Abstract
We previously demonstrated that the peptidergic neurotransmitter pituitary adenylate cyclase-activating polypeptide (PACAP) affects the autonomic system and contributes to the control of metabolic and cardiovascular functions. Previous studies have demonstrated the importance of centrally-mediated sympathetic effects of leptin for obesity-related hypertension. Here we tested whether PACAP signaling in the brain is implicated in leptin-induced sympathetic excitation and appetite suppression. In anesthetized mice, intracerebroventricular (ICV) pre-treatment with PACAP6-38, an antagonist of the PACAP receptors (PAC1-R and VPAC2), inhibited the increase in white adipose tissue sympathetic nerve activity (WAT-SNA) produced by ICV leptin (2 μg). In contrast, leptin-induced stimulation of renal sympathetic nerve activity (RSNA) was not affected by ICV pre-treatment with PACAP6-38. Moreover, in PACAP-deficient (Adcyap1−/−) mice, ICV leptin-induced WAT-SNA increase was impaired, whereas RSNA response was preserved. The reductions in food intake and body weight evoked by ICV leptin were attenuated in Adcyap1−/− mice. Our data suggest that hypothalamic PACAP signaling plays a key role in the control by leptin of feeding behavior and lipocatabolic sympathetic outflow, but spares the renal sympathetic traffic.
Keywords: leptin, autonomic nerve, kidney, white adipose tissue, feeding behavior, pituitary adenylate cyclase-activating polypeptide
INTRODUCTION
The hormone leptin is produced mainly in the white adipose tissue (WAT), released into the circulation, and acts on the central nervous system. Leptin is involved in appetite suppression, thermogenesis, and lipolysis acceleration (Shen et al., 2007; Kalil and Haynes, 2012). Leptin stimulation of the sympathetic nerve activity (SNA) to brown adipose tissue, and WAT is important for the metabolic regulation. In addition, leptin causes sympathetic activation to the kidney, resulting in blood pressure elevation (Shen et al., 2007; Rahmouni, 2010). In obese hypertensive animals, the high levels of plasma leptin are associated with the inability of leptin to properly regulate thermogenic sympathetic traffic and feeding behavior, but intact renal sympathetic and arterial pressure responses to leptin (Rahmouni et al., 2005; Tanida et al., 2006), implicating selective leptin resistance as a major mechanism in obesity-associated hypertension.
The pituitary adenylate cyclase-activating polypeptide (PACAP), a neuropeptide and member of the vasoactive intestinal polypeptide (VIP)/secretin/glucagon family (Miyata et al., 1989; Hashimoto et al., 2001), was recently identified by immunohistochemical study in the hypothalamus (Hannibal, 2002), which is major site of leptin action (Kim et al., 2011). Central PACAP was found to regulate homeostatic functions such as appetite (Mounien et al., 2009), body temperature (Hawke et al., 2009; Resch et al., 2011), energy metabolism (Inglott et al., 2011), the cardiovascular system (Tanida et al., 2010; Farnham et al., 2012), and the autonomic nervous system (Tanida et al., 2010). In particular, we recently found that intracerebroventricular (ICV) injection of PACAP stimulated SNA to the kidney and adipose tissue, and raised arterial pressure in anesthetized rats (Tanida et al., 2010). Furthermore, sympathetic and hyperglycemic responses to some stressful stimuli including strong light exposure (Hatanaka et al., 2008), immobilization, and/or ether exposure (Tanida et al., 2010) were disrupted in Adcyap1−/− mice.
With regard to PACAP neurotransmission in the brain, it is considered that PACAP-induced responses result from interaction with three receptor types expressed in the hypothalamus: thePACAP-specific receptor (PAC1-R); the PACAP/VIP mutual receptors, VPAC1-R, and VPAC2-R (Ishihara et al., 1992; Lutz et al., 1993; Spengler et al., 1993). Of note, in mice ICV PACAP-evoked suppression of appetite and elevation of body temperature were abolished by pre-injection of PACAP6-38, an antagonist of PAC1-R and VPAC2 (Hawke et al., 2009). In addition, light-induced phase delay was also attenuated by ICV PACAP6-38 (Bergström et al., 2003).
There is evidence for the regulation ofPACAP signaling by leptin in the brain: ICV injection of leptin increased the expression of PACAP mRNA in the ventromedial hypothalamus, and leptin-induced appetite suppression and body temperature elevation were eliminated by pretreatment with PACAP6-38 (Hawke et al., 2009). In addition, neuroanatomical studies showed that PACAP receptors (Usdin et al., 1994; Hashimoto et al., 1996; Vertongen et al., 1997) or leptin receptor-containing hypothalamic nuclei (Elmquist et al., 1998) are expressed in the hypothalamus that innervates the kidney and WAT through the sympathetic nerves (Bamshad et al., 1998; Cano et al., 2004). These lines of evidence suggested that hypothalamic PACAP signaling may be an important mediator of central leptin actions on food intake, cardiovascular, and metabolic regulation through the autonomic nervous system; however, it remains unclear whether leptin-induced sympathetic activation is mediated by the hypothalamic PACAP pathway. Thus, in this study, we evaluated the effects of ICV injection of PACAP6-38 on leptin-induced SNA subserving the kidney and WAT. Moreover, we compared regional sympathetic responses to ICV injection of leptin in the Adcyap1−/− mice and wild-type mice.
EXPERIMENTAL PROCEDURES
Animals
We used male CD1 (ICR) mice (10 weeks, n=34) for electrophysiological experiment. Generation of Adcyap1−/− mice by a gene targeting technique has been reported previously (Hashimoto et al., 1996). The null mutation was backcrossed onto the genetic background of Crlj:CD1 (Institute of Cancer Research, Charles River, Tokyo, Japan) at least 10 times. All wild-type control (n=29) and Adcyap1−/− mice (n=17) used were obtained from the intercross of animals heterozygous for the mutant PACAP gene, and experiments were conducted with naive male mice 10 weeks of age. Animals were housed in a temperature-controlled room with a 12-h light–dark cycle. Food and water were freely available. Animals were adapted to the experimental environment for at least 1 week prior to the experiment. All animal care and handling procedures were approved by the Institutional Animal Care and Use Committee of the Ritsumeikan University.
Recording of sympathetic nerve discharge and cardiovascular parameters
On the day of the experiment, food was removed 5 h prior to surgery. Under anesthesia induced by intraperitoneal (IP) injection of 1 g/kg urethane (when it was insufficient, 0.2–0.3 g/ kg of urethane was added), a polyethylene catheter was inserted into the jugular vein for intravenous injection, and another catheter was inserted into the carotid artery for blood pressure (BP) determination. The mice were then cannulated through the trachea and fixed in a stereotaxic apparatus. Body temperature was maintained at 36.5–37.0 °C using a heating pad and monitored with a thermometer inserted into the rectum. Using a dissecting microscope, the left sympathetic nerve innervating the kidney was exposed through an incision to the left flank and a recording of the renal SNA (RSNA) was made. For recording WAT-SNA, an abdominal testicular blood vessel supplying the testis and adipose tissue of the epididymis was located and the nerve bundle was exposed. The nerve was attached to a pair of stainless-steel electrodes, and then hooked up to electrodes for recording. The electrodes were fixed with a silicon gel (liquid A & liquid B, Kagawa kikai Co., JAPAN) to prevent dehydration and for electrical insulation. The mouse was allowed to stabilize for 10–20 min after being placed on the recording electrodes.
Electrical changes in RSNA and WAT-SNA were amplified 2000–5000 times with a band path of 100–1000 kHz, and monitored by an oscilloscope as described previously (Tanida et al., 2008). Raw data of nerve activity were converted to standard pulses by a window discriminator, which separated discharge from electrical background noise which remained post-mortem. Both the discharge rates and the neurogram were sampled with a Power-Lab analog-to-digital converter for recording and data analysis on a computer. Background noise, which was determined 30–60 min after the animal was euthanized, was subtracted. Nerve activity was rectified and integrated, and baseline nerve activity was normalized to 100%. A catheter inserted in the carotid artery was connected to a blood pressure transducer (DX-100, Nihon Kohden, Japan), and the output signal of the transducer amplified (AP641G, Nihon Kohden, Japan), monitored with an oscilloscope, sampled with the Power-Lab, and stored on a hard disk for off-line analysis to calculate mean arterial pressure (MAP) and heart rate (HR).
Baseline measurements of RSNA, WAT-SNA MAP and HR were made 5 min prior to ICV injection of leptin (2 μg/2 μl vehicle) or vehicle (Phosphate Buffered Saline 2 μl). The dose of leptin was that used for effect in a previous study (Rahmouni et al., 2003). After the start of the injection, these parameters were recorded for 240 min. The effects of PACAP6-38 (2.4 nmol/2 μl), antagonist of a specific PAC-1R, on changes in sympathetic activities and cardiovascular parameters by leptin were examined. At the end of the experiment, hexamethonium chloride (10 mg/kg) was administered intravenously to ensure that post-ganglionic efferent sympathetic nerve activity had been recorded.
ICV cannulation
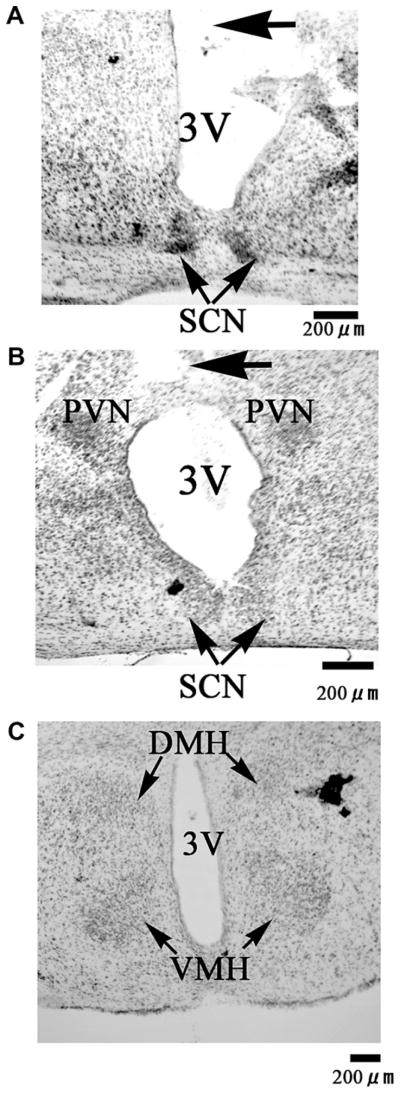
One week before the experiment, a 25-gauge stainless steel guide cannula with a stylet was implanted into the third ventricle area by using a stereotaxic apparatus (coordinates: AP, 0.7 mm posterior to the bregma; L, 0 mm; V, 3.0 mm) under anesthesia (IP injection of ketamine/xylazine) as previously described (Rahmouni et al., 2003). After recovery, the stylet was removed from the guide cannula, and in turn, a 33-gauge injection cannula attached to a 25 μl Hamilton syringe was inserted. The injection cannula was designed to be 1.0 mm longer than the guide cannula in order to reach the floor of the third ventricle area. Subsequently, 2 μl solution was injected during 1 min using a micro syringe pump. After the experiment, in order to check correct cannula placement, the brain was removed and histologically examined by Cresyl Violet staining (Fig. 5).
Fig. 5.
Photomicrographs of representative coronal brain sections including the SCN (A, B), PVN (B), DMH and VMH (C) of a mouse received ICV injection. The injection tract (arrow) was described in the panel (A, B). 3V, third ventricle; SCN, suprachiasmatic nucleus; PVN, paraventricular nucleus; DMH, dorsomedial hypothalamus; VMH, ventromedial hypothalamus.
Determination of plasma leptin, glucose and triglyceride
Food was removed 5 h prior to blood sampling, and under anesthesia induced by IP injection of ketamine/xylazine, blood samples were withdrawn from the celiac vein using the syringe 298 M. Tanida et al. / Neuroscience 238 (2013) 297–304 attached needle. Measurements of plasma glucose and triglyceride were conducted by FUJI DRY-CHEM (Fujifilm Corporation, Japan), and measurement of plasma leptin was conducted by a mouse leptin RIA kit (IBL, Japan).
Data analyses
The RSNA, WAT-SNA, MAP, and HR data measured during each 5-min period after injection of all agents were analyzed by digital signal processing and appropriate statistical analyses. All data were expressed as means±standard error of the mean (SEM). Percent changes from baseline values were calculated for the SNA. When comparing the responses of SNA, body weight and food intake to leptin between groups, analysis of variance (ANOVA) with the Bonferroni post hoc test was applied to compare group responses. When comparing the basal level data of body weight, abdominal fat weight, blood parameters and cardiovascular parameters between wild-type and Adcyap1−/−, Student t-test was used. P<0.05 was considered statistically significant.
RESULT
Effects of ICV PACAP6-38 on leptin-induced increase in regional SNA
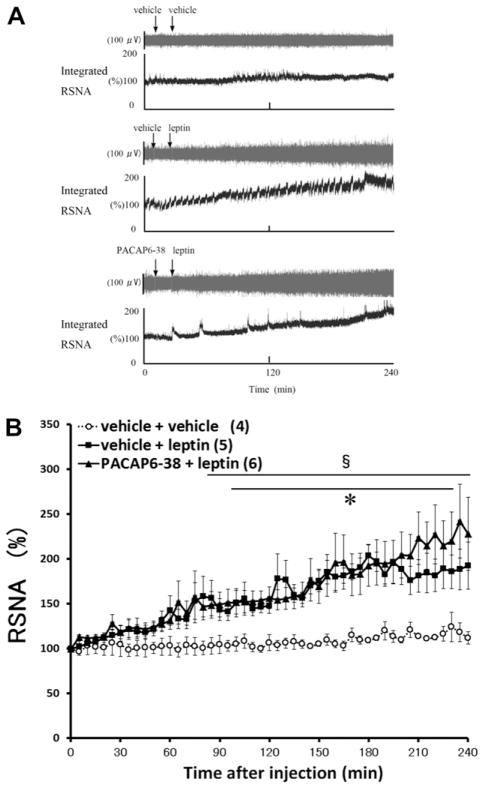
We determined the possible involvement of PACAP receptors in leptin-induced regional sympathetic responses. Typical recordings of renal SNA (RSNA) (Fig. 1) before and after ICV injection of vehicle or leptin are shown in Fig. 1A. In the vehicle-pretreated group, vehicle injection did not cause a significant alteration in RSNA, whereas injection of leptin resulted in a gradual increase in RSNA, with the greatest elevation occurring at 180 min (203.9±12.3%). Moreover, pretreatment with PACAP6-38, did not block the effects of leptin on RSNA (Fig. 1B).
Fig. 1.
Effects of ICV injection of PACAP6-38 on leptin induced-increase in renal sympathetic nerve activity (RSNA). Representative neurograms (A) from RSNA recordings in each group (vehicle+ vehicle, vehicle+leptin, PACAP6-38+leptin). Time-course data of change in RSNA following ICV injection of leptin (B). RSNA changes are expressed as mean±SEM. ICV injection of vehicle or PACAP6-38 was given 15 min before ICV injection of either vehicle or leptin. Numbers of animals used are shown in the parentheses. *P<0.05 (vehicle+vehicle vs. vehicle+leptin); §P<0.05 (vehicle+ vehicle vs. PACAP6-38+leptin).
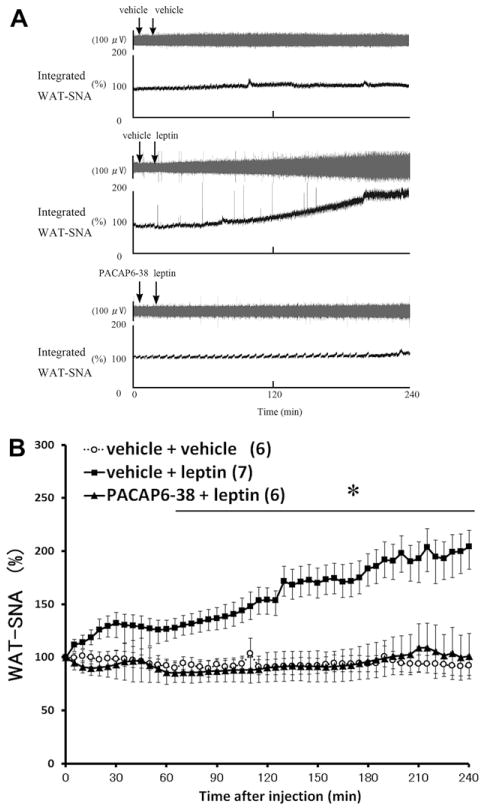
Next, we examined the possible role of PACAP receptors in mediating the effect of leptin on WAT-SNA. Typical recordings of WAT-SNA before and after ICV injection of vehicle or leptin are displayed in Fig. 2A. In the vehicle-pretreated group, vehicle injection did not alter the WAT-SNA, whereas the injection of leptin resulted in a gradual increase in WAT-SNA, with a peak (204.0±17.3%) at 215 min (Fig. 2B). Interestingly, pretreatment with PACAP6-38 abolished the effects of leptin on WAT-SNA (Fig. 2B).
Fig. 2.
Effects of ICV injection of PACAP6-38 on leptin induced-increase in white adipose tissue sympathetic nerve activity (WAT-SNA). Representative neurograms (A) from recordings of WAT-SNA in each group (vehicle+vehicle, vehicle+leptin, PACAP6-38+leptin). Time-course data of change in WAT-SNA following ICV injection of leptin (B). WAT-SNA changes are expressed as mean±SEM. ICV injection of vehicle or PACAP6-38 was given 15 min before ICV injection of either vehicle or leptin. Numbers of animals used are shown in the parentheses. *P<0.05 (vehicle+vehicle vs. vehicle+leptin).
Responses of RSNA and WAT-SNA to leptin administration in PACAP-deficient mice
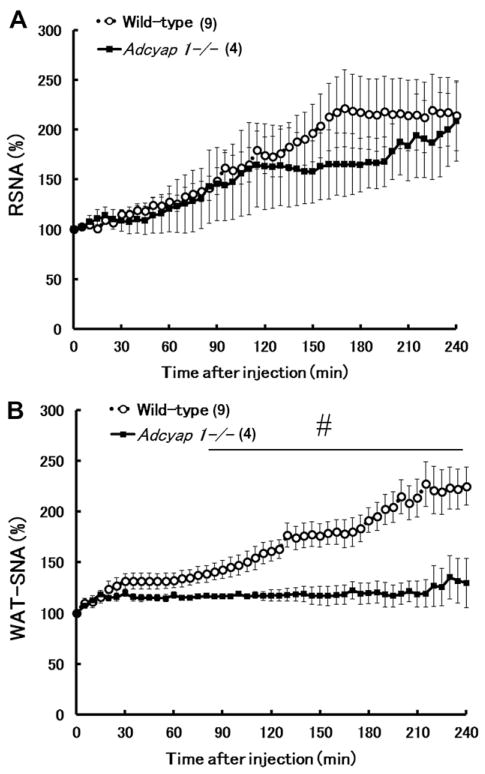
Leptin-induced sympathoexcitation in Adcyap1−/− mice and wild-type controls was investigated. Fig. 3 shows the stimulatory effects of ICV administration of leptin on RSNA and WAT-SNA. Injection of leptin caused a gradual and robust increase in the discharge rate of neural outflow to the kidney in both wild-type and Adcyap1−/− mice (RSNA at 240 min vs. the baseline level; Adcyap1−/− mice, 208.1±39.9%; wild-type mice, 214.1±35.1%). In contrast, the stimulatory effect of leptin on WAT-SNA was significantly attenuated in Adcyap1−/− mice (WAT-SNA at 240 min vs. the baseline level; Adcyap1−/− mice, 129.7±24.4%; wild-type mice, 225.1±18.9%) (Fig. 3).
Fig. 3.
Effects of ICV injection of leptin on RSNA and WAT-SNA in wild-type mice and Adcyap1−/− mice. Time-course data of changes in RSNA (A) and WAT-SNA (B) following ICV injection of leptin wild-type mice and Adcyap1−/− mice. Number of animals used are shown in the parentheses. Data are expressed as mean±SEM. #Significant differences between wild-type mice and Adcyap1−/− mice (P<0.05).
Basal levels of MAP and HR were not significantly different between the two groups of mice (Table 1).
Table 1.
Body weight, abdominal fat weight and plasma leptin, glucose, triglyceride levels and cardiovascular parameters in wild-type and Adcyap1−/− mice
| Wild-type | Adcyap1−/− | |||
|---|---|---|---|---|
| Body weight (g) | 41.7±1.1 | [12] | 34.8±1.9* | [8] |
| Abdominal fat weight# (g) | 1.79±0.19 | [12] | 0.35±0.03* | [8] |
| Plasma leptin (pg/ml) | 121.3±11.4 | [5] | 71.9±17.9* | [5] |
| Plasma glucose (mg/dl) | 184.6±24.3 | [5] | 154.5±16.0 | [5] |
| Plasma triglyceride (mg/dl) | 110.0±8.9 | [5] | 101.5±47.0 | [5] |
| Mean arterial pressure (mmHg) | 66±5 | [18] | 60±3 | [8] |
| Heart rate (bpm) | 611±31 | [18] | 583±7 | [8] |
Data are shown as means±SEM.
Numbers of animals used are shown in the parentheses.
Abdominal fat; epididymal fat, retroperitoneal fat, mesenteric fat.
P <0.05 vs. wild-type mice.
Food intake and body weight responses to leptin in PACAP-deficient mice
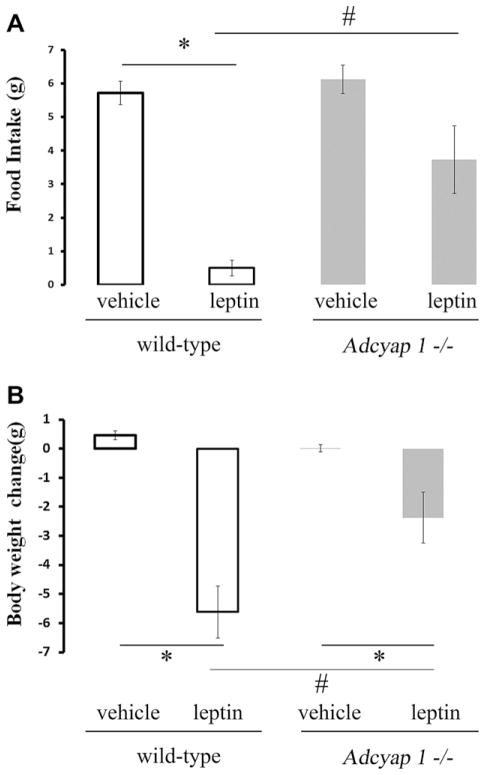
Finally, the effects of leptin on food intake and body weight in Adcyap1−/− mice and wild-type controls were examined. In wild-type mice, significant decreases in body weight and food intake was measured 24 h after ICV leptin injection (Fig. 4A, B). Relative to vehicle control, ICV administration of leptin had no significant effect on food intake in Adcyap1−/− mice (Fig. 4A), but resulted in a decrease in body weight (Fig. 4B). The reductions in food intake and body weight in leptin-treated wild-type mice were greater than those observed in Adcyap1−/− mice (Fig. 4A, B). In addition, as previously reported, the body weight and abdominal fat content were significantly reduced in Adcyap1−/− mice compared with wild-type littermates (Table 1). Plasma levels of leptin were significantly lower in Adcyap1−/− mice compared with wild-type controls (Table 1). There was no difference in plasma levels of glucose or triglycereides in Adcyap1−/− mice relative to wild-type controls (Table 1).
Fig. 4.
Effects of ICV injection of leptin on food intake (A) and body weight (B) in Adcyap1−/− mice (gray bars) vs. wild-type mice (white bars). Data are means±SEM. n=6 in wild-type mice and n=4 in Adcyap1−/− mice. *P<0.05 (vehicle vs. leptin); #P<0.05 (wild-type mice vs. Adcyap1−/− mice).
DISCUSSION
It is now well established that leptin activation of the sympathetic nervous system contributes to the regulation of a number of physiological processes including cardiovascular function, thermogenesis, and lipolysis (Shen et al., 2007; Kalil and Haynes, 2012). Hypothalamic PACAP signaling has recently been implicated in leptin-induced anorexia and hyperthermia (Hawke et al., 2009). In the present study, we examined a possible role for central PACAP signaling in sympathetic activation and feeding decrease evoked by leptin. The results demonstrated for the first time that pharmacological blockade of the PACAP receptors by ICV injection of PACAP6-38 attenuated the leptin-induced increase in WAT-SNA, but not in RSNA. Moreover, deletion of PACAP in mice eliminated the increase in WAT-SNA in response to leptin, whereas the leptin-induced RSNA response was preserved. In addition, PACAP deletion substantially attenuated the reduction of body weight and food intake caused by ICV leptin. To the best of our knowledge, this is the first in vivo evidence implicating PACAP signaling in leptin-induced increase in WAT-SNA as well as decrease in body weight and food intake.
We found that pharmacological blockade of the PACAP receptors, with PACAP6-38 significantly attenuated the WAT-SNA response to leptin, but not the RSNA response, suggesting that lepin may act on PACAP signaling in the hypothalamus and affect sympathetic outflow to WAT through central PACAP receptors. Previous studies have confirmed that there are three types of PACAP receptors in the animal hypothalamus (Ishihara et al., 1992; Lutz et al., 1993; Spengler et al., 1993): PAC-1, VPAC1, and VPAC2. In addition, recent observation that PACAP6–38 treatment blocks increases in phospho-Extracellular Signal-regulated Kinase by cocaine- and amphetamine-regulated transcript (CART), one of neuropeptides related to appetite regulation, suggests that PACAP6–38 may function as a CART receptor antagonist (Lin et al., 2011). Although the actions of PACAP6-38 suggest involvement of central PACAP receptors, this compound has been shown to interact with at least one other neuropeptide receptor (Lin et al., 2011). Since in fact no specific blockers for the PACAP PAC1 receptor exist, we employed PACAP-deficient mice to validate our pharmacological results with PACAP6-38. Our findings in the present study are consistent with a recent report showing that ICV administration of leptin increased PACAP expression in ventromedial hypothalamus and that leptin-induced anorexia and weight loss were eliminated by pre-treatment with PACAP6-38 (Hawke et al., 2009). In contrast to WAT-SNA response to leptin, we found that leptin-induced RSNA increase was preserved in the presence of PACAP6-38. Thus, central neurotransmission between PACAP and PACAP receptors appears to be involved in mediating SNA.
The altered sympathetic responses to leptin in Adcyap1−/− mice were region-specific: the stimulatory effects of leptin on WAT-SNA were abolished, but the RSNA response to leptin was unaffected. Leptin resistance and hyperactivity of the sympathetic nervous system have previously been implicated in the attenuated metabolic sympathetic responses to leptin in obese animals (Rahmouni et al., 2005; Tanida et al., 2006). However, in the current study, the level of plasma leptin in Adcyap1−/− mice was lower than that in control mice. In addition, previous studies reported that plasma levels of adrenaline and noradrenaline in mice lacking PACAP did not differ from those in control mice (Hamelink et al., 2002). Thus, the attenuated WAT-SNA response to leptin in Adcyap1−/− mice cannot be attributed to either hyperactivity of sympathetic nerves or leptin resistance.
Interestingly, in Adcyap1−/− mice, although leptin-induced WAT-SNA increase and decrease in food intake and body weight were eliminated or attenuated, body weight and abdominal fat were decreased in Adcyap1−/− mice relative to controls. We have no clear explanation for such a discrepancy. It is possible that Adcyap1−/− mice are resistant only to the metabolic effects of exogenous leptin administered acutely. Alternatively, compensatory mechanisms such as enhanced responses to other adipokines may have offset the loss of leptin action on metabolism in Adcyap1−/− mice. Interestingly, the food intake of young (7–12 weeks old) Adcyap1−/− mice was found to be similar to that of wild-type mice, but as these mice get older (>13 weeks old) they eat significantly less than the wild-type controls (Tomimoto et al., 2008). Because in the present study we used mice that are 9–10 weeks old, presumably with similar food intake, abnormal basal feeding behavior may not have contributed to the reduction of body weight and fat tissue content in Adcyap1−/− mice. Although the status of energy metabolism has not been reported for Adcyap1−/− mice; the energy expenditure of mice lacking the VPAC2 receptor (one of PACAP receptors) was found to be higher than that in wild-type mice (Asnicar et al., 2002), raising the possibility that energy expenditure may be increased in Adcyap1−/− mice.
As observed in our previous study (Hatanaka et al., 2008), the basal levels of arterial pressure and HR in anesthetized Adcyap1−/− mice were not different from the levels recorded in wild-type mice. These data are consistent with the previous finding that plasma catecholamine levels, which regulates arterial pressure, were similar in Adcyap1−/− and wild-type mice (Hamelink et al., 2002). Thus, deletion of PACAP may have no effect on the regulation of BP and HR in the anesthetized state. On the other hand, even though leptin-induced RSNA activation was detected in both Adcyap1−/− and wild-type mice in the current study, BP and HR were not elevated by leptin injection in either group (data not shown). A previous study using anesthetized mice showed that ICV administration of leptin did not increase BP, whereas RSNA was elevated (Hilzendeger et al., 2012), indicating that the sympathetic nervous system may be sparing contribution to mouse BP regulation under anesthesia. Moreover, with respect to blood parameters, blood sampling was done under anesthesia and present study has not performed measurement of blood parameters in conscious mice. Thus, it will be needed to examine cardiovascular function and blood parameters in Adcyap1−/− and wild-type mice under awake status for modifying the issue of experimental condition.
The results from the present study support the involvement of PACAP signaling in leptin regulation of food intake and autonomic function. The effects of ICV administration of leptin on WAT-SNA, food intake, and body weight were attenuated by pharmacological blocking of central PACAP receptors by PACAP6-38, as well as in mice lacking PACAP (Adcyap1−/− mice). In contrast, the RSNA response to leptin was preserved following PACAP6-38 treatment and in the absence of PACAP. These findings suggest that PACAP signaling in the brain is implicated in the control of SNA by leptin in a region-specific manner.
Leptin stimulates SNA and regulates cardiovascular and metabolic functions with the result that RSNA or WAT-SNA is associated with BP elevation or body weight reduction, respectively. Our findings support the notion that the sympathetic nervous system is differentially regulated. Our data support the compelling argument that leptin controls the various physiological processes including regional SNA through overlapping yet distinct neuronal and molecular circuits. A better understanding of these circuits will make it possible to enhance those mediating the beneficial actions of leptin on metabolism while sparing those that may detrimental such as the RSNA. Thus, targeting brain PACAP signaling will be important for the development of hypotensive and antiobesity drugs by blocking the hypertensive effect of leptin and preserving the metabolic and slimming actions of leptin.
Acknowledgments
We acknowledge the very kind technical advice of Drs. Paul N. Pilowsky and Melissa Farnham.
Grants: This research was supported by Grants from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant-in-Aid for Young Scientists to TM, 23689008).
Abbreviations
- BP
blood pressure
- CART
cocaine- and amphetamine-regulated transcript
- HR
heart rate
- ICV
intracerebroventricular
- IP
intraperitoneal
- MAP
mean arterial pressure
- PAC1-R
PACAP-specific receptor
- PACAP
pituitary adenylate cyclase-activating polypeptide
- RSNA
renal sympathetic nerve activity
- SEM
standard error of the mean
- SNA
sympathetic nerve activity
- VIP
vasoactive intestinal polypeptide
- WAT-SNA
white adipose tissue sympathetic nerve activity
References
- Asnicar MA, Köster A, Heiman ML, Tinsley F, Smith DP, Galbreath E, Fox N, Ma YL, Blum WF, Hsiung HM. Vasoactive intestinal polypeptide/pituitary adenylate cyclase-activating peptide receptor 2 deficiency in mice results in growth retardation and increased basal metabolic rate. Endocrinology. 2002;143:3994–4006. doi: 10.1210/en.2002-220354. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Aoki VT, Adkison MG, Warren WS, Bartness TJ. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am J Physiol. 1998;275:R291–R299. doi: 10.1152/ajpregu.1998.275.1.R291. [DOI] [PubMed] [Google Scholar]
- Bergström AL, Hannibal J, Hindersson P, Fahrenkrug J. Light-induced phase shift in the Syrian hamster (Mesocricetus auratus) is attenuated by the PACAP receptor antagonist PACAP6-38 or PACAP immunoneutralization. Eur J Neurosci. 2003;18:2552–2562. doi: 10.1046/j.1460-9568.2003.03000.x. [DOI] [PubMed] [Google Scholar]
- Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol. 2004;471:462–481. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Bjørbaek C, Ahima RS, Flier JS, Saper CB. Distributions of leptin receptor mRNA isoforms in the rat brain. J Comp Neurol. 1998;395:535–547. [PubMed] [Google Scholar]
- Farnham MM, Lung MS, Tallapragada VJ, Pilowsky PM. PACAP causes PAC1/VPAC2 receptor mediated hypertension and sympathoexcitation in normal and hypertensive rats. Am J Physiol Heart Circ Physiol. 2012;303:H910–H917. doi: 10.1152/ajpheart.00464.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Pituitary adenylate cyclase-activating peptide in the rat central nervous system: an immunohistochemical and in situ hybridization study. J Comp Neurol. 2002;453:389–417. doi: 10.1002/cne.10418. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Nogi H, Mori K, Ohishi H, Shigemoto R, Yamamoto K, Matsuda T, Mizuno N, Nagata S, Baba A. Distribution of the mRNA for a pituitary adenylate cyclase-activating polypeptide receptor in the rat brain: an in situ hybridization study. J Comp Neurol. 1996;371:567–577. doi: 10.1002/(SICI)1096-9861(19960805)371:4<567::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Shintani N, Tanaka K, Mori W, Hirose M, Matsuda T, Sakaue M, Miyazaki J, Niwa H, Tashiro F, Yamamoto K, Koga K, Tomimoto S, Kunugi A, Suetake S, Baba A. Altered psychomotor behaviors in mice lacking pituitary adenylate cyclase-activating polypeptide (PACAP) Proc Natl Acad Sci U S A. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka M, Tanida M, Shintani N, Isojima Y, Kawaguchi C, Hashimoto H, Kakuda M, Haba R, Nagai K, Baba A. Lack of light-induced elevation of renal sympathetic nerve activity and plasma corticosterone levels in PACAP-deficient mice. Neurosci Lett. 2008;444:153–156. doi: 10.1016/j.neulet.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Hawke Z, Ivanov TR, Bechtold DA, Dhillon H, Lowell BB, Luckman SM. PACAP neurons in the hypothalamic ventromedial nucleus are targets of central leptin signaling. J Neurosci. 2009;29:14828–14835. doi: 10.1523/JNEUROSCI.1526-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin–renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197–H206. doi: 10.1152/ajpheart.00974.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglott MA, Farnham MM, Pilowsky PM. Intrathecal PACAP-38 causes prolonged widespread sympathoexcitation via a spinally mediated mechanism and increases in basal metabolic rate in anesthetized rat. Am J Physiol Heart Circ Physiol. 2011;300:H2300–H2307. doi: 10.1152/ajpheart.01052.2010. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Shigemoto R, Mori K, Takahashi K, Nagata S. Functional expression and tissue distribution of a novel receptor for vasoactive intestinal polypeptide. Neuron. 1992;8:811–819. doi: 10.1016/0896-6273(92)90101-i. [DOI] [PubMed] [Google Scholar]
- Kalil GZ, Haynes WG. Sympathetic nervous system in obesity-related hypertension: mechanisms and clinical implications. Hypertens Res. 2012;35:4–16. doi: 10.1038/hr.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Zhao L, Donato J, Jr, Kohno D, Xu Y, Elias CF, Lee C, Parker KL, Elmquist JK. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci U S A. 2011;108:10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Hall RA, Kuhar MJ. CART peptide stimulation of G protein-mediated signaling in differentiated PC12 cells: identification of PACAP 6-38 as a CART receptor antagonist. Neuropeptides. 2011;45:351–358. doi: 10.1016/j.npep.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz EM, Sheward WJ, West KM, Morrow JA, Fink G, Harmar AJ. The VIP2 receptor: molecular characterisation of a cDNA encoding a novel receptor for vasoactive intestinal peptide. FEBS Lett. 1993;334:3–8. doi: 10.1016/0014-5793(93)81668-p. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jégou S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology. 2009;34:424–435. doi: 10.1038/npp.2008.73. [DOI] [PubMed] [Google Scholar]
- Rahmouni K. Leptin-induced sympathetic nerve activation: signaling mechanisms and cardiovascular consequences in obesity. Curr Hypertens Rev. 2010;6:104–209. doi: 10.2174/157340210791170994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci. 2003;23:5998–6004. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- Resch JM, Boisvert JP, Hourigan AE, Mueller CR, Yi SS, Choi S. Stimulation of the hypothalamic ventromedial nuclei by pituitary adenylate cyclase-activating polypeptide induces hypophagia and thermogenesis. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1625–R1634. doi: 10.1152/ajpregu.00334.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Tanida M, Niijima A, Nagai K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett. 2007;416:193–197. doi: 10.1016/j.neulet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Tanida M, Iwashita S, Terui N, Ootsuka Y, Shu M, Kang D, Suzuki M. Effect of peripheral administration of leptin on the renal sympathetic nerve activity in high-fat diet-related hypertensive rats. Life Sci. 2006;78:1149–1154. doi: 10.1016/j.lfs.2005.05.058. [DOI] [PubMed] [Google Scholar]
- Tanida M, Shen J, Niijima A, Yamatodani A, Oishi K, Ishida N, Nagai K. Effects of olfactory stimulations with scents of grapefruit and lavender oils on renal sympathetic nerve and blood pressure in Clock mutant mice. Auton Neurosci. 2008;139:1–8. doi: 10.1016/j.autneu.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Tanida M, Shintani N, Morita Y, Tsukiyama N, Hatanaka M, Hashimoto H, Sawai H, Baba A, Nagai K. Regulation of autonomic nerve activities by central pituitary adenylate cyclase-activating polypeptide. Regul Pept. 2010;161:73–80. doi: 10.1016/j.regpep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Tomimoto S, Ojika T, Shintani N, Hashimoto H, Hamagami K, Ikeda K, Nakata M, Yada T, Sakurai Y, Shimada T, Morita Y, Ishida C, Baba A. Markedly reduced white adipose tissue and increased insulin sensitivity in adcyap1-deficient mice. J Pharmacol Sci. 2008;107:41–48. doi: 10.1254/jphs.fp0072173. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Bonner TI, Mezey E. Two receptors for vasoactive intestinal polypeptide with similar specificity and complementary distributions. Endocrinology. 1994;135:2662–2680. doi: 10.1210/endo.135.6.7988457. [DOI] [PubMed] [Google Scholar]
- Vertongen P, Schiffmann SN, Gourlet P, Robberecht P. Autoradiographic visualization of the receptor subclasses for vasoactive intestinal polypeptide (VIP) in rat brain. Peptides. 1997;18:1547–1554. doi: 10.1016/s0196-9781(97)00229-5. [DOI] [PubMed] [Google Scholar]