Abstract
Intradiscal O2–O3 injections are conventionally used as a minimally invasive treatment for lumbar disc herniation in patients not responding to conservative treatments. The aim of the present study is to report data of long-term imaging follow-up (3 years) of patients treated with intradiscal O2–O3 lumbar chemiodiscolysis. We evaluated the changes of disc volume and the modifications in disc appearance (in terms of disc degeneration) and endplate changes (according to Modic), comparing the results with a control group of patients. Our results showed a stable reduction of the disc herniation volume in patients treated compared with the control group, while we did not find statistically significant differences in terms of disc degeneration and endplate changes (Modic). We concluded that the O2–O3 discolysis, despite leading to a significant shrinkage of the disc herniation, does not involve – in the long term – biomechanical changes of the spine in terms of acceleration of the disc degeneration process in comparison with the natural course.
Keywords: MRI, lumbar disc herniation, oxygen–ozone, Modic changes
Introduction
Symptomatic lumbar disc herniation is a degenerative disease of the intervertebral disc that may lead to clinical symptoms such as back pain, sciatica, or compressive radiculopathy and functional limitations. Besides being the most common cause of lumboradicular pain, it is also the most frequent indication for spine surgery.1–13
Studies on the natural history of disc herniation have shown that the herniated intervertebral disc can often resorb spontaneously, completely or in part, and also the associated clinical picture frequently subsides with conservative therapies.11,14 Treatment of symptomatic lumbar disc herniation should also begin with conservative measures prior to surgery, but the variability of interventions and treatment offered for lumbar disc herniation reflects the uncertainty regarding the indications for surgery. In recent years there have been an increasing number of studies on the use of oxygen–ozone injections for lumboradicular pain caused by disc herniation.2–13 Although the therapeutic efficacy of computed tomography (CT)-guided intradiscal injection of an oxygen–ozone mixture in patients with lumbar disc herniation is established, few data are found in literature reporting the long-term magnetic resonance imaging (MRI) findings after intradiscal ozone treatment.
This report updates our published data detailing our experience with intradiscal ozone injections to include data from long-term follow-up (3 years). We have supplemented our initial results to quantify the change of disc volume and modifications in disc appearance (in terms of disc degeneration) and endplate changes (according to Modic) 3 years after ozone–oxygen nucleolysis.
Materials and method
Patients
We retrospectively evaluated MRI examinations of the patients included in our previous studies about the efficacy of O2–O3 discolysis in the treatment of lumbar disc herniation.
From these series, we selected 50 patients (28 males, 22 females, mean age 44.4 ± 9.5) who underwent, in the period from July 2012 to June 2013, percutaneous CT-guided oxygen–ozone discolysis (10 ml of 28 µg/ml concentration O2–O3). Inclusion criteria were lumbar disc protrusion or herniation detected on previous MR or CT studies, and discogenic pain persistent for at least 8 weeks despite conservative medical therapy.
As a control group, we selected 50 patients who underwent in the same period percutaneous treatment with periradicular injection of steroids only (24 males, 26 females, mean age 43.8 ± 11.2), without intradiscal injection of O2–O3. Inclusion criteria for the control group were the same as the study group.
Exclusion criteria included surgical treatment during MRI follow-up. All patients gave informed consent.
MR protocol
The MRI exams were performed using GE Healthcare – Signa EXCITE 1.5T with a phased-array spine coil. Axial FSE T2-weighted images (T2-WI) were acquired with a field of view (FOV) of 17 cm, with 4000/108/4 (TR/TE/NEX) matrix (256 × 192), slice thickness 5 mm and gap 1 mm. Sagittal FSE-T2 and T2 Fat-weighted images were acquired with FOV of 26 cm and the remaining parameters of axial T2-WI. Sagittal SE-T1-WI images were acquired with 500/20/2(TR/TE/NEX) and with same remaining parameters of sagittal FSE-T2 and T2 Fat-WI.
Image analysis
Two neuroradiologists with, respectively, 25 and 5 years’ experience, blinded to the kind of treatment, independently reviewed the MR images. MRI image analysis was performed at a workstation by comparing baseline and 3-year follow-up MR images.
Intervertebral Disc Volumetric Analysis (IDVA) was manually done by defining regions of interest (ROIs) on the axial FSE-T2 MRI images. Entire disc and herniated portion were included in the ROI on all slices of axial images.
Modic change
Modic change was assessed at baseline and follow-up using the original classification by Modic et al. which consists of three types – type 1: hypointense on T1 and hyperintense on T2 images; type 2: hyperintense on T1 and isointense/hyperintense on T2images; type 3: hypointense on both T1 and T2 images. Images were assessed in the sagittal plane and scored at both the proximal and distal endplates within the same vertebrae.
Disc degeneration
Grading of disc degeneration was assessed from T2-weighted sagittal images based on the Pfirrmann method, which was endorsed as a valid and reliable method of assessing the intervertebral disc using MRI in a systematic review of existing grading systems for lumbar disc degeneration. This method utilised a constellation of qualitative MRI features including the appearance of the disc structure, the distinction between the nucleus and the annulus, the signal intensity and the intervertebral disc height, to give a 5-point grading system ranging from grade 1 (homogeneous hyperintense disc with normal height) to grade 5 (inhomogeneous hypointense disc, collapsed disc space).
Matching between study and control groups
Before treatment, the clinical neuroradiologist matched study and control group patients to exclude potential confounding factors, according to the criteria of same sex, age (differing by no more than ± 5 years) and disc level, similar body mass index (BMI) (differing by no more than ± 5 kg/m2), and same type of disc herniation.
Data and statistical analysis
The intra and interobserver reliability of the MRI evaluations for qualitative analysis was estimated using agreement percentage and kappa statistics according to Landis and Koch. For comparison of categorical variables, the χ2 test was used, while for quantitative variables using an unpaired two-tailed t-test. SPSS version 11.5 (SPSS, Chicago, Ill, USA) was used for all calculations.
Results
At presentation, the study and control group were not significantly different (p = 0.45 to p = 0.88) in terms of demographic data (sex, age), disc level affected, BMI, and herniation type.
Lumbar disc herniation was treated in 21% of patients at level L3/L4, in 49% of patients at level L4/L5, and in 30% of patients at level L5/S1.
Intervertebral Disc Volumetric Analysis (IDVA)
Initial mean disc volume was 18.35 ± 1.62 (range 8.12–29.15 cm3) in the study group and 18.84 ± 1.45 in the control group.
At the follow-up, the mean disc volume was 15.96 ± 3.72 cm3 in the study group (treatment with intradiscal ozone) and 17.45 ± 4.23 cm3 in the control group (Figures 1 and 2). Disc volume reductions evaluated with IDVA were significantly more frequent in the study group than in the control group. Student’s t-test showed a statistically significant reduction (p < 0.05) after O2–O3 discolysis, with an average reduction of whole discs with herniation volumes around 4–5% in the study group patients.
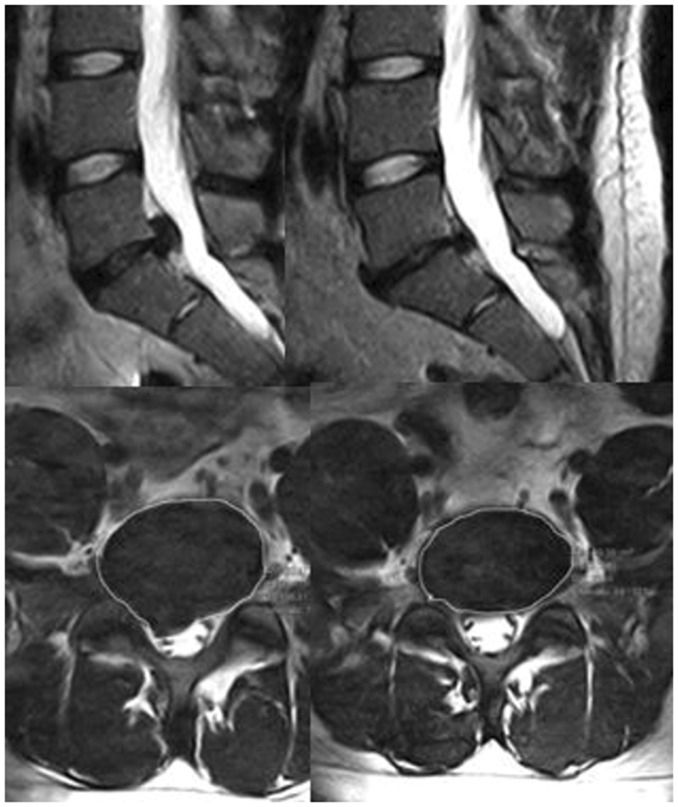
Figure 1.
Sagittal and axial images in a patient treated with intradiscal O2–O3 before (images on the left) and at 3-year follow-up (images on the right). Note the shrinkage of the herniated disc with reduction of the disc volume. The above L4-L5 disc grade of degeneration (Pfirmann II) is the same before and after treatment.
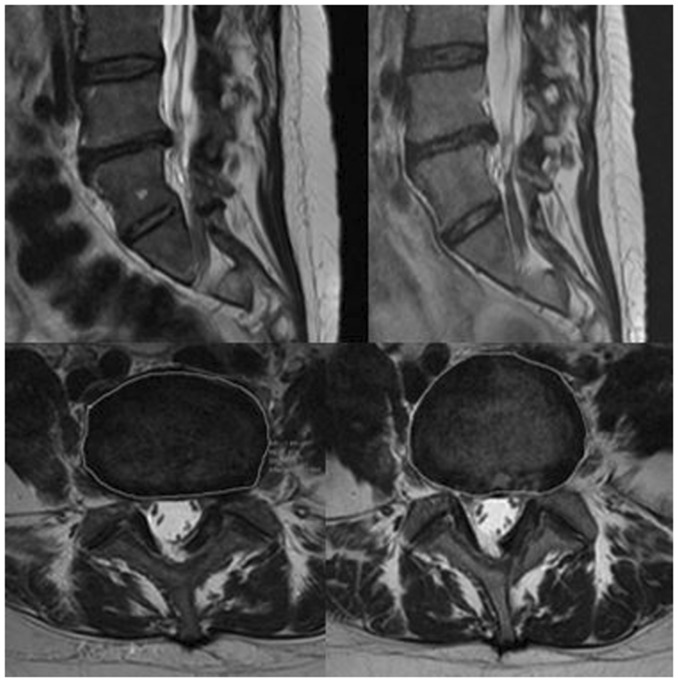
Figure 2.
Sagittal and axial images in a patient treated with periganglionic injection of corticosteroids, before (images on the left) and at 3-year follow-up (images on the right), showing non-significant reduction of the herniated L4–L5 disc.
A disc volume reduction was seen in 84% of the treated herniated lumbar discs at 3 years after therapy. The greatest mean reduction was observed at L4/L5, but one-way analysis of variance revealed no significant differences in mean volume reduction depending on disc location (p ≤ 0.525). In total, 40 patients of the study group (81%) had a reduction of 50% or more; eight patients (16%) had no observable change in the herniation.
Modic changes
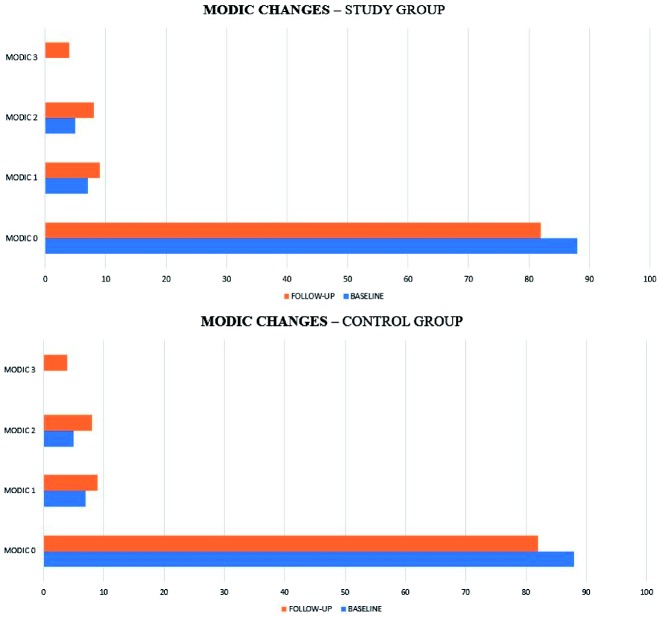
A total of 250 endplates in each group were studied. The overall prevalence of Modic changes at baseline was 23%. In the study group at baseline 88% were type 0, 7% type I, and 5% type II.
Of the endplates showing no Modic changes, at the follow-up 6% developed Modic type I and 1% developed type II. Of the endplates with Modic I changes at the baseline, 50% remained the same, 36% progressed to Modic type II, 8% progressed to Modic type III and 5% were found to be normal (type 0) on the repeated scan (Figure 3). Of the endplates initially reported as Modic II, 81% remained unchanged, 0% converted to Modic III and 18% converted to Modic I (Table 1).
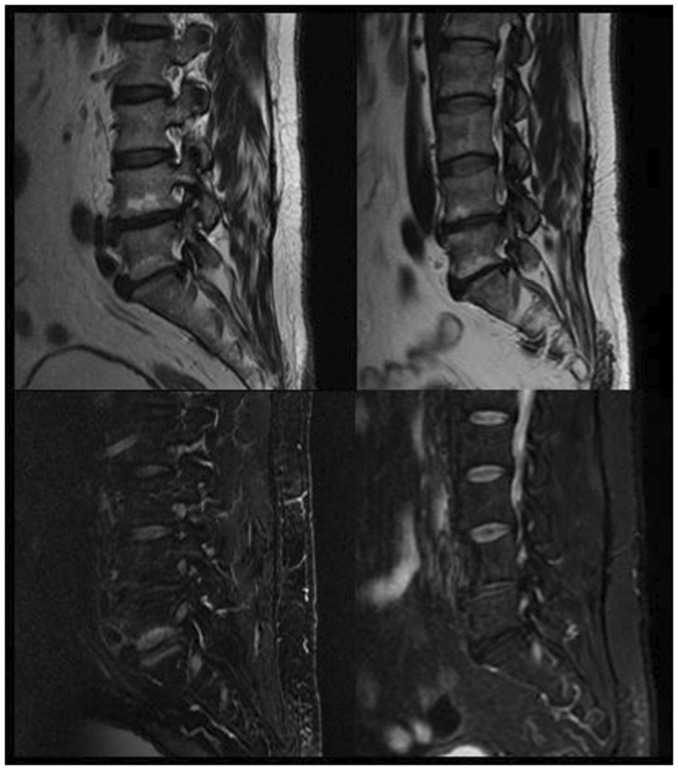
Figure 3.
Sagittal T2 (above) and T2 with fat saturation (below) images showing evolution of Modic endplate changes (from Modic I to Modic II) 3 years after intradiscal O2–O3 injection.
Table 1.
Distribution of disc degeneration changes in adjacent levels (according to Pfirmann classification from 1 to 5). The results show that the distribution of degenerative changes is similar in both study group and control group at any level.
| PFIRMANN |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| DISC LEVEL | |||||
| L1-L2 | 57 | 36 | 7 | 0 | 0 |
| L2-L3 | 0 | 64 | 32 | 4 | 0 |
| L3-L4 | 0 | 35 | 49 | 15 | 1 |
| L4-L5 | 0 | 19 | 51 | 30 | 1 |
| L5-S1 | 0 | 39 | 35 | 25 | 3 |
| Baseline study group (data are showed as %) | |||||
| DISC LEVEL | |||||
| L1-L2 | 60 | 32 | 8 | 0 | 0 |
| L2-L3 | 0 | 63 | 33 | 4 | 0 |
| L3-L4 | 0 | 34 | 51 | 15 | 1 |
| L4-L5 | 0 | 17 | 52 | 31 | 1 |
| L5-S1 | 0 | 36 | 35 | 27 | 4 |
| Follow-up study group (data are showed as %) | |||||
| DISC LEVEL | |||||
| L1-L2 | 57 | 36 | 7 | 0 | 0 |
| L2-L3 | 0 | 64 | 32 | 4 | 0 |
| L3-L4 | 0 | 35 | 49 | 15 | 1 |
| L4-L5 | 0 | 19 | 51 | 30 | 1 |
| L5-S1 | 0 | 39 | 35 | 25 | 3 |
| Baseline control group (data are showed as %) | |||||
| DISC LEVEL | |||||
| L1-L2 | 57 | 36 | 7 | 0 | 0 |
| L2-L3 | 0 | 62 | 33 | 5 | 0 |
| L3-L4 | 0 | 34 | 51 | 14 | 1 |
| L4-L5 | 0 | 18 | 50 | 32 | 1 |
| L5-S1 | 0 | 36 | 36 | 27 | 3 |
| Follow-up control group (data are showed as %) | |||||
In the control group, at baseline 4% were type I, 15% were type II and 81% were type 0.
Of the endplates showing no Modic changes, at the follow-up 3% developed Modic type I and 2% developed type II. Of the endplates with Modic I changes at the baseline, 54% remained the same, 33% progressed to Modic type II, 6% progressed to Modic type III and 7% were found to be normal (type 0) on the repeated scan.
Of the endplates initially reported as Modic II, 84% remained unchanged, 0% converted to Modic III and 16% converted to Modic I.
No statistically significant difference was detected in Modic changes between the two groups during follow-up (p > 0.05)
Disc degeneration
Results of Pfirmann changes are summarised in Figure 4. No statistically significant difference was detected in Pfirmann changes between the two groups during follow-up (p > 0.05), especially in the discs adjacent to the treated levels.
Figure 4.
Distribution of Modic changes in study and control group at baseline and follow-up.
Discussion
The prevalence rate of low back pain (LBP) is approximately 29–70% over the lifetime, and in 5–15% of patients LBP is associated with degenerative joints and disc disease.1,14,15 Although the clinical outcomes of surgical discectomy are generally satisfactory, there are several procedure-related complications including re-herniations, accelerated disc degeneration, and post-operative LBP.16 The use of open surgical approaches for disc herniations, however, has reduced since new percutaneous, imaging-guided techniques allowing disc shrinkage have been developed.9,10,16 The therapeutic efficacy of oxygen–ozone has been known for over 25 years, and many studies have demonstrated its validity for the treatment of herniated discs.1,3–5,7,8
Ozone (O3) is an unstable, strongly oxidising gas with antiseptic, immunomodulating, analgesic and anti-inflammatory properties. Intradiscal ozone has a direct effect on the proteoglycans composing the nucleus pulposus, resulting in release of water molecules and subsequent matrix, replaced by fibrous tissues within 5 weeks. Together, these changes result in a reduction of disc volume.2–5 The disc volume reduction is one of the main therapeutic targets of intradiscal O2–O3 injections, since disc shrinkage may reduce nerve root compression.
Making a comparison with observations in cases of spontaneous resorption of disc herniation, the effects of ozone can be considered as an acceleration of the normal process. After intradiscal injection, ozone can accelerate the degradation of proteoglycans of the nucleus pulposus, leading to its reabsorption and dehydration, with consequent volume reduction.7,8,11–13,16–18
Several studies showed a significant correlation between the patient’s age and disc volume changes after ozone–oxygen nucleolysis,2–5,18 with significant reduction in disc shrinkage in older patients. This is probably a result of a normal age-related process of desiccation of the intervertebral discs, with a reduced direct effect of intradiscal ozone on the release of water molecules from the degenerated nucleus pulposus. For similar reasons, the initial disc volume and the grade of disc dehydration was shown to represent a second factor that can affect the volume changes after treatment. This was confirmed by our results.
The main limits of previous studies reporting a long-term follow-up in patients treated with intradiscal ozone11,13 was the lack of a control group, leaving the questions of whether the patients may have improved spontaneously without any treatment at all and of how much volume change was related to ozone–oxygen therapy versus physiological shrinkage.
Moreover, compared with previous studies that have only made a clinical evaluation, we showed the instrumental MRI results regarding disc volumetric changes and biomechanical changes of the spine in terms of modifications in endplate and disc degeneration.
In recent years numerous studies on the natural history of disc herniation have shown that disc herniations may decrease in size and even disappear spontaneously.14 Bozzao et al.19 reported on a series of 69 non-operated patients with discal herniations of various locations and sizes proven at MRI imaging. The mean interval between the two scans was 11 months. Reduction of more than 70% was observed in 48% of patients, and 30–70% in 15%. No change or increase was observed, respectively, in 29% and 8% of the remaining patients. There was no significant correlation between the location of the herniations and the reduction of the herniation. In contrast, a high rate of resorption (over 70%) was observed in the large and medium herniations. In our series, a disc volume reduction was seen in 84% of the treated herniated lumbar discs at 3 years after therapy, with 81% of the discs having a reduction of 50% or more; moreover, we found disc volume reductions significantly more frequently in the study group than in the control group, in which the treatment did not involve the intervertebral disc. These results may suggest that ozone is also effective in hernias of lower dimensions that less frequently tend to shrink spontaneously.
Shape and volume of the intervertebral discs influence the load-carrying capacity of the spinal column, and morphologic abnormalities such as intervertebral disc space narrowing and thinning have been associated with acute or chronic disabilities of the lumbar spine.17,18 The influence of ozone-induced disc volume changes on lumbar spine stability has to be clarified, particularly because published data indicate that progressive disc degeneration leads to a decrease in axial rotation stability. Our study has documented that ozone treatment does not involve biomechanical abnormalities of the spine in terms of acceleration of the disc degeneration process compared with the natural course; in particular, we have not observed statistically significant changes in terms of disc degeneration (Pfirmann) in adjacent discs to the treated levels in comparison with the control group (representing the natural course of disc degeneration, as this group was treated only with periradicular steroids, without injection of O2–O3 into the disc).
Modic changes are dynamic markers of the age-related degenerative process affecting the lumbar spine.20–25 These lesions can convert from one type to another with time (usually within 3 years). Patients with Modic I signal changes (oedema) exhibit more frequent clinical and biological inflammatory features. Modic II (fatty replacement) and Modic III (sclerosis) signal changes are considered more quiescent phases of the process, associated with fewer inflammatory features and less painful symptoms, and thus may represent the healing stages of the disease. The potential clinical relevance of MRI Modic findings has been of great interest because of the increasing acceptance of discogenic pain as a cause of LBP. With an understanding of the spontaneous natural course of vertebral endplate Modic signal change, treatments aimed at accelerating the course of Modic I lesions may be relevant.23
Vital et al.26 suggested that spinal fusion and instrumented stabilisation may accelerate this natural history of Modic type I signal anomalies. It has also been suggested that successful treatment is associated with Modic II changes, and adverse surgical outcome (e.g. nonfusion) is associated with the persistence of Modic type I abnormalities. There is, therefore, circumstantial evidence indicating the potential clinical relevance of Modic changes on MR imaging to the extent that management decisions have been influenced by Modic changes seen on MR images.
Nguyen et al.23 reported a case report of Modic switch from I to 0, associated with regression of clinical symptomatology, after intradiscal corticosteroid injection. We advocate that the same effect could be achieved with intradiscal ozone injection, given its known anti-inflammatory effect.
To our knowledge, there are no studies in the literature that have evaluated Modic changes in patients treated with intradiscal ozone. Our results showed that no statistically significant difference was detected in Modic changes between patients treated with intradiscal ozone and the control group (p > 0.05). However, given the lack of a short-term MRI control, we cannot demonstrate if the Modic changes modifications could have been in some way influenced or accelerated by the treatment or are the results of the natural course. A follow-up pain measure may also have enabled the relationship between Modic lesions and pain to be characterised.
In this study we did not considered the clinical outcome of patients, already evaluated in our previous trials; however, the long-term clinical efficacy of intradiscal ozone injection has already been confirmed in other studies.
Conclusions
Intradiscal ozone administration it is useful in the acute and chronic setting in cases in which conservative treatment has failed, especially in patients with frequent relapses. In addition, the pain and functional outcomes are similar to those of lumbar disc treatment with surgical discectomy, but the complication rate is much lower (0.1%) and the recovery time is significantly shorter.
The effect of disc herniation shrinkage with no complications combined with an easy-to-perform treatment under CT guidance make oxygen–ozone therapy a viable alternative to surgery in the management of disc herniation.
The results of the present study indicate that intradiscal administration of intradiscal ozone can not only be associated with a statistically significant volume reduction of the herniated lumbar disc, with stable effects in the time, but also that the discolysis does not involve biomechanical changes of the spine in terms of acceleration of the disc degeneration process compared with the control group.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Gallucci M, Limbucci N, Paonessa A, et al. Degenerative disease of the spine. Neuroimaging Clin N Am 2007; 17(1): 87–103. [DOI] [PubMed] [Google Scholar]
- 2.Splendiani A, Perri M, Conchiglia A, et al. MR assessment of lumbar disk herniation treated with oxygen-ozone diskolysis: The role of DWI and related ADC versus Intervertebral Disk Volumetric Analysis for detecting treatment response. Neuroradiol J 2013; 26(3): 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perri M, Grattacaso G, Di Tunno V, et al. MRI DWI/ADC signal predicts shrinkage of lumbar disc herniation after O2-O3 discolysis. Neuroradiol J 2015; 28: 198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perri M, Grattacaso G, di Tunno V, et al. T2 shine-through phenomena in diffusion-weighted MR imaging of lumbar discs after oxygen-ozone discolysis: A randomized, double-blind trial with steroid and O2-O3 discolysis versus steroid only. Radiol Med 2015; 120(10): 941–950. [DOI] [PubMed] [Google Scholar]
- 5.Perri M, Marsecano C, Varrassi M, et al. Indications and efficacy of O2-O3 intradiscal versus steroid intraforaminal injection in different types of disco vertebral pathologies: A prospective randomized double-blind trial with 517 patients. Radiol Med 2016; 121(6): 463–471. [DOI] [PubMed] [Google Scholar]
- 6.Kelekis AD, Filippiadis DK, Martin JB, et al. Standards of practice: quality assurance guidelines for percutaneous treatments of intervertebral discs. Cardiovasc Intervent Radiol 2010; 33: 909–913. [DOI] [PubMed] [Google Scholar]
- 7.Buric J, Rigobello L, Hooper D. Five and ten year follow-up on intradiscal ozone injection for disc herniation. Int J Spine Surg 2014; 8: Article 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreula CF, Simonetti S, de Santis F, et al. Minimally invasive oxygen-ozone therapy for lumbar disk herniation. Am J Neuroradiol 2003; 24: 996–1000. [PMC free article] [PubMed] [Google Scholar]
- 9.De Oliveira Magalhaes FN, Dotta L, Sasse A, et al. Ozone therapy as a treatment for low back pain secondary to herniated disc: A systematic review and meta-analysis of randomized controlled trials. Pain Phys 2012; 15: 115–129. [PubMed] [Google Scholar]
- 10.Hashemi M, Poorfarokh M, Mohajerani SA, et al. Injection of intradiscal O2-O3 to reduce pain and disability of patients with low back pain due to prolapsed lumbar disk. Anesth Pain Med 2014; 4(5): e19206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonetti M. Herniated discs unchanged over the time: size reduced after oxygen-ozone therapy. Int J Ozone Ther 2013; 12: 83–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardi M, Simonetti L, Raffi L, et al. Mini-invasive treatment of herniated disc by oxygen-ozone injection. Intervent Neuroradiol 2003; 9(2): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alexandre A, Buric J, Paradiso R, et al. Intradiscal injection of O2-O3 to treat lumbar disc herniations: Results at five years. Riv Ital Oss Ozonoter 2002; 1: 165–169. [Google Scholar]
- 14.Benoist M. The natural history of lumbar disc herniation and radiculopathy. Joint Bone Spine 2002; 69(2): 155–160. [DOI] [PubMed] [Google Scholar]
- 15.Splendiani A, Ferrari F, Barile A, et al. Occult neural foraminal stenosis caused by association between disc degeneration and facet joint osteoarthritis: Demonstration with dedicated upright MRI system. Radiol Med 2014; 119(3): 164–174. [DOI] [PubMed] [Google Scholar]
- 16.Briseno MR, Phukan RD, Leonard DA, et al. The influence of adjacent level disc disease on discectomy outcomes. Eur Spine J 2016; 25: 230–234. [DOI] [PubMed] [Google Scholar]
- 17.Neubert A, Fripp J, Engstrom C, et al. Validity and reliability of computerized measurement of lumbar intervertebral disc height and volume from magnetic resonance images. Spine J 2014; 14: 2773–2781. [DOI] [PubMed] [Google Scholar]
- 18.Lehnert T, Naguib NNN, Wutzler S, et al. Analysis of disk volume before and after CT-guided intradiscal and periganglionic ozone-oxygen injection for the treatment of lumbar disk herniation. J Vasc Interv Radiol 2012; 23: 1430–1436. [DOI] [PubMed] [Google Scholar]
- 19.Bozzao A, Gallucci M, Masciocchi C, et al. Lumbar disk herniation: MR imaging assessment of natural history in patients treated without surgery. Radiology 1992; 185(1): 135–141. [DOI] [PubMed] [Google Scholar]
- 20.Teichtahla AJ, Finnina MA, Wanga Y, et al. The natural history of Modic changes in a community-based cohort. Joint Bone Spine 2016. (article in press). [DOI] [PubMed] [Google Scholar]
- 21.Kuisma M, Karppinen J, Niinimäki J, et al. A three-year follow-up of lumbar spine endplate (Modic) changes. Spine 2006; 31(15): 1714–1718. [DOI] [PubMed] [Google Scholar]
- 22.Hutton MJ, Bayer JH, Powell JM, et al. Modic vertebral body changes: The natural history as assessed by consecutive magnetic resonance imaging. Spine 2011; 26(36): 2304–2307. [DOI] [PubMed] [Google Scholar]
- 23.Nguyen C, Be´nichou M, Revel M, et al. Association of accelerated switch from vertebral end-plate Modic I to Modic 0 signal changes with clinical benefit of intradiscal corticosteroid injection for chronic low back pain. Arthritis Rheum 2011; 63(9): 2828–2831. [DOI] [PubMed] [Google Scholar]
- 24.Weiner BK, Vilendecic M, Ledic D, et al. Endplate changes following discectomy: Natural history and associations between imaging and clinical data. Eur Spine J 2015; 24: 2449–2457. [DOI] [PubMed] [Google Scholar]
- 25.Järvinen J, Karppinen J, Niinimäki J, et al. Association between changes in lumbar Modic changes and low back symptoms over a two-year period. BMC Musculoskel Disord 2015; 16: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vital JM, Gille O, Pointillart V, et al. Course of Modic 1 six months after lumbar posterior osteosynthesis. Spine (Phila Pa 1976) 2003; 1;28(7): 715–720. discussion 721. [DOI] [PubMed] [Google Scholar]