Abstract
Introduction
Blunt head trauma can injure the cavernous segment of the internal carotid artery (ICA). This may result in a carotid cavernous fistula (CCF). Rarely, a traumatic aneurysm may bleed medially causing massive epistaxis.
Case presentation
We present two cases of traumatic intracavernous carotid pseudoaneurysms with delayed massive epistaxis. The patients were managed with endovascular treatment involving coil embolization with parent vessel sparing and detachable balloon occlusion with carotid sacrifice. Early clinical outcome was good in both patients. Wherever possible, the CARE1 guidelines were followed in the reporting.
Conclusion
These cases illustrate the delayed nature of traumatic aneurysms and the need for a high index of suspicion in the presence of skull base fractures. The use of endovascular detachable balloon occlusion and coil embolization treatment with parent vessel preservation is shown.
Keywords: Cerebrovascular trauma, aneurysm, carotid, epistaxis
Introduction
Traumatic carotid false aneurysms are a rare but potentially fatal cause of epistaxis, having a mortality rate as high as 30%–50%.2,3 Presentation is typically delayed, with an interval of three days to six months; 50%–80% present by three weeks.3 Epistaxis may be massive and recurrent if the diagnosis and treatment are delayed. Endovascular therapy is the treatment of choice, with success reported with the use of detachable coils, stenting with carotid sparing and balloon occlusion of the parent vessel.4,5
We report two unusual cases of delayed massive epistaxis from cavernous carotid false aneurysms after blunt trauma.
Case 1
A 32-year-old male sustained blunt head injury after a motor vehicle accident. His Glasgow Coma Score (GCS) was 14 on admission, with rapid improvement to 15/15. A computerized tomography (CT) scan revealed extensive skull base fractures involving the ethmoid, maxillary and sphenoid sinuses. He was managed conservatively and subsequently discharged. Four months later he represented with a right-side hemiparesis and dysphasia. A new CT scan revealed a left wedge middle cerebral artery (MCA) territory ischemic infarction with hemorrhagic conversion (Figure 1). No CT angiogram (CTA) was performed at this stage as the presentation was at a lower-level district hospital with limited radiology support. He was mistakenly managed medically for an ischemic stroke with aspirin 75 mg daily and Simvastatin 10 mg daily. One month subsequent, he re-presented with massive epistaxis, losing more than 1000 ml of blood, and requiring resuscitation. He had two further episodes of epistaxis, both significant. A CTA performed revealed a left-side carotid cavernous aneurysm (Figure 1(a)), prompting referral to our institution.
Figure 1.
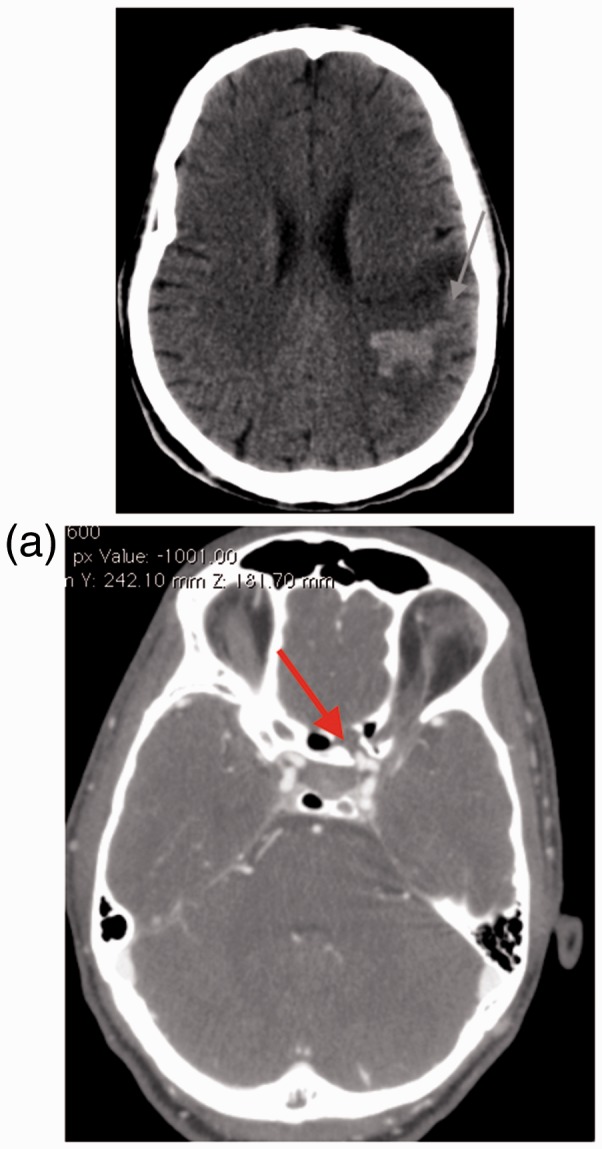
Patient 1 uncontrasted computed tomography (CT) scan of the brain revealing a left focal hypodensity with hemorrhagic conversion (arrow). (a) Patient 1 CT angiogram of cerebral vessels depicting a left carotid artery false aneurysm.
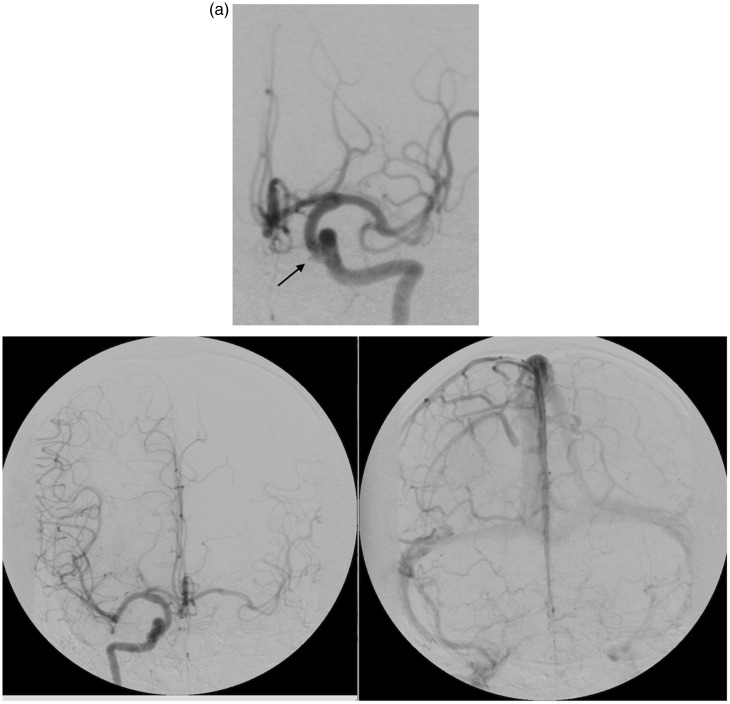
He was admitted awake and alert, with a right arm monoplegia and ipsilateral leg paresis. A digital subtraction angiogram (DSA) showed an irregularity of the cavernous segment of the left internal carotid artery (ICA) (Figure 2). A balloon test occlusion was performed which confirmed adequate cross-flow with a 2 second delay venous phase filling on ipsilateral carotid artery occlusion (Figure 2(a)). A detachable balloon was positioned adjacent to the dilatation; on deploying the balloon it herniated into the sphenoid sinus. This was left in place and a second safety balloon deployed proximally in the cervical ICA to secure occlusion, sacrificing the parent vessel. The remainder of his hospital stay was unremarkable and he was discharged on day 4 with no new neurological deficit but persistence of the presenting dysphasia and hemiparesis.
Figure 2.
Patient 1. A left internal carotid artery injection shows a small irregularity of the medial wall of the internal carotid artery representing the area of wall disruption. (a) Patient 1. Right internal carotid injection after the left internal carotid was occluded with balloon inflation. Late arterial and venous phases.
Case 2
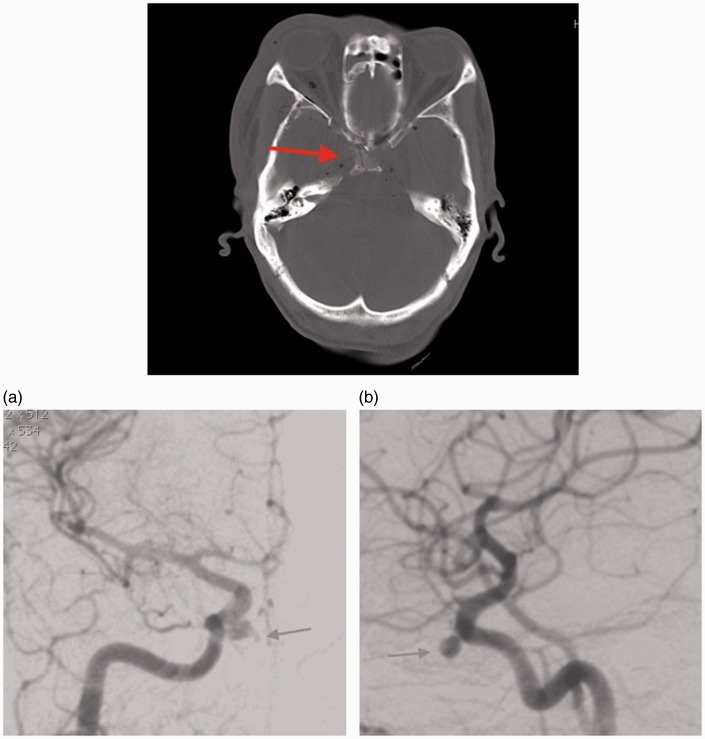
A 50-year-old male was involved in a motor vehicle accident and sustained blunt head trauma with a sphenoid body fracture involving the right carotid canal and a degloving scalp injury (Figure 3). He was admitted to the plastic surgery unit for wound management. Initially, no angiographic study was performed. On day 9 of his admission, he developed massive epistaxis requiring a blood transfusion. An emergency angiogram revealed bilateral cavernous carotid false aneurysms. Opacification of the right sphenoid sinus on uncontrasted CT and irregularity of the aneurysm suggested the right-sided lesion to be the bleeding source (Figure 3(a)). A balloon occlusion test of the right ICA showed good cross-flow from the left without development of symptoms from the patient. Right-side parent vessel balloon occlusion was performed without complication here again a proximal safety balloon was placed. The left-side false aneurysm (Figure 3(b)) was treated with detachable coil embolization with preservation of carotid flow.
Figure 3.
Patient 2, uncontrasted computed tomography (CT) of skull base depicting a fracture through the sphenoid bone (arrow). (a) Patient 2. Right internal carotid artery injection. A medially directed false aneurysm projecting into the sphenoid sinus. (b) Patient 2. Left internal carotid lateral view showing the small false aneurysm and narrow area of wall disruption.
He recovered well post-operatively and was discharged without development of new neurological deficits.
Discussion
With extensive traumatic skull base fractures, injury to the carotid artery may occur when the forces are transmitted to where the artery is fixed in the skull base. The cavernous sinus is one of these areas. Traumatic intracranial aneurysms are classified pathologically into true, false and mixed aneurysms, depending on extent of vessel wall disruption. Focal luminal dilatation from injury to the intima, internal elastic layer and media leads to a true saccular aneurysm. A traumatic false aneurysm has injury to all three layers with formation of a false lumen, contained by adventitia.6 Such lesions may be asymptomatic or commonly present after contained rupture as a carotid-cavernous fistula. Less frequently, these lesions may present with epistaxis from an aneurysmal rupture into the disrupted sphenoid sinus, which can be life threatening. These aneurysms are called false aneurysms as they lack the three-layer integrity of true aneurysms. Traumatic aneurysm walls are composed of thrombus and some extravascular tissue;7,8 this also contributes to the higher rupture risk.
Although protocols exist that help identify patients at risk for blunt cerebrovascular injury,9 the delay in onset and presentation of traumatic cavernous carotid aneurysms may result in false-negative CT angiographic studies in the acute period. At our institution, we have a selective approach to screening for cerebrovascular injury. All patients with traumatic brain injury who require a CT scan have bone window included. If this modality reveals fracture to the sphenoid or petrous bones, then a CTA is performed. If a CTA shows a false aneurysm, vessel spasm, luminal filling defect or cut-off, then we proceed to DSA. This is performed early in the injury period and when negative, follow-up imaging is not performed. Although there is a risk of missing delayed aneurysm formation, subsequent imaging after negative initial CTA has not been cost effective.
A delayed presentation is not uncommon but our first patient not only presented late but suffered a thrombo-embolic complication that was not considered to be related to the trauma he had sustained four months prior. It was only when he presented with epistaxis that it became apparent that he might have a pseudoaneurysm of the cavernous carotid. Although no dissecting flap was clearly identified on the vascular study, it was thought that the etiology of his infarction was embolic and related to the vessel injury. The side of the lesion also lateralized to the location of the aneurysm. While the second patient’s presentation was more typical, it is unusual to have bilateral carotid injury. It seemed that the right pseudoaneurysm was responsible for the epistaxis as the sphenoid sinus on the right was opacified. The carotid on the right was sacrificed as this was thought to be the quickest and safest way to prevent further hemorrhage. It was essential to preserve the left ICA and for this reason simple coiling of the false aneurysm was chosen. Although selective coiling is not an adequate treatment for a false aneurysm, in this case the area of disruption was small and felt likely to heal. The need for anti-platelet medication was also avoided. A shortcoming, specifically for the second patient treated with detachable coils only, was the inability to perform follow-up imaging to ensure sustainability of treatment. Both patients were referred from remote provinces, making close follow-up difficult.
Interventional approaches to traumatic aneurysms have evolved from Huntarian parent vessel ligation to a largely endovascular paradigm. Success has been reported with the use of Guglielmi detachable coils (GDCs), detachable balloons (outside of the United States (US)) and flow-diverting stents for parent vessel reconstruction.10–13 Because a false aneurysm is essentially an encapsulated hematoma communicating with the vessel lumen, treatment with coil embolization only carries risk of rupture or recanalization. We chose this modality for the left-side aneurysm in the second patient because it was the safest route after right-side vessel occlusion. We have been unable to assess the durability of this choice. Stent-assisted coiling has been preferred with parent vessel preservation or carotid artery sacrifice using either coils or balloons.14 Although detachable balloon devices are not approved in the US, these are in use in Europe, South Africa and Asia.15 Balloon test occlusion for evaluating adequacy of cross perfusion was performed in both patients, allowing for safe parent vessel occlusion. This technique was validated by van Rooij et al., who defined it as synchronous venous phase filling with <0.5 second delay.16 This strict angiographic criteria was expanded on by Abud et al., who in their series defined a negative test allowing for safe carotid occlusion as delay of <2 seconds’ filling between injected and occluded hemisphere. They also demonstrated the feasibility of performing test occlusion under general anesthesia, obviating the need for clinical examination.17 It is the authors’ practice to routinely deploy a proximal safety balloon when using this technique for vessel sacrifice. Detachable balloons are also our preference because of relative affordability and allowing for rapid occlusion.
Conclusion
Patients with traumatic carotid injury and a false aneurysm directed into the sphenoid sinus may be missed on presentation. This is because they may not have active bleeding or only minor epistaxis at the time of injury. The combination of epistaxis and a skull base fracture through the sphenoid should prompt immediate CTA. Even sphenoid body fracture alone may be associated with carotid injury and warrants investigation with CTA. A CTA as an initial screening tool is appropriate as it is fast and can be performed immediately on recognition of high-risk fractures. A DSA is indicated if CTA reveals a lesion or is inconclusive in the patient with a high-risk mechanism and fractures. When patients present with massive epistaxis from a cavernous carotid injury, urgent treatment is required and where possible carotid closure offers the most rapid and safe way to stop hemorrhage.
Acknowledgment
Author contributions are as follows: NM designed, compiled the manuscript and reviewed the literature. DL and AT provided expert opinion and manuscript editorial expertise.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: Consensus-based clinical case report guideline development. J Clin Epidemiol 2014; 67: 46–51. [DOI] [PubMed] [Google Scholar]
- 2.Fontela PS, Tampieri D, Atkinson JD, et al. Posttraumatic pseudoaneurysm of the intracavernous internal carotid artery presenting with massive epistaxis. Pediatr Crit Care Med 2006; 7: 260–262. [DOI] [PubMed] [Google Scholar]
- 3.Simpson RK, Harper RL, Bryan RN. Emergency balloon occlusion for massive epistaxis due to traumatic carotid-cavernous aneurysm. Case report. J Neurosurg 1988; 68: 142–144. [DOI] [PubMed] [Google Scholar]
- 4.Kadyrov NA, Friedman JA, Nichols DA, et al. Endovascular treatment of an internal carotid artery pseudoaneurysm following transsphenoidal surgery. Case report. J Neurosurg 2002; 96: 624–627. [DOI] [PubMed] [Google Scholar]
- 5.Zhang CW, Xie XD, You C, et al. Endovascular treatment of traumatic pseudoaneurysm presenting as intractable epistaxis. Korean J Radiol 2010; 11: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talamonti G, Aliberti GD and Collice M. Management of traumatic intracranial aneurysms. In: Schmidek and Sweets operative neurosurgical techniques: Indications, methods and results. 6th ed. Philadelphia, USA: Elsevier, 2012, pp.1611–1618.
- 7.Krings T, Mandell DM, Kiehl TR, et al. Intracranial aneurysms: From vessel wall pathology to therapeutic approach. Nat Rev Neurol 2011; 7: 547–559. [DOI] [PubMed] [Google Scholar]
- 8.Larson PS, Reisner A, Morassutti D, et al. Traumatic intracranial aneurysms. Neurosurg Focus 2000; 8: 1–6. [DOI] [PubMed] [Google Scholar]
- 9.Fusco MR, Harrigan MR. Cerebrovascular dissections: A review. Part II: Blunt cerebrovascular injury. Neurosurgery 2011; 68: 517–530. discussion 530. [DOI] [PubMed] [Google Scholar]
- 10.Eckard DA, O’Boynick PL, McPherson CM, et al. Coil occlusion of the parent artery for treatment of symptomatic peripheral intracranial aneurysms. AJNR Am J Neuroradiol 2000; 21: 137–142. [PMC free article] [PubMed] [Google Scholar]
- 11.Lempert TE, Halbach VV, Higashida RT, et al. Endovascular treatment of pseudoaneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol 1998; 19: 907–911. [PMC free article] [PubMed] [Google Scholar]
- 12.Mortimer AM, Klimczak K, Nelson RJ, et al. Endovascular management of cavernous internal carotid artery pseudoaneurysms following transsphenoidal surgery: A report of two cases and review of the literature. Clin Neuroradiol 2014; 25: 295–300. [DOI] [PubMed] [Google Scholar]
- 13.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen JE, Gomori JM, Segal R, et al. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery 2008; 63: 476–485. [DOI] [PubMed] [Google Scholar]
- 15.Alaraj A, Wallace A, Dashti R, et al. Balloons in endovascular neurosurgery: History and current applications. Neurosurgery 2014; 74(2 Suppl): 163–190. [DOI] [PubMed] [Google Scholar]
- 16.Van Rooij WJ, Sluzewski M, Slob MJ, et al. Predictive value of angiographic testing for tolerance to therapeutic occlusion of the carotid artery. AJNR Am J Neuroradiol 2005; 26: 175–178. [PMC free article] [PubMed] [Google Scholar]
- 17.Abud DG, Spelle L, Piotin M, et al. Venous phase timing during balloon test occlusion as a criterion for permanent internal carotid artery sacrifice. AJNR Am J Neuroradiol 2005; 26: 2602–2609. [PMC free article] [PubMed] [Google Scholar]