expression level was normalized to the expression of Ribosomal protein S18 and quantified using comparative threshold cycle method.
Echocardiographic evaluation
Rats were lightly anaesthetized with 3% inhaled isoflurane and set in a supine position. Two‐dimensional M‐mode and Doppler echocardiography was performed with a Vevo2100 Imaging System (Visual Sonics Inc., Toronto, Canada). LV at the papillary muscle level was imaged in the parasternal short‐axis view. LV fractional shortening and LV mass were calculated with equipped software. All evaluations were performed by one investigator blinded to the experimental group (KA).
Histopathological evaluations of kidney and heart
Formalin fixed tissue sections were embedded in paraffin, cut into 5‐μm‐thick sections. Kidney samples were stained with periodic acid‐schiff staining and periodic acid silver‐methenamine staining for histopathological analysis. At least 50 glomeruli in each kidney were graded from 0 to 4 according to the severity of the glomerular sclerosis (Kasiske et al. 1988): 0 = normal; 1 = slight glomerular damage, the mesangial matrix and/or hyalinosis with focal adhesion, involving sclerosis of <25% of the glomerulus; 2 = sclerosis of 25 to 50%; 3 = sclerosis of 50 to 75%; and 4 = sclerosis of >75% of the glomerulus. To assess the extent of tubular dilation, tubular epithelial cell flattening and interstitial fibrosis, 20 fields of tublulointerstitial area in each sample (magnification, ×400) were examined and graded as follows (Kasiske et al. 1988): 0 = no lesions; 1 = very mild focal dilation of tubules; 2 = larger number of dilated tubules with widening interstitium; 3 = fairly extensive dilation of tubules with cystic formation and widening of interstitium; and 4 = entire atrophy of tubules. Heart sample was supplied to the preparation of transverse transmural slices of the LV and stained with Masson's trichrome staining. To assess the extent of myocardial fibrosis, 20 fields of LV wall in each sample (magnification, ×400) were examined and graded as follows (Radovits et al. 2013): 0 = absent; 1 = slight; 2 = moderate; and 3 = intense. All samples were examined by one investigator blinded to the treatment protocol of the animals (YA).
Statistical analysis
Data are shown as the means ± SD. Comparison of survival periods was performed by Kaplan–Meier analysis. Changes in blood pressure, urinary protein excretion, serum creatinine concentration, LV fractional shortening, and LV mass were analyzed by two‐way repeated‐measures ANOVA, and variables were compared by one‐way ANOVA. The difference between the two groups was analyzed by the Bonferroni–Dunn test. The histopathological scores were compared using Kruskal–Wallis test, and the Mann–Whitney U test with Bonferroni correction. A value of P < 0.05 was considered to be significant.
Results
Experiment 1
Survival rate and blood pressure
In this study, mean survival times {8 weeks (56 days) after subtotal nephrectomy +days after dosing} in the first and the second trials were 102.4 and 100.0 days (vehicle), 116.3 and 112.6 days (valsartan‐15 mg/kg), and 138.8 and 129.7 days (LCZ696‐30 mg/kg). Data of the survival in the first and the second trials were combined and analyzed. Compared to the vehicle, valsartan‐(5 mg/kg) slightly and valsartan‐(15 mg/kg) significantly prolonged the survival of the animals (Fig. 1). LCZ696‐(10 mg/kg and 30 mg/kg) also significantly prolonged their survival compared to the vehicle. The effect of LCZ696‐(30 mg/kg) on survival was significantly greater than that of valsartan‐(15 mg/kg).
Figure 1.
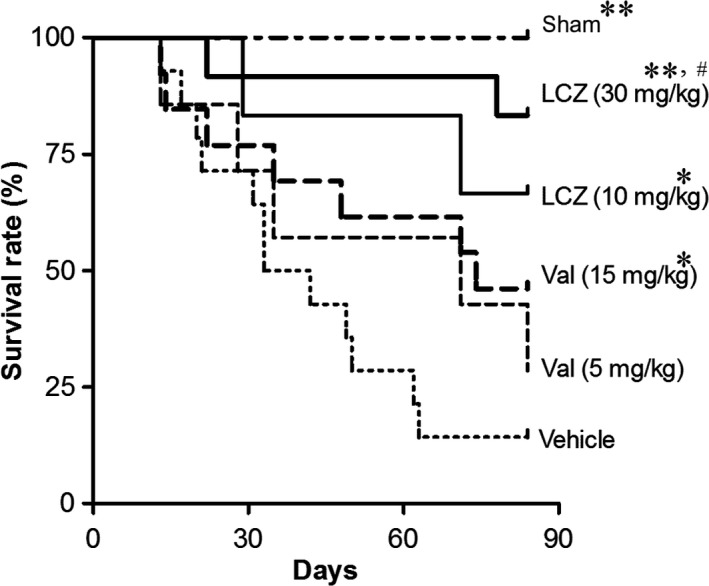
Survival rate during the repeated dosing of LCZ696 and valsartan for 12 weeks. Initial number of animals in each group: n = 8 in the sham, n = 18 in the vehicle, n = 9 in the valsartan‐(5 mg/kg), n = 13 in the valsartan‐(10 mg/kg), n = 6 in the LCZ696‐(10 mg/kg) and n = 10 in the LCZ696‐(30 mg/kg) Mean survival period in rats with vehicle was 100 days {8 weeks (56 days) after subtotal nephrectomy +34 days after dosing of vehicle}. *P < 0.05, **P < 0.01 versus vehicle; #P < 0.05 versus valsartan‐(15 mg/kg).
LCZ696‐(30 mg/kg) and valsartan‐(15 mg/kg) significantly decreased systolic BP in rats with subtotal nephrectomy, but the parameter in the drug‐treated groups was still higher (P < 0.01) than that in the sham‐operated group (Fig. 2). BP‐lowering effect of LCZ696‐(30 mg/kg) and valsartan‐(15 mg/kg) did not significantly differ.
Figure 2.
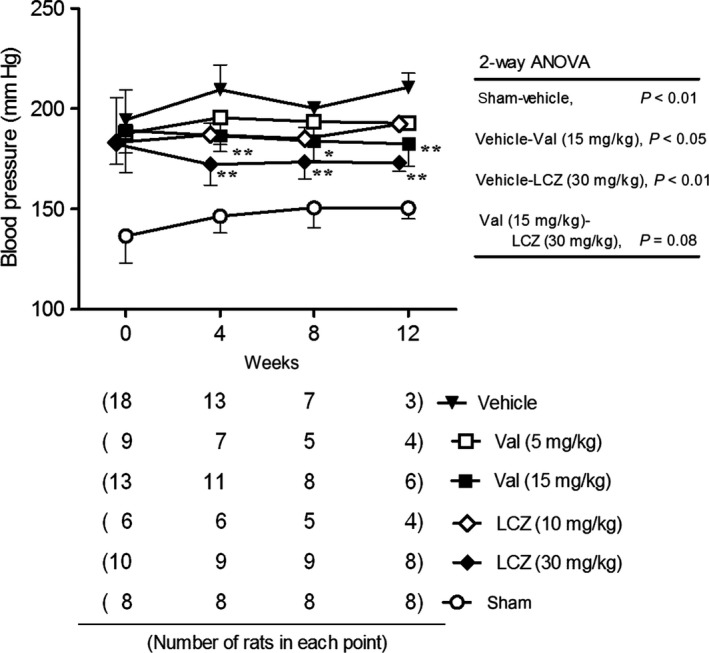
Systolic blood pressure during the repeated dosing of LCZ696 and valsartan for 12 weeks. All data obtained at each observation point were used. The number of rats of each point was described in the bottle panel. *P < 0.05, **P < 0.01 versus vehicle; Mean ± SD
Serum creatinine and urinary protein excretion
Before the initiation of the experiment, while serum creatinine and urinary protein excretion in rats with subtotal nephrectomy were significantly elevated compared to the sham‐operated group, there were no significant differences in these variables among the groups with subtotal nephrectomy (Fig. 3). During the treatment period, serum creatinine and urinary protein excretion further elevated in the vehicle‐treated rats with subtotal nephrectomy. While valsartan and LCZ696 did not suppress the elevation in serum creatinine, these drugs dose dependently diminished the elevations of urinary protein excretion. In addition, protective effect of LCZ696‐(30 mg/kg) against urinary protein excretion was significantly greater than that of valsartan‐(15 mg/kg). Body weight in each subtotal nephrectomized group was almost same level or slightly decreased during 12 weeks of dosing period (Fig. S1).
Figure 3.
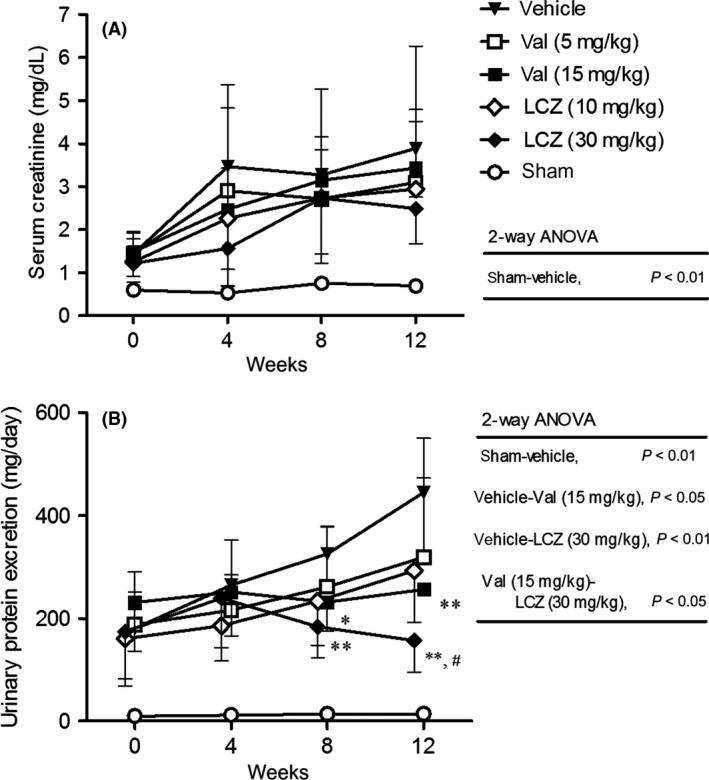
Serum creatinine (A) and urinary protein excretion (B) during the repeated dosing of LCZ696 and valsartan for 12 weeks. All data obtained at each observation point were used. The number of rats of each point was described in Fig. 2. *P < 0.05, **P < 0.01 versus vehicle; #P < 0.05 versus valsartan‐(15 mg/kg); Mean ± SD.
Experiment 2
Laboratory tests, mRNA expressions and renal histology
In Experiment 2, Body weight and serum creatinine concentration did not differ among the subtotal nephrectomized groups (Fig. S2). Compared to the sham‐operated group, serum ANP and urinary ET‐1 excretion were significantly higher, whereas urinary excretions of ANP, cGMP, and nitric oxide were significantly lower in the vehicle group (Fig. 4). Valsartan‐(15 mg/kg) did not correct these changes except for urinary ET‐1 excretion. On the other hand, LCZ696‐(30 mg/kg) significantly decreased serum ANP and urinary ET‐1 excretion, and significantly increased urinary ANP and nitric oxide excretions compared to the vehicle group. LCZ696‐(30 mg/kg) also tended to increase urinary cGMP excretion. Serum ANP in subtotal nephrectomized rats with LCZ696‐(30 mg/kg) was higher, but not significantly, than that of the sham‐operated animals (pg/ml; 40 ± 10 vs. 29 ± 7).
Figure 4.
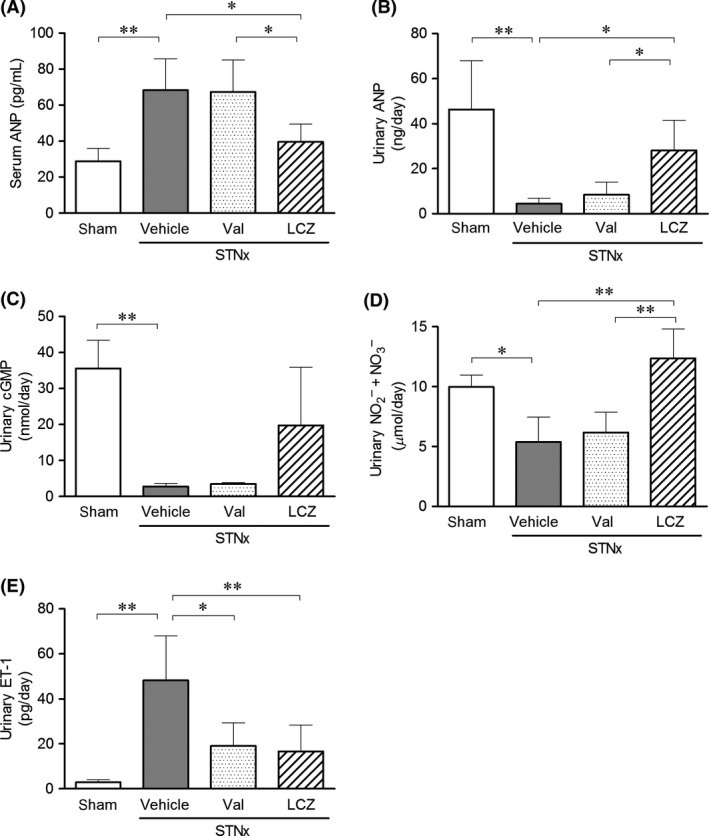
(A) Serum ANP concentration, (B) urinary excretions of ANP, (C) cGMP, (D) nitric oxide, and (E) ET‐1 after the repeated dosing of valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg) for 8 weeks. Data were obtained in rats survived at 8 weeks. Number of animals; n = 4 in the vehicle and valsartan‐(15 mg/kg) groups, and n = 5 in the LCZ696‐(30 mg/kg) and sham groups. STNx; subtotal nephrectomy, *P < 0.05, **P < 0.01; Mean ± SD.
Expression levels of neprilysin (NEP), angiotensin‐converting enzyme2 (ACE2), and natriuretic peptide receptor (NPR)‐C mRNAs in renal cortex of rats with subtotal nephrectomy were significantly decreased compared to those in the sham‐operated rats (Fig. 5). On the other hand, mRNA expression level of NPR‐A in renal cortex did not differ between the sham‐operated and subtotal nephrectomized rats. While valsartan‐(15 mg/kg) did not affect these mRNA expressions, LCZ696‐(30 mg/kg) significantly increased NPR‐C mRNA expression level compared to that in the vehicle and valsartan‐(15 mg/kg) groups.
Figure 5.
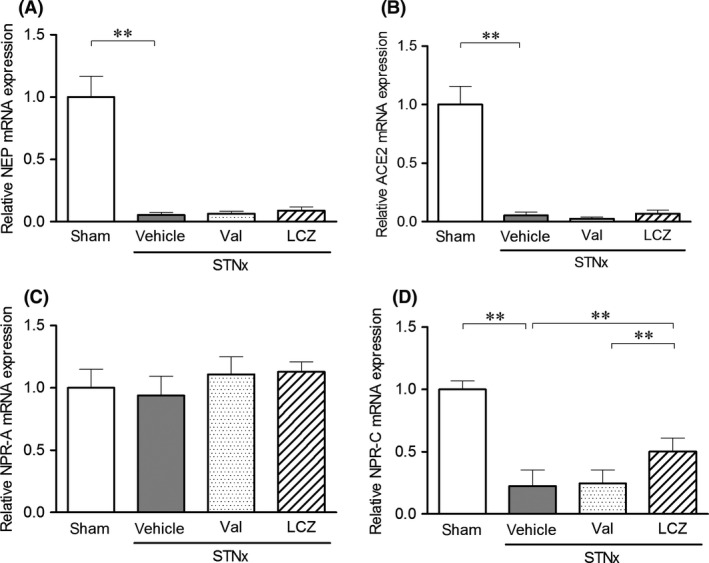
The mRNA expressions of (A) NEP, (B) ACE2, (C) NPR‐A, and (D) NPR‐C in renal cortex after the repeated dosing of valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg) for 8 weeks. Data were obtained in rats survived at 8 weeks. Number of animals; n = 4 in the vehicle and valsartan‐(15 mg/kg) groups, and n = 5 in the LCZ696‐(30 mg/kg) and sham groups. STNx; subtotal nephrectomy, Mean expression level of sham group was set to 1.0. **P < 0.01; Mean ± SD.
Severe glomerular sclerosis, extensive dilation of tubules, and widening of interstitium were detected in rats with subtotal nephrectomy (Fig. 6). Both glomerulosclerosis and tubulointerstitial scores in the LCZ696‐(30 mg/kg) group were significantly lower than those in the vehicle group. Valsartan‐(15 mg/kg) significantly lowered tubulointerstitial score, but not glomerulosclerosis score. Thus, protective effect of LCZ696‐(30 mg/kg) against progression in glomerular sclerosis was significantly greater than that of valsartan‐(15 mg/kg).
Figure 6.
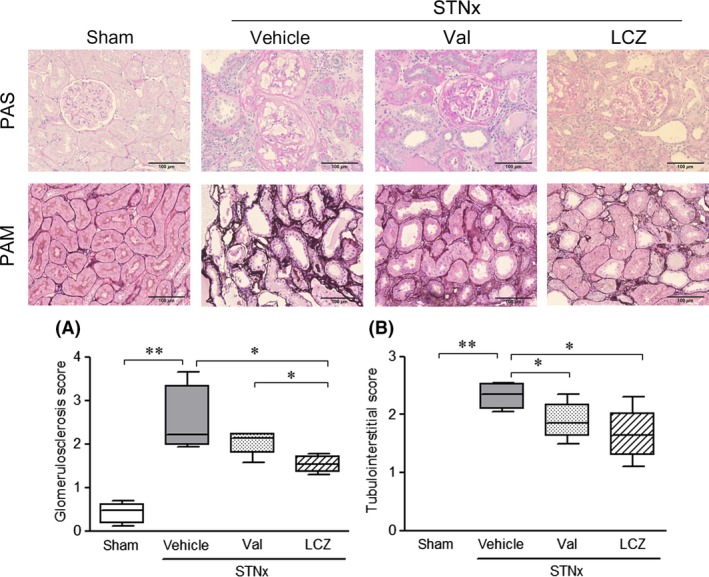
Renal histological changes after the repeated dosing of valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg) for 8 weeks. Data were obtained in rats survived at 8 weeks. Representative images of periodic acid‐schiff staining (PAS, A) and periodic acid silver‐methenamine (PAM, B) were shown in the upper panel. Scale bar: 100 μm, Number of animals; n = 4 in the vehicle and valsartan‐(15 mg/kg) groups, and n = 5 in the LCZ696‐(30 mg/kg) and sham groups. STNx; subtotal nephrectomy, *P < 0.05, **P < 0.01; Bars indicate 90% tile and 10% tile, and boxes indicate 75% tile to 25% tile with lines of median values inside the boxes.
Cardiac function and fibrosis
Compared to the sham‐operated group, fractional shortening significantly decreased and left ventricular mass significantly increased in the vehicle group (Fig. 7A, B). No significant differences were detected in body weight among the treated groups {vehicle, valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg)} during the study period (data not shown). Valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg) did not correct the changes in the values of fractional shortening and left ventricular mass. Cardiac fibrosis detected in the vehicle group was not significantly improved by valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg) (Fig. 7C).
Figure 7.
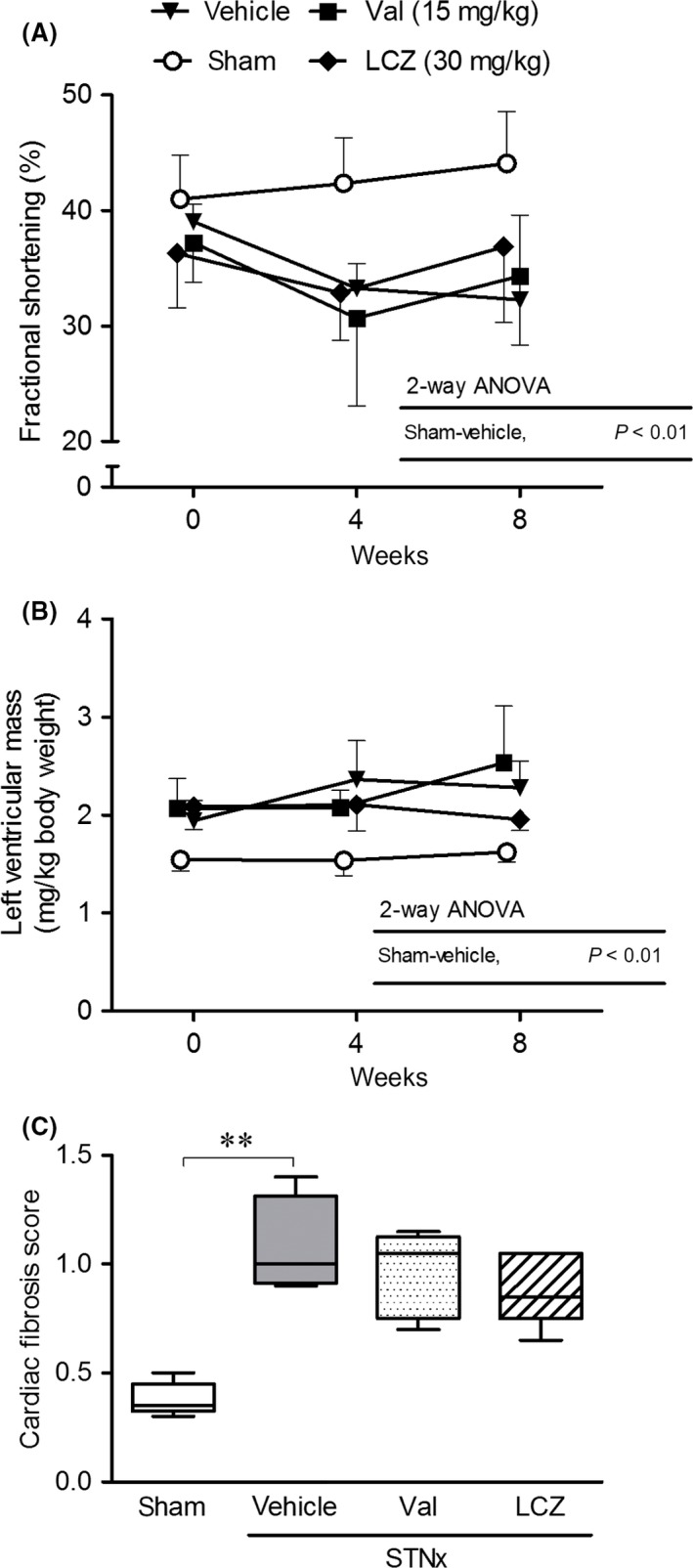
Cardiac function during the repeated dosing of valsartan‐(15 mg/kg) and LCZ696‐(30 mg/kg) for 8 weeks, and cardiac fibrosis at the end of the treatment. All echocardiographic data obtained at each observation point were used. Number of animals in each group (week 0‐week 4‐week 8); n = 5‐5‐5 in the sham, n = 6‐4‐4 in the vehicle, n = 6‐5‐4 in the valsartan‐(15 mg/kg), and n = 6‐6‐5 in the LCZ696‐(30 mg/kg). STNx; subtotal nephrectomy, **P < 0.01; (A, B) Mean ± SD, (C) Bars indicate 90% tile and 10% tile, and boxes indicate 75% tile to 25% tile with lines of median values inside the boxes.
Discussion
Mean survival time in the vehicle‐treated rats with subtotal nephrectomy was about 100 days in this study, which is similar to previous finding (mean 92 days) in 5/6 nephrectomized rats (Satoh et al. 1995). LCZ696 and valsartan dose dependently prolonged the survival of these animals, and the effect of LCZ696‐(30 mg/kg) was significantly greater than that of valsartan‐(15 mg/kg). Subtotal nephrectomy causes the elevations in serum creatinine concentration, urinary protein excretion, and blood pressure in rats (Koleganova et al. 2009), which are confirmed in this study. In this study, LCZ696 and valsartan dose dependently reduced urinary protein excretion in rats with subtotal nephrectomy, and the effect of LCZ696‐(30 mg/kg) was significantly greater than that of valsartan‐(15 mg/kg). In addition, compared to the vehicle group, glomerular sclerosis was significantly alleviated in the LCZ696‐(30 mg/kg), but not the valsartan‐(15 mg/kg) groups at 8 weeks in Experiment‐2. These data indicate that the renoprotective effect of LCZ696‐(30 mg/kg) was greater than that of valsartan‐(15 mg/kg), which might, at least in part, explain different effects of LCZ696‐(30 mg/kg) and valsartan‐(15 mg/kg) on the survival of subtotal nephrectomized rats detected in this study. Blood pressure lowering effect of LCZ696‐(30 mg/kg) was slightly greater than that of valsartan‐(15 mg/kg) in rats with subtotal nephrectomy (P = 0.08), which might also contribute to different effects of the dosage regimens on the survival of these animals.
Previous study showed that the progressive renal disease in rats with subtotal nephrectomy is associated with the increased renal synthesis of ET‐1 and the decreased generation of nitric oxide in the remnant kidney (Benigni et al. 2004). ET‐1 is considered to be involved in the mechanism of progressive renal injury in chronic disease (Benigni 2000). As the renoprotective effect of an endothelin receptor antagonist partly depends on the increased renal nitric oxide generation in rats with subtotal nephrectomy, excessive renal ET‐1 could be responsible for the defective renal nitric oxide in these animals (Aiello et al. 1998). On the other hand, nitric oxide might decrease the ET‐1 synthesis in subtotal nephrectomized rats (Benigni et al. 1999). Thus, the imbalance of renal ET‐1/nitric oxide could contribute to the progression of renal injury in the animals with kidney diseases. In this study, urinary excretion of ET‐1, the marker of renal ET‐1 synthesis, increased and urinary nitrite/nitrate excretion, the marker of nitric oxide synthesis, decreased in the vehicle‐treated group which are similar to previous data (Benigni et al. 2004). LCZ696‐(30 mg/kg) corrected the elevation of urinary ET‐1 excretion and the decrease in urinary nitrite/nitrate excretion, whereas valsartan‐(15 mg/kg) only blunted the elevation of urinary ET‐1 excretion in this study. Based on the findings, the renoprotective effect of LCZ696‐(30 mg/kg) is speculated to be greater than that of valsartan‐(15 mg/kg) in rats with subtotal nephrectomy. The present finding supports the idea that the protective effect of LCZ696 against renal tissue damages is greater than that of valsartan in human CKD.
In this study, urinary ET‐1 excretion was significantly decreased by the treatment with valsartan‐(15 mg/kg), whereas urinary nitric oxide excretion did not increase. Imai T demonstrated that angiotensin II exhibited a stimulatory effect on prepro‐ET‐1 mRNA expression in cultured endothelial cells (Imai et al. 1992). In addition, a blockade of angiotensin II type 1 receptor inhibited ET‐1 expression in rat vascular smooth muscle cells (Sung et al. 1994). Thus, these observations and the present findings suggest that valsartan decreased urinary ET‐1 excretion mainly through the blockade of angiotensin II type 1 receptor in rats with subtotal nephrectomy.
In this study, serum ANP increased and urinary nitric oxide decreased in the vehicle‐treated rats. ANP is mainly degraded through two processes: (1) natriuretic peptide receptor (NPR)‐C‐mediated internalization followed by lysosomal degradation in organs including kidney and (2) enzymatic degradation by NEP (Volpe et al. 2016). Hydrosoluble, filtered ANP is also degraded by NEP in the kidney (Roques et al. 1993). In the subtotal nephrectomized rats, NEP and NPR‐C mRNA expression levels in renal cortex were significantly decreased. These alterations of the mRNA expressions correlated with the reduction in the degradation of ANP in the kidney which, in turn, led to the elevation of serum ANP in the vehicle‐treated rats. Although serum ANP elevated and renal NPR‐A mRNA expression did not change in the vehicle group in this study, urinary nitric oxide excretion did not increase, but decreased. These data indicate that the renal response to ANP is blunted in rats with subtotal nephrectomy. In this study, ACE2 mRNA expression significantly decreased in the vehicle‐treated rats. This enzyme catabolizes angiotensin II to produce Ang1‐7, which exhibits renoprotective action by upregulating nitric oxide and prostaglandins (Santos et al. 2013; Mizuiri and Ohashi 2015). Therefore, reduced ACE2 mRNA expression in renal cortex might contribute to the decrease in urinary nitric oxide excretion in the vehicle‐treated rats.
In this study, LCZ696‐(30 mg/kg), but not valsartan‐(15 mg/kg) corrected the changes in serum ANP and urinary excretions of ANP and cGMP. Previous studies showed that NEP inhibition potentiates the renal actions of ANP and increases urinary ANP and cGMP excretions (Margulies et al. 1990; Haloui et al. 2001). In addition, this study showed that LCZ696 increased the renal NPR‐C mRNA expression, which might contribute to the decrease in serum ANP and increase in urinary ANP in rats with subtotal nephrectomy.
Recently, cardioprotective effects of LCZ696 were reported by several groups. von Lueder et al. (2015) showed that 68 mg/kg of LCZ696 reduced cardiac hypertrophy and fibrosis in Sprague–Dawley rats after myocardial infarction. Kusaka et al. (2015) observed that 60 mg/kg of LCZ696 ameliorated cardiac fibrosis in spontaneously hypertensive rats on a high‐salt diet. The following cardiac changes are reported after subtotal nephrectomy in rats; increased left ventricular mass, decreased left ventricular fractional shortening, and increased fibrosis (Koleganova et al. 2009), which were also detected in this study. It was expected that LCZ696‐(30 mg/kg) might improve these cardiac changes in rats with subtotal nephrectomy. However, LCZ696‐(30 mg/kg) did not exert cardioprotective effects under the present condition. We do not have any definite explanations for diverse findings, but differences in the dose of LCZ696, strain of animals or animal models might be involved.
In summary, this study showed that the renoprotective effects of LCZ696‐(30 mg/kg) were stronger than those of valsartan‐(15 mg/kg) in rats with subtotal nephrectomy, which might be involved in the mechanism of the prolonged survival of the animals treated with LCZ696‐(30 mg/kg). These findings provide the idea that, compared to valsartan, LCZ696 is more effective for the treatment of human CKD.
Author Contributions
Participated in research design: Ushijima, Ando, Aizawa, Tsuruoka, and Fujimura. Conducted experiments: Ushijima, Arakawa, Aizawa, Suzuki, and Shimada. Contributed new reagents or analytic tools: Ushijima and Tsuruoka. Performed data analysis: Ushijima and Suzuki, Shimada. Wrote or contributed to the writing of the manuscript: Ushijima, Ando, Arakawa, and Fujimura.
Disclosure
All authors declare no conflict of interest.
Supporting information
Table S1. Sequences of primers for real‐time PCR analysis.
Figure S1. Body weight of rat during the repeated dosing of LCZ696 and valsartan for 12 weeks in Experiment 1.
Figure S2. (A) Body weight of rats during the repeated dosing of LCZ696 and valsartan for 8 weeks and (B) serum creatinine concentration at 8 weeks in Experiment 2.
Acknowledgement
We thank Novartis Phama AG for supplying LCZ696 and valsartan.
Ushijima K., Ando H., Arakawa Y., Aizawa K., Suzuki C., Shimada K., Tsuruoka S.‐i., Fujimura A.. Prevention against renal damage in rats with subtotal nephrectomy by sacubitril/valsartan (LCZ696), a dual‐acting angiotensin receptor‐neprilysin inhibitor, Pharma Res Per, 5(4), 2017,e00336, https://doi.org/10.1002/prp2.336
References
- Ahmed A, Jorna T, Bhandari S (2016). Should we stop angiotensin converting enzyme inhibitors/angiotensin receptor blockers in advanced kidney disease? Nephron 133: 147–158. [DOI] [PubMed] [Google Scholar]
- Aiello S, Remuzzi G, Noris M (1998). Nitric oxide/endothelin balance after nephron reduction. Kidney Int 53: S63–S67. [PubMed] [Google Scholar]
- Benigni A (2000). Endothelin antagonists in renal disease. Kidney Int 57: 1778–1794. [DOI] [PubMed] [Google Scholar]
- Benigni A, Zoja C, Noris M, Corna D, Benedetti G, Bruzzi I, et al. (1999). Renoprotection by nitric oxide donor and Lisinopril in the remnant kidney model. Am J Kidney Dis 33: 746–753. [DOI] [PubMed] [Google Scholar]
- Benigni A, Zoja C, Zatelli C, Corna D, Longaretti L, Rottoli D, et al. (2004). Vasopeptidase inhibitor restores the balance of vasoactive hormones in progressive nephropathy. Kidney Int 66: 1959–1965. [DOI] [PubMed] [Google Scholar]
- Gu J, Noe A, Chandra P, Al‐Fayoumi S, Ligueros‐Saylan M, Sarangapani R, et al. (2010). Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual‐acting angiotensin receptor‐neprilysin inhibitor (ARNi). J Clin Pharmacol 50: 401–414. [DOI] [PubMed] [Google Scholar]
- Haloui M, Messika‐Zeitoun D, Louedec L, Philippe M, Michel JB (2001). Potentiation of urinary atrial natriuretic peptide interferes with macula densa function. Cardiovasc Res 51: 542–552. [DOI] [PubMed] [Google Scholar]
- Imai T, Hirata Y, Emori T, Yanagisawa M, Masaki T, Marumo F (1992). Induction of endothelin‐1 gene by angiotensin and vasopressin in endothelial cells. Hypertension 19: 753–757. [DOI] [PubMed] [Google Scholar]
- Judge P, Haynes R, Landray MJ, Baigent C (2015). Neprilysin inhibition in chronic kidney disease. Nephrol Dial Tranplant 30: 738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiske BL, O'Donnell MP, Garvis WJ, Keane WF (1988). Pharmacologic treatment of hyperlipidemia reduces glomerular injury in rats 5/6 nephrectomy model of chronic renal failure. Circ Res 62: 367–374. [DOI] [PubMed] [Google Scholar]
- Koleganova N, Piecha G, Ritz E, Bekeredjian R, Schirmacher P, Schmitt CP, et al. (2009). Interstitial fibrosis and microvascular disease of the heart in uremia: amelioration by a calcimimetic. Lab Invest 89: 520–530. [DOI] [PubMed] [Google Scholar]
- Kusaka H, Sueta D, Koibuchi N, Hasegawa Y, Nakagawa T, Lin B, et al. (2015). LCZ696, angiotensinII receptor‐neprilysin inhibitor, ameliorates high‐salt‐induced hypertension and cardiovascular injury more than valsartan alone. Am J Hypertens 28: 1409–1417. [DOI] [PubMed] [Google Scholar]
- Lopez‐Novoa JM, Martinez‐Salgado C, Rodriguez‐Pena AB, Hernandes FJL (2010). Common pathophysiological mechanisms of chronic kidney disease: therapeutic perspectives. Pharmacol Ther 128: 61–81. [DOI] [PubMed] [Google Scholar]
- von Lueder TG, Wang BH, Kompa AR, Huang L, Webb R, Jordaan P, et al. (2015). Angiotensin receptor neprilysin inhibitor LCZ696 attenuates cardiac remodeling and dysfunction after myocardial infarction by reducing cardiac fibrosis and hypertrophy. Circ Heart Fail 8: 71–78. [DOI] [PubMed] [Google Scholar]
- Margulies KB, Cavero PG, Seymour AA, Delaney NG, Burnett JC (1990). Neutral endopeptidase inhibition potentiates the renal actions of atrial natriuretic factor. Kidney Int 38: 67–72. [DOI] [PubMed] [Google Scholar]
- McMurray JJV, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. (2014). Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 371: 993–1004. [DOI] [PubMed] [Google Scholar]
- Mizuiri S, Ohashi Y (2015). ACE and ACE2 in kidney disease. World J Nephrol 4: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radovits T, Oláh A, Lux Á, Németh BT, Hidi L, Birtalan E, et al. (2013). Rat model of exercise‐induced cardiac hypertrophy: hemodynamic characterization using left ventricular pressure‐volume analysis. Am J Physiol Heart Circ Physiol 305: H124–H134. [DOI] [PubMed] [Google Scholar]
- Roques BP, Noble F, Dauge V, Fournie‐Zaluski MC, Beaumont A (1993). Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev 45: 87–146. [PubMed] [Google Scholar]
- Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowits MP (2010). Blood‐pressure reduction with LCZ696, a novel dual‐acting inhibitor of the angiotensin II receptor and neprilysin: a randomized, double‐blind, placebo‐controlled, active comparator study. Lancet 375: 1255–1266. [DOI] [PubMed] [Google Scholar]
- Santos RA, Ferreira AJ, Verano‐Braga T, Bader M (2013). Angiotensin‐converting enzyme 2, angiotensin‐(1‐7) and Mas: new players of the renin‐angiotensin system. J Endocrinol 216: R1–R17. [DOI] [PubMed] [Google Scholar]
- Satoh S, Kaneko T, Omori S, Sugimura J, Ujiie T, Fujioka T, et al. (1995). The effect of enalapril and sairei‐to on survival‐time for the rat with subtotal nephrectomy. Nihon Jinzo Gakkai Shi 37: 112–118. [PubMed] [Google Scholar]
- Sung CP, Arleth AJ, Storer BL, Ohlstein EH (1994). Angiotensin type 1 receptors mediate smooth muscle proliferation and endothelin biosynthesis in rat vascular smooth muscle. J Pharmacol Exp Ther 271: 429–437. [PubMed] [Google Scholar]
- Taal MW, Nenov VD, Wong W, Satyal SR, Sakharova O, Choi JH, et al. (2001). Vasopeptidase inhibition affords greater renoprotection than angiotensin‐ converting inhibition alone. J Am Soc Nephrol 12: 2051–2059. [DOI] [PubMed] [Google Scholar]
- Volpe M, Carnovali M, Mastromarino V (2016). The natriuretic peptides system in the pathophysiology of heart failure: from molecular basis to treatment. Clin Sci 130: 57–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sequences of primers for real‐time PCR analysis.
Figure S1. Body weight of rat during the repeated dosing of LCZ696 and valsartan for 12 weeks in Experiment 1.
Figure S2. (A) Body weight of rats during the repeated dosing of LCZ696 and valsartan for 8 weeks and (B) serum creatinine concentration at 8 weeks in Experiment 2.


