Abstract
Purpose:
ASCO identified oncologist-patient conversations about cancer costs as an important component of high-quality care. However, limited data exist characterizing the content of these conversations. We sought to provide novel insight into oncologist-patient cost conversations by determining the content of cost conversations in breast cancer clinic visits.
Methods:
We performed content analysis of transcribed dialogue from 677 outpatient appointments for breast cancer management. Encounters featured 677 patients with breast cancer visiting 56 oncologists nationwide from 2010 to 2013.
Results:
Cost conversations were identified in 22% of visits (95% CI, 19 to 25) and had a median duration of 33 seconds (interquartile range, 19 to 62). Fifty-nine percent of cost conversations were initiated by oncologists (95% CI, 51 to 67), who most commonly brought up costs for antineoplastic agents. By contrast, patients most frequently brought up costs for diagnostic tests. Thirty-eight percent of cost conversations mentioned cost-reducing strategies (95% CI, 30 to 46), which most commonly sought to lower patient costs for endocrine therapies and symptom-alleviating treatments. The three most commonly discussed cost-reducing strategies were: switching to a lower-cost therapy/diagnostic, changing logistics of the intervention, and facilitating copay assistance.
Conclusion:
We identified cost conversations in approximately one in five breast cancer visits. Cost conversations were mostly oncologist initiated, lasted < 1 minute, and dealt with a wide range of health care expenses. Cost-reducing strategies were mentioned in more than one third of cost conversations and often involved switching antineoplastic agents for lower-cost alternatives or altering logistics of diagnostic tests.
INTRODUCTION
Health care–related financial distress remains prevalent in the United States, despite policy efforts to contain costs and increase insurance enrollment. In 2014, more than one quarter of Americans reported having difficulty paying their medical bills.1-3 This hardship is disproportionately common and severe in patients with cancer, who face significantly greater out-of-pocket costs than those without cancer who are healthy or chronically ill with conditions such as diabetes, cardiovascular disease, or mental illness.4,5 Health care–related financial distress is not just an economic concern but has also been associated with worse quality of life, lower adherence, and excess mortality.6-11
Given the high financial burden associated with cancer diagnosis and treatment, ASCO released a statement in 2009 that affirmed a critical role for oncologists in addressing out-of-pocket costs with their patients. Calling for greater integration of cost considerations into decision-making efforts, ASCO recommended oncologist-patient communication about cancer costs as a key component of high-quality care.12 Recognizing the paucity of data on this issue, as well as the centrality of oncologist-patient communication for out-of-pocket cost management, ASCO recommended cost communication as a primary target for future research.
Since that seminal report, several surveys have been published characterizing patient and oncologist preferences and experiences with cost communication. In general, results from these surveys suggest the following: the majority of patients want to discuss out-of-pocket costs with their oncologists,13,14 the majority of oncologists believe that it is important to help manage their patients’ out-of-pocket costs,15 and oncologist-patient cost discussions occur infrequently, with more than two thirds of patients reporting rarely or never speaking with oncologists about their costs.7,13,16 Although these surveys have provided helpful information about attitudes and experiences with cost communication, few data exist describing the content of these discussions. To address this knowledge gap, we analyzed transcribed patient-physician dialogue from 677 outpatient breast oncology visits occurring across the United States.
METHODS
Sample Description
Visits were selected from the Verilogue Point-of-Practice database, an international corpus of audio-recorded and transcribed clinical encounters. We have described this database and our sample in detail elsewhere.17,18 Briefly, Verilogue recruited board-certified physicians and compensated them to audio-record full clinic visits with patients they were seeing for routine clinical care. Patients gave consent before visits using a double opt-in method and were not compensated in any way.
We obtained from Verilogue the most recent 1,000 encounters for management of breast cancer. We excluded visits with nonphysician providers and those that occurred outside of the United States, leaving a final sample of 677 encounters, which occurred across 56 oncologists.
Analytic Approach
With no detailed or validated definition of cost conversation in the extant literature, we developed a novel, rigorous definition for our analyses. We defined cost conversation as any mention of the patient’s out-of-pocket expenses or insurance coverage for a past, present, or potential health care service (example quotes in Results and Table 1). This definition is grounded in communication theory,19,20 informed by examples used in previous studies,21,22 and shaped by team members’ clinical and research experience.7,23-26 This definition, as well as its underlying conceptual framework, has been described in detail in prior publications18 (Appendix, online only).17,27
Table 1.
Example Quotes for Cost-Reducing Strategies

Visits were first reviewed to determine whether a cost conversation occurred. If a cost discussion was present, we identified who initiated the cost talk (defined as the party making the first explicit statement related to the patient’s health care costs). In addition, we determined the total time spent discussing health care costs and identified strategies discussed to reduce the patient’s costs.17
Furthermore, we determined which intervention the cost conversation was related to and categorized it in one of the following four groups: antineoplastic therapies, symptom or comorbidity management, diagnostic tests, ancillary services and supplies, and other. To minimize individual coder biases and mitigate error, transcripts were analyzed independently by at least two team members, and all decisions were assessed for agreement. In cases of agreement, the corresponding decision was assigned as final. When discrepant, the final coding decision was decided by group consensus.
Statistical Analysis
We used Pearson’s χ2 test and Fisher’s exact test to compare code frequencies and calculated 95% CIs for all proportions using Clopper and Pearson’s exact method.28 Distributions of cost discussion durations were nonnormal, so nonparametric Kruskal-Wallis and Mann-Whitney U tests were used to compare cost discussion durations.
RESULTS
Study Population
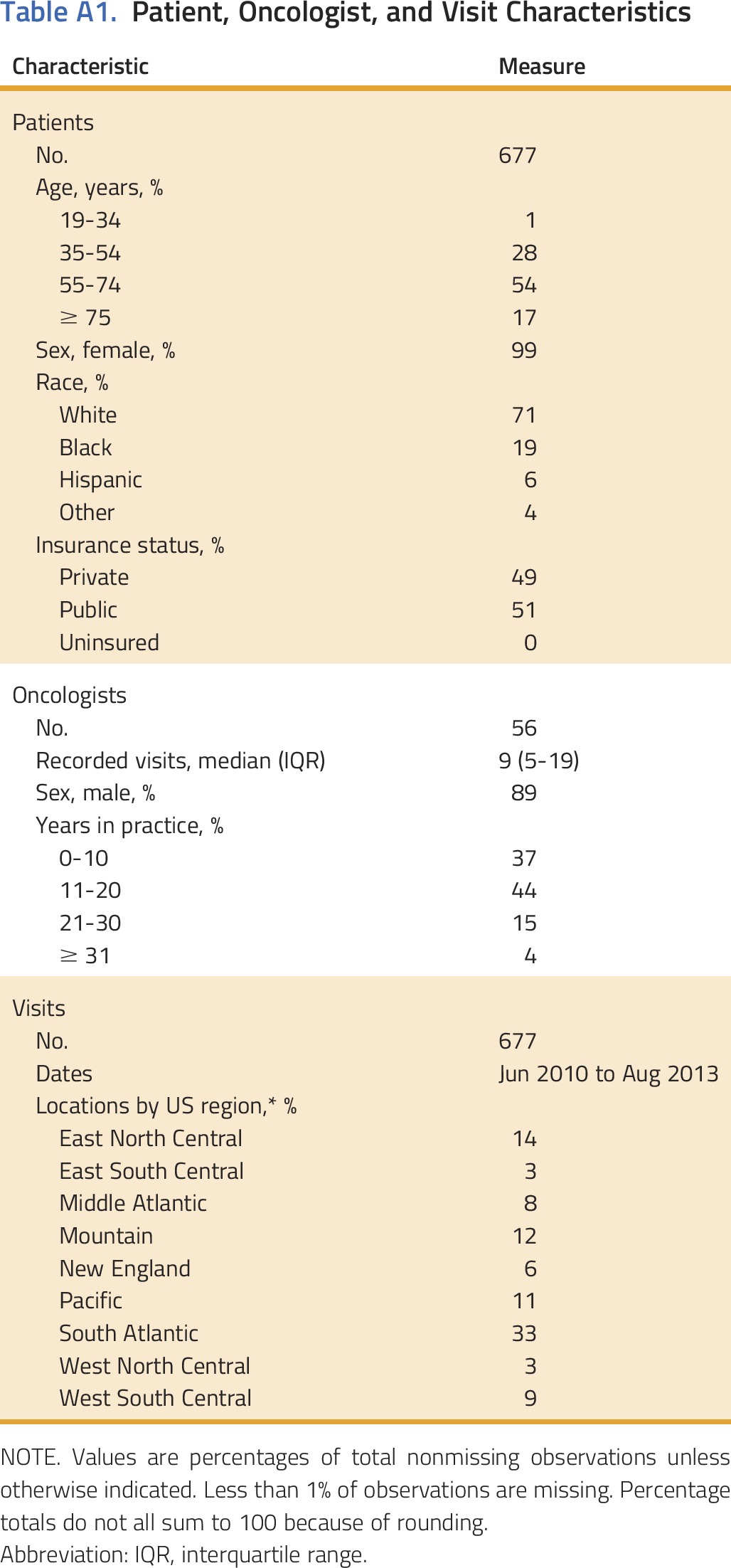
A full description of patient, oncologist, and visit characteristics is presented in the online supplement (Appendix Table A1, online only). Most patients were 55 to 74 years old (54%), white (71%), and female (99%). Only three patients (< 1%) reported being uninsured, which is significantly lower than national estimates.29 Oncologists were predominantly male (89% in our sample; 70% nationally),30 and most had < 20 years of clinical experience (81%). Visits were located in 19 different states and occurred between June 2010 and August 2013 in community-based, private practice oncology clinics across the United States.
Frequency of Cost Conversations by Clinical Topic
Overall, 147 of 677 clinic visits (22%; 95% CI, 19 to 25) contained a cost conversation. Cost conversations occurred more commonly in encounters involving female oncologists (30%) than those involving male oncologists (21%; P = .054). Cost conversation frequency did not differ significantly across months of the year (P = .90) or by the year in which visits took place (P = .14). A significantly greater percentage of cost conversations were initiated by physicians than by patients (59% v 37%, respectively; P < .001; Fig 1). Patient caregivers or companions initiated 3% of cost conversations and nurses initiated 1%. Patient-initiated cost conversations occurred more frequently in visits involving white patients (12% of visits) than those involving black patients (3% of visits; P = .001).
Fig 1.
Cost conversation initial topics and initiators. Initial topics of cost conversations were classified into five mutually exclusive categories shown above, with relative frequencies displayed as percentages above each bar. Colors in each bar demonstrate who initiated each cost conversation. Topic percentages do not add to 100 because of rounding. (*) Patient-initiated category comprises 54 patient-initiated cost conversations and five initiated by caregiver/companions; these were combined into one category here to simplify data presentation. (†) Percentages do not sum to 100 because one cost conversation was nurse-initiated. MGT, management.
The most common cost conversation topic was antineoplastic therapy (39% of initial cost conversation topics). The second most common topic was diagnostic testing (27%), followed by symptom/comorbidity treatments (17%), ancillary services/supplies (10%), and various other interventions (6%). Among the antineoplastic therapies, the cost of endocrine therapies (eg, tamoxifen, letrozole, anastrozole) was most frequently discussed (14% of all clinical topics), followed by targeted agents (eg, trastuzumab, lapatinib; 11% of all clinical topics), chemotherapy (eg, docetaxel, carboplatin; 7%), and bone therapies (eg, zoledronic acid, denosumab; 7%). Among the diagnostic tests, the costs of staging/restaging scans were most commonly discussed (eg, positron emission tomography [PET] scan, computed tomography scan; 16% of all clinical topics), followed by laboratory tests (eg, blood counts, BRCA testing, Oncotype DX; 6%) and cardiac function tests (eg, echocardiography, multigated acquisition scan; 4%). The cost of surgical procedures, radiation therapy, and hospital visits was discussed in < 3% of cost conversations. Notably, 10% of cost conversations addressed two different clinical topics, and 2% mentioned three different clinical topics.
Oncologist-Initiated Versus Patient-Initiated Cost Conversation Topics
Oncologists and patients differed with respect to the initial cost conversation topics they brought up (Fig 1). The majority of oncologist-initiated cost conversations (62%) addressed expenses for pharmacotherapy of some kind, whereas the majority of patient-initiated cost conversations (57%) were concerned with costs of nonpharmacologic interventions. A significantly greater percentage of patient-initiated cost conversations addressed other topics (a variety of infrequently discussed topics, such as physician office visits, cost of surgery, radiation therapy, and miscellaneous fees) than physician-initiated cost conversations (13% v 2%, respectively; P = .03)
Cost Conversation Duration
The median duration of cost conversations was 33 seconds (interquartile range, 19-62 seconds), out of a median appointment time of 12 minutes and 2 seconds (interquartile range, 6 minutes and 54 seconds–15 minutes and 57 seconds). No significant difference in cost conversation duration was observed between cost conversations initiated by physicians versus patients (median duration, 34 v 31 seconds, respectively; P = .88).
Cost conversation duration differed significantly by initial cost topic discussed (P = .03), with the longest cost conversations observed for discussions of bone therapies (median duration, 60 seconds) and endocrine therapies (median duration, 52 seconds) and the shortest cost conversations observed for symptom/comorbidity treatments (median duration, 29 seconds). In addition, cost conversations containing discussion of cost-reducing strategies were significantly longer than those without such discussions (median duration, 58 v 30 seconds, respectively; P < .001; selected quotes with durations in Table 2).
Table 2.
Types and Frequencies of Cost-Reducing Strategies Discussed for Various Cost Topics in Breast Cancer Visits (N = 677)

Discussion of Cost-Reducing Strategies
Thirty-eight percent of cost conversations contained discussion of cost-reducing strategies (95% CI, 30 to 46). Two main categories of cost-reducing strategies were identified: those that lowered patient costs by changing the care plan and those that reduced patient costs without changing the care plan. Overall, these two categories of cost-reducing strategies were discussed with nearly equal frequency: 51% of cost-reducing strategies involved care-plan changes, and 49% did not involve care-plan changes (Table 2). The greatest portion of cost-reducing strategies addressed antineoplastic therapies (46% of all cost-reducing strategies), followed by symptom/comorbidity management therapies (29% of cost-reducing strategies) and diagnostic tests (20% of cost-reducing strategies; P = .004; post hoc comparison between antineoplastics and diagnostics, P = .002). Specifically, cost-reducing strategies most commonly addressed endocrine therapies (26% of cost-reducing strategies) and symptom-alleviating treatments (22% of cost-reducing strategies).
Cost-reducing strategies involving care-plan changes
Overall, the most commonly discussed cost-reducing strategy was switching to a lower-cost intervention (Table 2). In the context of antineoplastic therapies, switching to lower-cost alternatives often involved transitioning from oral to intravenous therapies in the same class (eg, risedronate to zoledronic acid). For symptom/comorbidity management therapies, reducing costs commonly involved transitioning from sustained-release oxycodone to either short-acting oxycodone or long-acting morphine and switching from ondansetron (especially sublingual formulation) to less-expensive antiemetics like prochlorperazine. For diagnostic tests, switching to a lower-cost alternative commonly involved using computed tomography scans instead of PET scans.
The second most commonly discussed cost-reducing strategy involving care-plan changes was switching to a generic formulation of the medication in question. This strategy was particularly prominent for endocrine therapies, in which branded aromatase inhibitors were exchanged for their generics. For example, one patient who had experienced high copays was switched from Femara (Novartis; Basel, Switzerland) to its generic, letrozole. In the context of symptom management therapy, switching to generic was discussed to reduce patient costs for antiemetics, especially Zofran (GlaxoSmithKline; Bretford, United Kingdom) and over-the-counter analgesics.
The third most frequently discussed cost-reducing strategy involving care-plan changes was altering the dosage or frequency of an intervention. On several occasions, this strategy was discussed as a means of ensuring coverage for potentially high-cost interventions. In the context of diagnostic testing, this sometimes involved scheduling PET scans less frequently. This strategy was also used in several visits to reduce cost or ensure coverage for opiate analgesics.
Cost-reducing strategies not involving care-plan changes
The most commonly discussed cost-reducing strategy not involving care-plan changes was altering logistics of the intervention to reduce costs (ie, changing the timing, location, or source; Table 2). This strategy occurred commonly in the setting of diagnostic tests, with oncologists and patients scheduling potentially expensive diagnostics (especially PET scans) late in the calendar year, after the patient had met her deductible (Table 1). This strategy was also used to reduce patient costs for pharmacotherapy and supportive equipment, such as compression sleeves.
Facilitating copay assistance or coupons was the second most frequently discussed strategy to reduce patient costs without changing the care plan. This strategy was particularly prominent in the context of endocrine therapies, for which copay-assistance cards and charity care programs were discussed. This strategy was also discussed as a means to reduce patient costs for expensive oral chemotherapeutics (eg, Xeloda [Genentech; San Francisco, CA]), targeted agents (eg, Tykerb [Novartis; Basel, Switzerland]), and bone therapies (eg, Xgeva [Amgen; Thousand Oaks, CA]).
DISCUSSION
In a content analysis of transcribed dialogue from clinic visits, we found that oncologist-patient cost conversations occurred in approximately one in five visits, were mostly initiated by oncologists, typically lasted < 1 minute, most frequently addressed the cost of antineoplastic therapies, and commonly involved discussion of cost-saving strategies. To our knowledge, this represents the largest investigation of cost conversation dialogue in the oncology setting to date. Overall, our results suggest that oncologists and patients with breast cancer are willing and able to discuss patient costs during outpatient encounters, despite time pressure and price opacity. Importantly, our findings also highlight oncologist-patient resourcefulness and awareness in discussing a wide variety of different cost-saving strategies.
These results are important for several reasons. First, they counter previous survey findings on the incidence of cost conversations. In a prior survey conducted by Irwin et al,13 14% of patients with breast cancer reported ever discussing costs with their oncologists. In a general oncology setting, Bestvina et al7 found that 19% of patients reported ever discussing costs with oncologists. By contrast, our study suggests that cost conversations are more common, occurring in approximately one in five visits. Notably, these cost conversations took place in relatively brief (median duration, 12 minutes 2 seconds) clinic visits with returning patients.
Second, our results counter prevailing opinions expressed by oncologists and patients about the utility of cost communication. One of the most commonly cited barriers to cost conversation is the perceived lack of viable solutions.14,24,31,32 Contrary to this notion, we found that cost-reducing strategies were discussed in more than one third of cost conversations, and that at least seven different subtypes of cost-reducing strategies were mentioned. This suggests that oncologists and patients are aware of myriad cost-saving strategies, and may be able to provide targeted solutions for patients’ cost problems. This is in alignment with other results published by our group, showing that the majority of patients reported lower out-of-pocket costs as a result of cost discussions.33 Altogether, these data may suggest potential utility of cost conversations; however, additional investigation is needed to verify a positive impact of cost conversations on economic and clinical outcomes.
Third, our study calls into question the frequently cited barrier of insufficient time by demonstrating that cost conversations have a median duration of 33 seconds in general and 58 seconds when involving cost-reducing strategies.14,24,31,32 Notably, some cost conversations lasted > 3 minutes, which could be prohibitive for some clinicians, especially in visits with medically complex patients. However, these relatively long cost conversations were rare (3% of all cost conversations) and often addressed critical problems of patient access and affordability. Per ASCO guidelines, addressing patient costs is a critical component of high-quality care12; with greater numbers of patients signing up for high-deductible health insurance plans,34 persistently high rates of health care–related financial distress,1-3 and myriad negative downstream effects of such financial distress,6-11 some may argue that addressing patient costs is a critical task for clinicians even if it takes 3 to 5 minutes in an already time-pressured clinic visit.25,23,35-37 Nevertheless, our results suggest that short, targeted cost conversations may be sufficient to assess for financial toxicity and deliver cost-saving measures directly, or indirectly by referring for financial counseling.
Although our data may suggest greater cost conversation incidence and more extensive knowledge of cost-saving solutions than previously expected, they do not suggest that cost conversations are occurring as often as needed or that cost conversations are as effective as possible when they do occur. On the contrary, we found several areas of cost communication in which oncologists may need improvement. For example, cost conversations sometimes lost focus, dwelling in patient and oncologist frustrations rather than pursuing solutions for cost or coverage.27 On other occasions, oncologists did not fully address patient cost concerns or even ignored them. In addition, we observed an asymmetry between oncologist- and patient-initiated cost topics; whereas oncologists most often initiated cost conversations about antineoplastic therapies, patients most often initiated cost talk related to diagnostic tests. This discrepancy may reflect prevalent desire or unmet need among patients related to the costs of their diagnostic tests, which could potentially be addressed by greater physician-initiated conversation on this topic.
Notably, there were a few aspects of cost conversation in which oncologists demonstrated remarkable skill. For example, oncologists frequently reaffirmed commitment to their patients and built rapport in the midst of addressing difficult or frustrating cost/coverage barriers. In addition, some oncologists demonstrated detailed knowledge of drug prices, despite significant price opacity and substantial variation among patients. Last, some oncologists demonstrated deep knowledge of local and national resources with which to connect their patients for financial assistance.
This investigation has several limitations. First, the study sample limits the generalizability of our findings because it was composed entirely of breast oncology visits. Cost conversations in this setting may not represent those in other oncology settings, where treatments, prognosis, and patient demographics may differ. For example, cost conversations in surgical oncology visits may vary considerably from those observed in medical oncology visits, given differences in patient characteristics and interventions being offered. Second, we did not have access to data detailing patients’ disease stage or previous therapies they received. Consequently, we could not assess how disease stage affected cost conversations. Third, although we sampled patients across a broad range of geographic regions and assessed insurance coverage, we did not evaluate patients’ incomes. Accordingly, our study sample may not be generalizable from a financial standpoint. Fourth, we had access to only one recording per patient; thus, it is unknown whether cost was discussed in visits before or after the one recorded. Because earlier cost conversations may obviate the need for future ones, our study may have underestimated cost conversation incidence. Fifth, we did not have access to follow-up data and could not determine the impact of cost conversations on actual cost, adherence, or clinical outcomes.
In conclusion, in what we believe is the largest analysis of cost conversation content in the oncology setting to date, we found that cost conversations are occurring in more than one in five clinic visits, are mostly initiated by physicians, and are relatively brief, typically lasting < 1 minute. Contrary to prior evidence, our results suggest that oncologists and patients with breast cancer are aware of a wide variety of potential cost-reducing solutions and mention them in more than one third of cost conversations. Altogether, these data may evidence the willingness and capability of oncologists and patients with breast cancer to engage in cost conversations despite time pressure and price opacity. Additional research is needed to determine the impact of cost conversations on salient clinical, economic, and patient-centered outcomes and investigate educational interventions to foster effective and efficient cost conversations along the spectrum of disease stage.
ACKNOWLEDGMENT
Supported by a grant from the Robert Wood Johnson Foundation (P.A.U.) and Grant No. TL1TR001116 from the National Center for Advancing Translational Sciences (W.G.H.). Presented in part at the AcademyHealth Annual Research Meeting, Minneapolis, MN, June 14-16, 2015. The content of this article is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or the Robert Wood Johnson Foundation. We thank Cecilia Z. Zhang, BS, Annabel Wang, BS, Carmen Cummings BA, MPP, and Robin Fail, BA, MPP, for their assistance in data analysis.
Appendix
Table A1.
Patient, Oncologist, and Visit Characteristics
Additional Details About the Study Sample
A total of 677 breast cancer visits were included in this study. Each visit featured a unique patient (n = 677), but oncologists (n = 56) contributed multiple records. Along with visit recordings, physicians submitted clinical and demographic data for patients and themselves. Neither party was aware of the specific research questions for which the data would be used. All protected health information was removed from visit transcripts and clinical data sets before our analysis. The Duke University Institutional Review Board approved this study.
Visit Locations
Visits were located in the United States in nine major geographic regions, 19 states, and 52 zip codes. Visit locations were classified as urbanized areas, urban clusters, or rural areas by matching zip codes with 2010 US Census population data and applying definitions in the Urban Area Criteria for the 2010 Census (Bureau of the Census: Federal Register 76;1-15, 2011). Less than 10% of visits occurred in rural areas (population < 2,500); > 90% occurred in urbanized areas or urban clusters (populations > 50,000, and 2,500 to 50,000, respectively).
Insurance Plans
Only three patients in our study sample reported being uninsured (< 1%). Among those with insurance, 51% were enrolled in public plans (eg, Medicare, Medicaid, Tricare, COBRA), and 49% were enrolled in private plans (eg, Aetna, Blue Cross Blue Shield; 49%).
Cost Conversation Definition Development
Briefly, we developed definitions of cost conversation phenomena using an inductive application of summative content analysis, which proceeded in several phases: review of published literature to identify extant definitions, immersion in the transcripts to create data-driven definitions of emerging concepts, and iterative rounds of inductive analysis to adjust, refine, merge, and split definitions. To enhance the breadth and depth of our analysis, these phases were carried out with a multidisciplinary group of undergraduate and graduate students (with training in health policy, consumer behavior, medicine, or linguistics), trained conversation analysts, and clinician investigators. This process and its theoretical underpinnings are discussed in detail in a recent publication from our group.18
Team members with clinical experience (P.A.U., W.G.H.) supervised coder training and discrepancy resolution to ensure proper interpretation of clinical matters. All coding was applied using NVivo software (Version 10, 2014; QSR International, Melbourne, Australia).
Cost Conversation Topic Categories: Descriptions and Examples
Five key cost conversation topic categories emerged from our content analysis. These are listed below, along with descriptions and specific examples.
Antineoplastic therapies—all pharmacologic cancer treatments, such as endocrine therapy (eg, tamoxifen, anastrozole), chemotherapy (eg, docetaxel, carboplatin), targeted agents (eg, trastuzumab), and bone therapy (eg, zoledronic acid, denosumab).
Symptom or comorbidity management—analgesics (eg, oral or transdermal opiates, nonsteroidal anti-inflammatory agents), antiemetics (eg, ondansetron, promethazine), and bowel therapies such as stool softeners or laxatives.
Diagnostic tests—all imaging and laboratory tests (eg, positron emission tomography, BRCA testing, blood chemistries, Oncotype DX).
Ancillary services and supplies—dental care, nursing and wound care, physical therapy, podiatry, acupuncture, as well as medical equipment and supplies (eg, wheelchair, cane, home oxygen, positive airway pressure machine, wig).
Other—a variety of topics discussed infrequently, such as cost of surgery, radiation therapy, physician office visits, and other fees.
Statistical analysis was performed using R software. All authors had full access to the data and take responsibility for its integrity.
AUTHOR CONTRIBUTIONS
Conception and design: Wynn G. Hunter, Jamison A. Barnett, Peter A. Ubel
Financial support: Wynn G. Hunter, Peter A. Ubel
Administrative support: Wynn G. Hunter, Ashley Hesson, J. Kelly Davis, Christine Kirby, Jamison A. Barnett, Peter A. Ubel
Provision of study materials or patients: Jamison A. Barnett
Collection and assembly of data: Wynn G. Hunter, Ashley Hesson, J. Kelly Davis, Christine Kirby, Jamison A. Barnett, Peter A. Ubel
Data analysis and interpretation: Wynn G. Hunter, S. Yousuf Zafar, Ashley Hesson, J. Kelly Davis, Christine Kirby, Peter A. Ubel
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Discussing Health Care Expenses in the Oncology Clinic: Analysis of Cost Conversations in Outpatient Encounters
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Wynn G. Hunter
No relationship to disclose
S. Yousuf Zafar
Employment: Novartis (I)
Stock or Other Ownership: Novartis (I)
Consulting or Advisory Role: Vivor, Family Reach Foundation, AIM Specialty Health
Research Funding: AstraZeneca (Inst)
Ashley Hesson
Research Funding: Verilogue
J. Kelly Davis
No relationship to disclose
Christine Kirby
Employment: Duke University
Jamison A. Barnett
Employment: Verilogue
Leadership: Verilogue
Travel, Accommodations, Expenses: Verilogue
Peter A. Ubel
Consulting or Advisory Role: Humana, Genomic Health
REFERENCES
- 1. Cohen RA, Kirzinger WK: Financial burden of medical care: A family perspective. NCHS Data Brief 142:1-8, 2014. [PubMed] [Google Scholar]
- 2.Richman IB, Brodie M: A national study of burdensome health care costs among non-elderly Americans. BMC Health Serv Res 14:435, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collin SR, Rasmussen PW, Doty MM, et al: Too high a price: Out-of-pocket health care costs in the United States. Findings from the Commonwealth Fund Health Care Affordability tracking survey. September-October 2014. Issue Brief (Commonw Fund) 29:1-11, 2014. [PubMed]
- 4.Davidoff AJ, Erten M, Shaffer T, et al. : Out-of-pocket health care expenditure burden for Medicare beneficiaries with cancer. Cancer 119:1257-1265, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Bernard DSM, Farr SL, Fang Z: National estimates of out-of-pocket health care expenditure burdens among nonelderly adults with cancer: 2001 to 2008. J Clin Oncol 29:2821-2826, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanratty B, Holland P, Jacoby A, et al. : Financial stress and strain associated with terminal cancer--a review of the evidence. Palliat Med 21:595-607, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bestvina CM, Zullig LL, Rushing C, et al. : Patient-oncologist cost communication, financial distress, and medication adherence. J Oncol Pract 10:162-167, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zafar SY, Abernethy AP: Financial toxicity, Part I: A new name for a growing problem. Oncology (Williston Park) 27:80-81, 2013. [PMC free article] [PubMed]
- 9.Kim P: Cost of cancer care: The patient perspective. J Clin Oncol 25:228-232, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Tucker-Seeley RD, Li Y, Subramanian SV, et al. : Financial hardship and mortality among older adults using the 1996-2004 Health and Retirement Study. Ann Epidemiol 19:850-857, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollitz K, Cox C, Lucia K, et al. : Medical debt among people with health insurance http://kff.org/private-insurance/report/medical-debt-among-people-with-health-insurance/
- 12.Meropol NJ, Schrag D, Smith TJ, et al. : American Society of Clinical Oncology guidance statement: The cost of cancer care. J Clin Oncol 27:3868-3874, 2009 [DOI] [PubMed] [Google Scholar]
- 13.Irwin B, Kimmick G, Altomare I, et al. : Patient experience and attitudes toward addressing the cost of breast cancer care. Oncologist 19:1135-1140, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullock AJ, Hofstatter EW, Yushak ML, et al. : Understanding patients’ attitudes toward communication about the cost of cancer care. J Oncol Pract 8:e50-e58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schrag D, Hanger M: Medical oncologists’ views on communicating with patients about chemotherapy costs: A pilot survey. J Clin Oncol 25:233-237, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Meisenberg BR, Varner A, Ellis E, et al. : Patient attitudes regarding the cost of illness in cancer care. Oncologist 20:1199-1204, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter WG, Zhang CZ, Hesson A, et al. : What strategies do physicians and patients discuss to reduce out-of-pocket costs? Analysis of cost-saving strategies in 1,755 outpatient clinic visits. Med Decis Making 36:900-910, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter WG, Hesson A, Davis JK, et al. : Patient-physician discussions about costs: Definitions and impact on cost conversation incidence estimates. BMC Health Serv Res 16:108, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsieh H-F, Shannon SE: Three approaches to qualitative content analysis. Qual Health Res 15:1277-1288, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Bradley EH, Curry LA, Devers KJ: Qualitative data analysis for health services research: Developing taxonomy, themes, and theory. Health Serv Res 42:1758-1772, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tarn DM, Paterniti DA, Heritage J, et al. : Physician communication about the cost and acquisition of newly prescribed medications. Am J Manag Care 12:657-664, 2006 [PubMed] [Google Scholar]
- 22.Beard AJ, Sleath B, Blalock SJ, et al. : Predictors of rheumatoid arthritis patient-physician communication about medication costs during visits to rheumatologists. Arthritis Care Res (Hoboken) 62:632-639, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Zafar SY, Tulsky JA, Abernethy AP: It’s time to have ‘the talk’: Cost communication and patient-centered care. Oncology (Williston Park) 28:479-480, 2014 [PMC free article] [PubMed] [Google Scholar]
- 24.Riggs KR, Ubel PA: Overcoming barriers to discussing out-of-pocket costs with patients. JAMA Intern Med 174:849-850, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunter WG, Ubel PA: The black box of out-of-pocket cost communication. A path toward illumination. Ann Am Thorac Soc 11:1608-1609, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann PJ, Palmer JA, Nadler E, et al. : Cancer therapy costs influence treatment: A national survey of oncologists. Health Aff (Millwood) 29:196-202, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Ubel PA, Zhang CZ, Hesson A, et al: Study of physician and patient communication identifies missed opportunities to help reduce patients’ out-of-pocket spending. Health Aff (Millwood) 35:654-661, 2016. [DOI] [PMC free article] [PubMed]
- 28.Clopper CJ, Pearson ES: The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika 26:404-413, 1934 [Google Scholar]
- 29.Kaiser Family Foundation : Key facts about the uninsured population http://files.kff.org/attachment/fact-sheet-key-facts-about-the-uninsured-population
- 30. Association of American Medical Colleges: 2014 Physician specialty data book. https://members.aamc.org/eweb/upload/Physician%20Specialty%20Databook%202014.pdf.
- 31.Alexander GC, Casalino LP, Tseng C-W, et al. : Barriers to patient-physician communication about out-of-pocket costs. J Gen Intern Med 19:856-860, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Danis M, Sommers R, Logan J, et al. : Exploring public attitudes towards approaches to discussing costs in the clinical encounter. J Gen Intern Med 29:223-229, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zafar SY, Chino F, Ubel PA, et al. : The utility of cost discussions between patients with cancer and oncologists. Am J Manag Care 21:607-615, 2015 [PubMed] [Google Scholar]
- 34.Dolan PL: High-deductible impact. Balancing rising out-of-pocket costs and outcomes. Med Econ 91:17-20, 2014 [PubMed] [Google Scholar]
- 35.Zafar SY: Financial toxicity of cancer care: It’s time to intervene. J Natl Cancer Inst 108:djv370, 2015 [DOI] [PubMed] [Google Scholar]
- 36.Ubel PA, Abernethy AP, Zafar SY: Full disclosure--out-of-pocket costs as side effects. N Engl J Med 369:1484-1486, 2013 [DOI] [PubMed] [Google Scholar]
- 37.Moriates C, Shah NT, Arora VM: First, do no (financial) harm. JAMA 310:577-578, 2013 [DOI] [PubMed] [Google Scholar]



