Abstract
Background: Previous studies have indicated that the thread carpal tunnel release (TCTR) is a safe and effective technique. Through a study on 11 cadaveric wrists, the TCTR procedure was modified and the needle control accuracy was improved to 0.15 to 0.2 mm, which is precise enough to preserve superficial palmar aponeurosis (SupPA), Berrettini branch, and common digital nerves. The aim of the present study was to verify the modified TCTR clinically. Methods: The modified TCTR was performed on 159 hands of 116 patients. The Boston Carpal Tunnel Syndrome Questionnaire was used for assessing the outcomes. Statistical analyses were used to compare the outcomes with the available data from the literature for the open and endoscopic techniques. Results: TCTR led to significant improvement in the short-term results, and the outcomes were better in long-term results compared with the open or endoscopic release. The SupPA, Berrettini branch, and common digital nerves were protected. There was no neurovascular complication for any case. Significant relief of symptoms was observed 3 to 5 hours post procedure. Most patients used their hands on the day of the procedure for simple daily activity. Patients reported their sleep quality was improved on the surgical day. Most patients with office jobs were able to return to work on postoperative day 1, and those with repetitive jobs returned to work in about 2 weeks. The statistical evidence proves that the modified TCTR procedure results in improved clinical outcomes as compared with open carpal tunnel release (CTR) and endoscopic CTR. Conclusions: The TCTR procedure has been shown to be a safe and effective technique for CTR. The modified TCTR procedure minimizes postoperative complications, such as pillar pain, scar tenderness, or functional weakness, by avoiding unnecessary injuries to the surrounding structures around the transverse carpal ligament during the procedure.
Keywords: carpal tunnel release, carpal tunnel syndrome, percutaneous procedure, thread dissecting procedure, ultrasound-guided procedure
Introduction
The thread carpal tunnel release (TCTR) is a minimally invasive procedure for transecting the transverse carpal ligament (TCL) by sawing the ligament with a piece of thread looped percutaneously under the guidance of ultrasound.15 The method ensures that the division happens only inside the loop of thread around the target without injuring adjacent tissues. The thread can be easily routed in the body using a spinal needle with only 2 punctures as entry and exit points. The advantage is to minimize iatrogenic injuries.
A previous study on 34 hands of 20 patients has indicated that TCTR procedure is safe and effective, using only 2 needle puncture sites, no incision, local anesthesia, and can be performed in the clinic without need for an operating room. Patients have no scarring and can return to work within a few days after the procedure.15
During the TCTR procedure, surgeons may have challenges in manipulating the routing needle to exit the skin at the exact spot at the palm of the hand because of the obstruction of the stiff and bulging distal portion of the TCL. There is a risk for injuring the superficial palmar arterial arch (SPA) if the needle exits too distally,12,16 or a risk of incomplete transaction of distal TCL if the needle exits too proximally. Other concerns include injury of the common digital branch, or the communicating branch between the ulnar nerve and median nerve, called Berrettini branch,32,35 if the needle control accuracy is not improved.
Recently, the TCTR procedure has been modified with the entry-at-palm approach, in which the routing needle enters the skin at the palm of the hand and exits near the wrist, rather than entering near the wrist and exiting at the palm (the entry-at-wrist approach). With this modification, not only does the needle easily exit near the wrist with the wrist dorsiflexion, but also the needle angulation and positioning becomes more accurate at this critical space near the entry point that contains “at-risk” structures, because it is easier to handle a flexible needle with its tip close to the entry than its tip away from the entry as the previous situation of needle exiting. Furthermore, instead of beginning with an 18G needle, a 27G needle is used to start the routing at the palm. Then, the 18G needle follows the track of the fine needle to continue further routing. Using a flexible fine needle to start routing is helpful for better coordination with the ultrasound probe and better control of the needle positioning near the entry.
In a recent study on 11 unembalmed cadaveric wrists, the modified TCTR procedure was verified and the needle control accuracy was measured to be 0.15 to 0.2 mm, which is precise enough to protect the superficial palmar aponeurosis (SupPA), Berrettini branch, and common digital nerves.14
The purpose of this study was to evaluate the clinical outcomes of the modified TCTR. We hypothesized that the modification would result in improved clinical outcomes as compared with open carpal tunnel release (OCTR) and endoscopic carpal tunnel release (ECTR).
In the present study, TCTR was performed on 159 hands of 116 patients. The Boston Carpal Tunnel Syndrome Questionnaire (BCTQ)19 was used for assessing the symptom severity and functional status of the patients to measure outcomes. The outcomes were then compared with the OCTR and ECTR outcomes in the literature through statistical analyses.
Materials and Methods
General
The study is under the surveillance of the Quality Committee. To date, a total of 159 TCTR procedures have been performed on 116 patients in 14 months by 3 surgeons. The average age of patients was 54.83 ± 12.72, with an age range of 26 to 84 years. There were 39 males and 77 females. Of these patients, 43 patients had TCTR on both hands on different dates. None of the hands had prior carpal tunnel release (CTR). There were 17 patients with diabetes. All of the patients either failed conservative management or requested a surgical release. The preprocedure sonographic evaluation showed that all patients were eligible for TCTR without exclusion.
The diagnosis of carpal tunnel syndrome (CTS) was based on standard clinical criteria, including history, physical examination, electromyography (EMG), and sonographic studies. All patients completed a preprocedure BCTQ and a 24-hour and 6-month postoperative phone call follow-up. Clinical postoperative evaluations were completed at 3 to 7 days, 1 month, 3 months, and 1 year. Patients completed the BCTQ at each follow-up (phone call and evaluation).
Equipment
The equipment utilized for the technique includes a musculoskeletal ultrasound system, a 30G 1-inch needle, a 27G 1.5-inch needle, 2 18G 3.5-inch spinal needles, a piece of surgical dissecting thread (Loop&Shear™, 0.009 inch in diameter; Ridge & Crest Company, Monterey Park, California), 1% lidocaine 5 mL, and 0.5% lidocaine 10 mL.
Limit Points of Division
The limit points of division determine the location and the size of the division on the TCL. The distal limit point was selected sonographically at the tip of the duck’s beak (DB), shaped at the distal portion of TCL together with palmar aponeurosis, blending with the SupPA at a more superficial level (Figure 1). The concept of the DB was first presented by RojoMnaute et al.30 The proximal limit of point was the exit point, which is at 2 cm proximal to the distal wrist crease.
Figure 1.
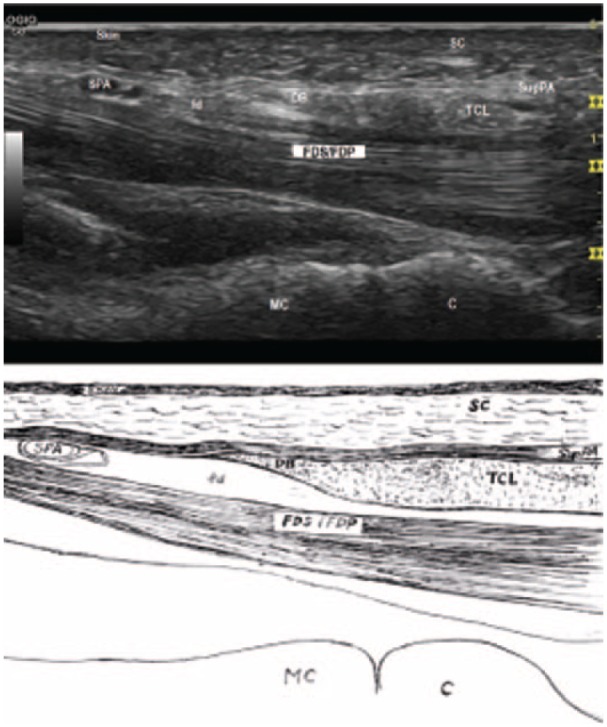
Duck’s beak (distal on left).
Note. The TCL blends with the superficial palmar aponeurosisas to show a hyperechoic area like a DB. The DB overlies the palmar fat pad, seen as a hypoechoic area between the DB and the flexor tendons. DB = the duck’s beak; SC = subcutaneous fibroadipose tissue of the palm; SPA = superficial palmar arterial arch; SupPA = superficial palmar aponeurosis; fd = fat pad; TCL = transverse carpal ligament; FDS = flexor digitorum superficialis tendons; FDP = flexor digitorum profundus tendons; MC = metacarpal bone; C = capitate bone.
Diagnosis
Most patients had suffered from CTS for at least 12 months and failed conservative treatments. There were 5 patients with severe thenar atrophy, abductor pollicis brevis weakness, and constant numbness and tingling in median nerve sensory distribution. There were 25 patients with impaired 2-point discrimination.
Preprocedure sonographic evaluation revealed anatomic variants of the patients, such as Berrettini branch, bifid median nerve, trifid median nerve, persistant median artery, variant superficial palmar arch, accessory abductor digiti minimi, and proximal origin of the lumbricals in the carpal tunnel. EMG was used to confirm the diagnosis.
Procedure and performance
The procedures were performed in a clinic procedure room by a surgeon with a medical assistant operating at the key board of the ultrasound machine.
An ultrasound was used to identify the median nerve, the third and fourth common digital nerves, Berrettini branch if it exists, the flexor tendons, the proximal and distal margins of the TCL, the bony marks of pisiform, tubercle of the scaphoid, hook of the hamate, tubercle of the trapezium, and the SPA.
Using a 30G 1-inch needle, 1 mL of 1% lidocaine was injected subcutaneously at the entry and exit points for local anesthesia. All patients were under local anesthesia, were conscious during the procedure, and were able to cooperate with the surgeon.
The routing needle traveled between the entry point on the palm and exit points at the wrist (Figure 2). The routing process includes 2 needle passes. The first pass is at the dorsal side of the TCL, and the second pass is at the palmar side. The 2 different routing paths are illustrated in Figure 3. The whole process was under the real-time visualization of ultrasound, and was involved with hydrodissecting all the time (injecting fluid to hydrostatically separate the tissues and create the space in between). The details are described as follows.
Figure 2.
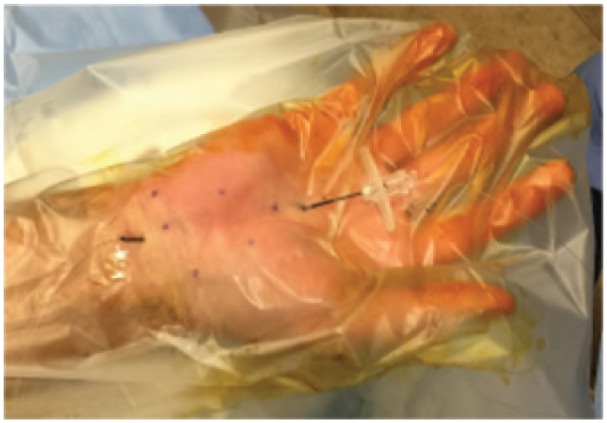
The routing needle traveled between the entry and exit points.
Figure 3.
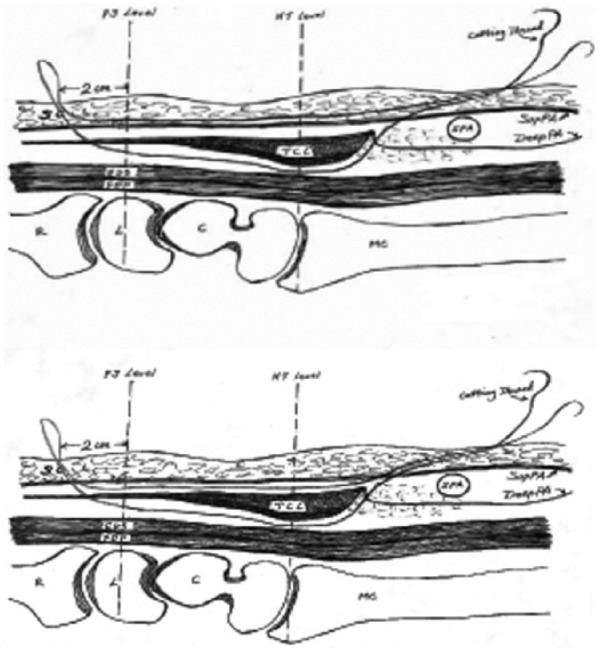
The routing path of thread with SupPA (top) or without SupPA (bottom) in the loop (distal on right).
Note. SupPA = superficial palmar aponeurosis; PS level = plane of the line connecting the pisiform and scaphoid bones; SC = subcutaneous fibroadipose tissue of the palm; HT level = plane of the line connecting the hook of hamate and trapezium tuberosity bones; SPA = superficial palmar arterial arch; DeepPA = deep palmar aponeurosis; TCL = transverse carpal ligament; FDS = flexor digitorum superficialis tendons; FDP = flexor digitorum profundus tendons; R = radius; L = lunate bone; C = capitate bone; MC = metacarpal bone.
Step 1. Inserted a fine needle into carpal tunnel at DB
A 27G needle was inserted subcutaneously at the palm of the hand, advanced proximally with hydrodissection with 1% lidocaine. After being passed over SPA (Figure 4), it was penetrated through the SupPA, then through the deep layer of palmar aponeurosis (DeepPA), then into the carpal tunnel at the tip of the DB (Figure 5). The needle was then removed.
Figure 4.
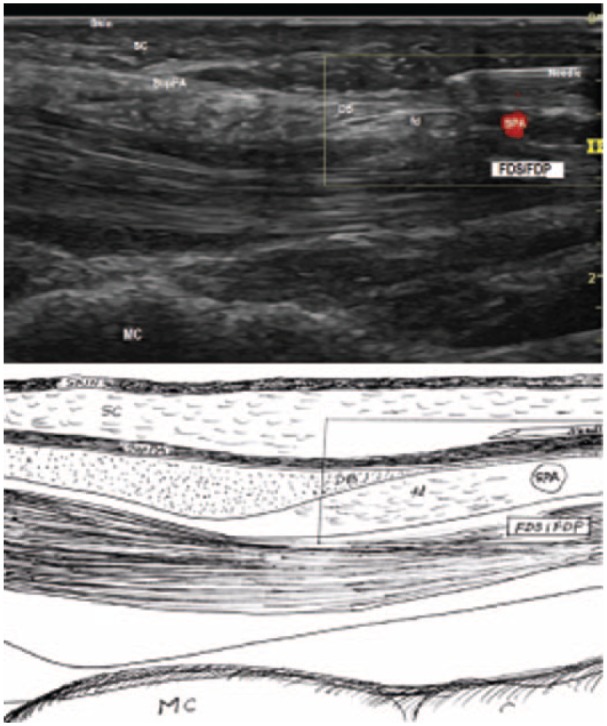
The 27G needle passed over the SPA.
Note. SPA = superficial palmar arterial arch; SC = subcutaneous fibroadipose tissue of the palm; SupPA = superficial palmar aponeurosis; DB = the duck’s beak; fd = fat pad; FDS = flexor digitorum superficialis tendons; FDP = flexor digitorum profundus tendons; MC = metacarpal bone.
Figure 5.
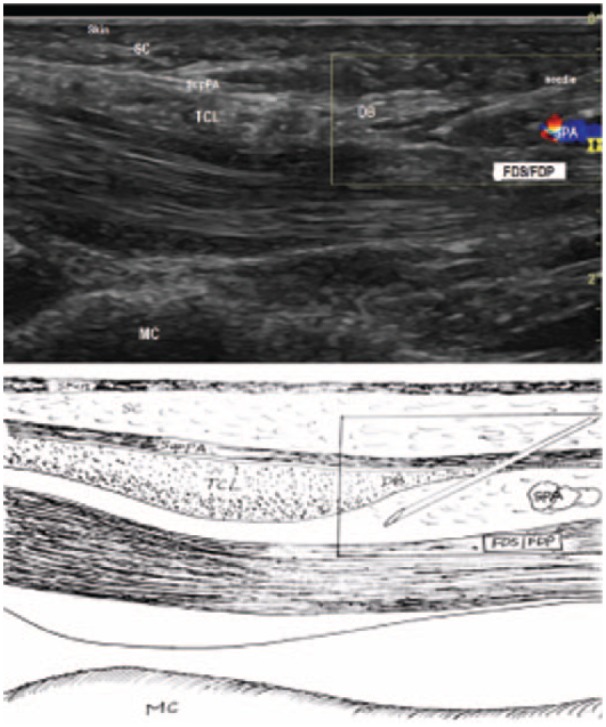
The 27G needle passed through the SupPA at the tip of DB.
Note. SupPA = superficial palmar aponeurosis; DB = the duck’s beak; SC = subcutaneous fibroadipose tissue of the palm; TCL = transverse carpal ligament; SPA = superficial palmar arterial arch; FDS = flexor digitorum superficialis tendons; FDP = flexor digitorum profundus tendons; MC = metacarpal bone.
Step 2. The first pass via the dorsal side of TCL
An 18G spinal needle was slightly curved at the tip and distal shaft. Following the track of the needle in step 1, the needle was placed into the carpal tunnel, and advanced along the dorsal surface of the TCL toward exit point (Figure 6). With dorsiflexion of the wrist, the needle exited at the exit point near the wrist. One end of the cutting thread was sent to the entry side from the exit side through the needle, and the needle was then withdrawn while leaving the thread in the carpal tunnel.
Figure 6.
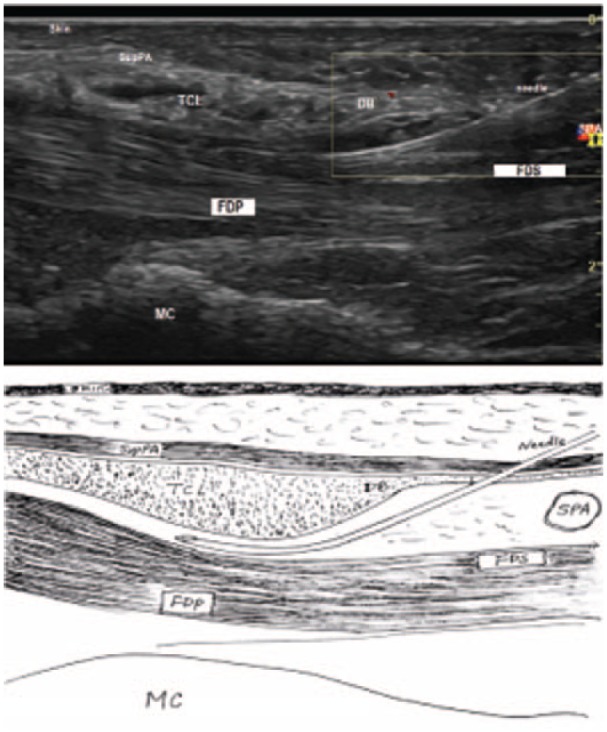
The 18G needle in the first pass.
Note. SupPA = superficial palmar aponeurosis; TCL = transverse carpal ligament; DB = the duck’s beak; SPA = superficial palmar arterial arch; FDS = flexor digitorum superficialis tendons; FDP = flexor digitorum profundus tendons; MC = metacarpal bone.
Step 3. The second pass via the palmar side of TCL
A straight 18G spinal needle was placed into the same entry point, advanced subcutaneously along palmar surface of SupPA, and exited at the same exit. The thread emerging from the hand was then passed through the needle, leaving the thread looped around the ligament (Figure 3, top).
Ultrasound was used to check the thread loop (Figure 7, top). The TCL was manually dissected by a reciprocating motion of the thread until the thread moved out of the hand. It took about 10 seconds.
Figure 7.
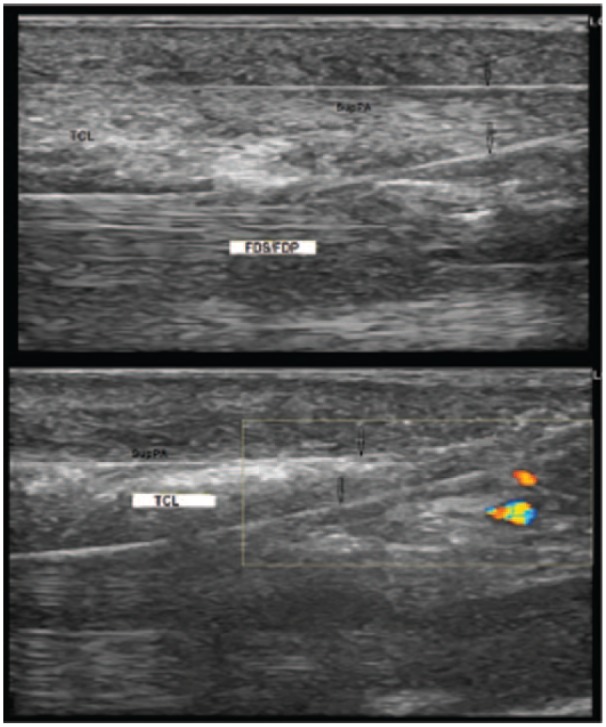
The sonographical view of the completed routing loop with (top) or without (bottom) the SupPA included.
Note. SupPA = superficial palmar aponeurosis; TCL = transverse carpal ligament; FDS = flexor digitorum superficialis tendons; FDP = flexor digitorum profundus tendons.
Modified step 3. The second pass with preserving SupPA
In this step, the SupPA will be excluded from the loop. After step 2, a straight 18G spinal needle followed the track of the needle in step 1 to be sent through the SupPA, and then advanced along the dorsal surface of the SupPA, instead of the palmar surface in step 3, until exiting at the same exit (Figure 3, bottom, and Figure 7, bottom).
The first 48 cases were performed with step 3, and the following cases were performed with a modified step 3.
Statistical Analyses
The statistical analyses were conducted on the BCTQ scores to compare the outcomes for different postoperative intervals with the data from ECTR and OCTR from the literature. The P values were calculated through z tests. The significant level used was .05.
Results
The TCTR procedure was performed with the modified technique as described on a total of 159 cases.
For the first 23 cases, Kenalog 20 mg mixed with 0.5% lidocaine was used as the hydrodissection fluid. Unexpectedly, there were 7 cases with mild clear fluid oozing at the exit point postoperatively. Five of these patients healed in 7 to 10 days whereas 2 developed infection. One infected case involved a patient with chronic pain syndrome and poor hygiene. She had not returned to the clinic for the routine 3- to 7-day follow-up. She returned to the clinic only after she was aware of the infection at the exit point. The patient was placed on 7 days of antibiotics and the exit point wound was treated and covered to keep dry and clean. The signs of infection disappeared in 2 days. Subsequent follow-up revealed her carpal tunnel symptoms had completely resolved with no scar at the exit point. The second infection case was related to a patient with type I diabetes. Her carpal tunnel symptoms had completely resolved on postoperative day 1; however, there was mild clear oozing at the exit point without infection. Although she was instructed to keep the wound dry during the follow-up visit on day 7, she returned to work on day 8 and washed her hands frequently as required by her job. She was immediately placed on antibiotics when erythema was noticed at the exit point on postoperative day 10. She ended up with open drainage.
It was decided that no steroid would be used for the subsequent cases, considering that it was possible that use of steroid was related to oozing and infection in previous cases. There were no infections in subsequent cases.
After completing the first 48 cases (26 with steriod and 22 without steriod), the TCTR procedure was modified to preserve SupPA for minimizing injury of the unnecessary surgical trauma. The Berrettini branch and common digital branch were protected for all the cases. We have successfully completed all TCTR procedures without neurovascular impairments or other postoperative complications of skin neuropraxia, sensory cutaneous neuroma–caused palmar symptoms, or reflex sympathetic dystrophy. There was no scarring.
The results of statistical analysis are presented as the means, standard deviations, and P value for the cases in scores of BCTQ to compare with the data of the open and endoscopic surgeries from Trumble et al34 in Table 1. There was no significant difference between TCTR and ECTR/OCTR groups for day 0 (before the procedure). There were significant differences between TCTR and ECTR/OCTR at all of the postoperative intervals through 1 year. The symptom severity mean score of TCTR for 1 day was significantly better than scores of ECTR/OCTR for 1 year, and the functional status score of TCTR for 1 month was better than scores of ECTR/OCTR for 1 year.
Table 1.
Comparisons.
A: Symptom severity mean (SD) scores.
| Guo et al |
Trumble et al34 |
Statistical significance |
|||||
|---|---|---|---|---|---|---|---|
| Postoperative interval | TCTR Mean (SD) | Cases | Endoscopic 97 cases | Open 95 cases |
P value |
Note | |
| Vs endoscopic | Vs open | ||||||
| 0 day | 3.19 (0.71) | 159 | 3.2 (0.14) | 3.1 (0.13) | >.05 | >.05 | |
| 1 day | 1.67 (0.48) | 159 | <.001 | <.001 | Vs Trumble’s data for 52 weeks | ||
| 1 to 2 weeks | 1.64 (0.44) One week |
154 | 2.3 (0.15) Two weeks |
3.1 (0.15) Two weeks |
<.001 | <.001 | Vs Trumble’s data for 2 weeks |
| 4 weeks | 1.48 (0.42) | 135 | 2.0 (1.4) | 3.0 (0.12) | <.001 | <.001 | |
| 8 weeks | 1.9 (0.12) | 2.7 (0.13) | |||||
| 12 weeks | 1.39 (0.73) | 111 | 1.8 (0.14) | 2.5 (0.11) | <.001 | <.001 | |
| 26 weeks | 1.19 (0.51) | 96 | 1.7 (0.13) | 1.8 (0.1) | <.001 | <.001 | |
| 52 weeks | 1.14 (0.30) | 41 | 1.8 (0.15) | 1.8 (0.1) | <.001 | <.001 | |
B: Functional status mean (SD) scores.
| Guo et al |
Trumble et al34 |
Statistical significance |
|||||
|---|---|---|---|---|---|---|---|
| Postoperative interval | TCTR Mean (SD) | Cases | Endoscopic 97 cases | Open 95 cases | P value | Note | |
| Vs endoscopic | Vs open | ||||||
| 0 day | 2.56 (0.89) | 159 | 2.7 (0.12) | 2.7 (0.08) | >.05 | >.05 | |
| 1 day | 2.29 (0.91) | 159 | |||||
| 1 to 2 weeks | 1.93 (0.78) One week | 154 | 2.2 (0.11) Two weeks | 3.0 (0.11) Two weeks | <.001 | <.001 | Vs Trumble’s data for 2 weeks |
| 4 weeks | 1.49 (0.59) | 135 | 1.9 (0.11) | 2.6 (0.08) | <.001 | <.001 | |
| 8 weeks | 1.9 (0.13) | 2.5 (0.12) | |||||
| 12 weeks | 1.30 (0.65) | 111 | 1.7 (0.1) | 2.4 (0.1) | <.001 | <.001 | |
| 26 weeks | 1.20 (0.66) | 96 | 1.8 (0.13) | 1.8 (0.09) | <.001 | <.001 | |
| 52 weeks | 1.11 (0.25) | 41 | 1.7 (0.1) | 1.7 (0.11) | <.001 | <.001 | |
Note. TCTR = thread carpal tunnel release.
Statistically, the evidence of the study supports the hypothesis that the modified TCTR would result in improved clinical outcomes as compared with OCTR and ECTR.
For most patients, the numbness and tingling was relieved 3 to 5 hours postoperative and sleep quality improved the night of the procedure. Only 2 patients took a couple of tramadol on the first night. Patients were able to use their hands the day of the procedure for simple daily activities such as eating, driving, or controlling a computer mouse. Most patients with office jobs returned to work or were eligible to return to work the day after the procedure. Some patient with repetitive work returned to work in a few days with some restrictions. Those with significantly repetitive jobs, such as factory jobs and any job with manual labor, returned to work in about 2 weeks. Two long-distance truck drivers returned to work within 4 days.
There were 8 patients who suffered from mild to moderate pillar-pain–like symptoms between 2 and 6 weeks. Sonographic evaluation revealed flexor carpi radialis tenosynovitis for 3 cases, flexor carpi ulnaris tenosynovitis for 2 cases, periostitis of hook of the hamate for 2 cases, and periostitis at the tuberosity of the trapezium for 1 case. Each case was treated with a 5-mg kenalog injection and the symptoms were completely relieved. There were 5 severe CTS cases in which patients with thenar atrophy and constant numbness and tingling in the median nerve distribution had complete tingling relief, considerable relief of numbness, and thumb function improvement with subsequent follow-ups. In addition, 4 patients with a history of needle faint and severe anxiety had the procedure successfully completed.
Discussion
Based on results of statistical analyses of the scores of BCTQ, a significant improvement was reported in 24 hours for the symptom severity and 1 week for functional status (P < .001, vs day 0), compared with the traditional procedures in which there were at least 3 weeks for obvious symptom relief and 4 to 6 weeks for functional improvement. The follow-ups for 1 month, 3 months, and 6 months also showed significantly better outcomes in the short term and better long-term outcomes than that for OCTR and ECTR. There were no postoperative complications (after the discontinuation of steroids in the procedures) and no scars; recovery time was short for all the cases.
Pillar pain is the most common complication after CTR, described as the pain between the thenar and hypothenar areas of the hand. The occurrence was estimated to be 28% to 30%,20 7% to 61%,2,4,7,9,11,25,26,28 36%,8 19% for open surgery and 28% for endoscopic surgery,17 or 6% to 36% regardless of the surgical technique.6 Although the etiology remains elusive, Ludlow et al summarized pillar pain into four categories: ligamentous or muscular, anatomic changes in the carpal arch, neurogenic cause, and edematous changes.21 Seitz et al hypothesized pillar pain was the result of a combination of ligamentous disruption with exposed nerve endings and loss of an anatomically covered carpal canal from loss of the biomechanical and neuroprotective qualities of the flexor retinaculum.31 Morrell et al assumed the etiologies are related to the biomechanical consequences of CTR, including structural alterations, muscle and tendon effects, and neurogenic and edematous effects.27
The OCTR requires a relatively large incision and causes surgical trauma. The ECTR occasionally makes iatrogenic injury to the median nerve and tendons by inserting a cannula into the pressurized and diseased carpal tunnel, which also increases the deformation of the carpal tunnel.
For the present 159 cases, no patient experienced skin neuroma pain, reflex symptomatic dystrophy pain, or pillar pain, although there were 8 cases that showed some pillar-pain–like symptoms. The result of zero complications was due to the extreme preciseness of TCTR, through which median nerve compression was relieved by dissecting exact TCL without injuring and disturbing adjacent structures. The postoperative swelling was minor at volar palm and wrist, especially when SupPA was preserved in the TCTR procedure.
There were cases associated with OCTR and ECTR in which injury to the Berrettini branch occurred and resulted in painful neuroma formation or alteration of the sensibility of ulnar side of middle and radial side of the ring finger.1,10,24 The incidence of the Berrettini branch is reported to be 67% to 100 %.3,5,10,13,22-24,29,32,33 Kolic et al found 81 Berrettini cases from 100 dissected palms.18 Many researchers mapped the Berrettini branch through different measurement or classification or defined a danger zone for avoiding iatrogenic injury.13
Ultrasound provides high-quality images and can track the whole course of the third common digital nerve from the median nerve branching off to the proper digital nerve, and the ulnar nerve and its sensory branches to ring finger and little finger, as well as Berrettini branch if it exists. In the procedure of TCTR, it is not important to identify whether the patient has Berrettini branch or which type it belongs to as the branch is under clear observation and a hydrodissecting maneuver can push the median nerve away from the TCL to create a hypoechoic fluid space between the dorsal surface of the TCL and the palmar surface of the median nerve smoothly and gently. The looping path is manipulated in the hydrodissected hypoechoic fluid space, and the Berrettini branch is excluded from the routing loop. Several Berrettini cases were encountered in the current study, and they showed no significant difference from other cases during the procedure. There was no postoperative sensory abnormality in any cases.
The study showed, through the technique of TCTR, that all of the possible postoperative complications, such as pillar pain, scar tenderness, or functional weakness, might be minimized by avoiding the unnecessary injuries and disturbances to the surrounding structures of the TCL during the procedure of decompressing the median nerve.
Acknowledgments
Many thanks to Karen W. Miller and Allison A. Reitzner for their effects in the quality monitor for the project. Thanks also to Lara Feldhausen and Cassie Toellner for assistance in language edit. Among the authors, three Guo brothers wish to remember their mother Wenying Song for her lifetime support, encourage, and love.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures in the study were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all patients for being included in the study. There is no identifying information of patients in this manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Joseph Guo has financial interest in the device for TCTR. Others have no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ridge & Crest Company provided the devices used in this study. Danqing Guo and Danzhu Guo received a research grant from BayCare Clinic, Green Bay, Wisconsin.
References
- 1. Agee JM, Peimer CA, Pyrek JD, Walsh WE. Endoscopic carpal tunnel release: a prospective study of complications and surgical experience. J Hand Surg Am. 1995;20(2): 165-171. [DOI] [PubMed] [Google Scholar]
- 2. Bande S, De Smet L, Fabry G. The results of carpal tunnel release: open versus endoscopic technique. J Hand Surg Br. 1994;19(1):14-17. [DOI] [PubMed] [Google Scholar]
- 3. Bas H, Kleinert JM. Anatomic variations in sensory innervation of the hand and digits. J Hand Surg Am. 1999;24(6): 1171-1184. [DOI] [PubMed] [Google Scholar]
- 4. Biyani A, Downes EM. An open twin incision technique of carpal tunnel decompression with reduced incidence of scar tenderness. J Hand Surg Br. 1993;18(3):331-334. [DOI] [PubMed] [Google Scholar]
- 5. Bonnel F, Vila RM. Anatomical study of the ulnar nerve in the hand. J Hand Surg Br. 1985;10(2):165-168. [DOI] [PubMed] [Google Scholar]
- 6. Brooks JJ, Schiller JR, Allen SD, Akelman E. Biomechanical and anatomical consequences of carpal tunnel release. Clin Biomech (Bristol, Avon). 2003;18(8):685-693. [DOI] [PubMed] [Google Scholar]
- 7. Brown RA, Gelberman RH, Seiler J, et al. Carpal tunnel release. A prospective, randomized assessment of open and endoscopic methods. J Bone Joint Surg Am. 1993;75(9):1265-1275. [DOI] [PubMed] [Google Scholar]
- 8. Buchanan RT, Blair WF, Higgs PE, Hurst LN, Nathan PA. Method, education and therapy of carpal tunnel patients. Hand Surg Quart. 1995. [Google Scholar]
- 9. Dumontier C, Sokolow C, Leclercq C, Chauvin P. Early results of conventional versus two-portal endoscopic carpal tunnel release. A prospective study. J Hand Surg Br. 1995;20(5):658-662. [DOI] [PubMed] [Google Scholar]
- 10. Ferrari GP, Gilbert A. The superficial anastomosis on the palm of the hand between the ulnar and median nerves. J Hand Surg Br. 1991;16(5):511-514. [DOI] [PubMed] [Google Scholar]
- 11. Foucher G, Braga DSJ. [Endoscopic opening of the carpal canal]. Chirurgie. 1993;120(12):100-104. [PubMed] [Google Scholar]
- 12. Gellman H, Botte MJ, Shankwiler J, Gelberman RH. Arterial patterns of the deep and superficial palmar arches. Clin Orthop Relat Res. 2001;383:41-46. [DOI] [PubMed] [Google Scholar]
- 13. Griot JPWD, Van Kooten EO, Zuidam JM, Prose LP, Hage JJ. Internal anatomy of the communicating branch between the ulnar and median nerves in the hand and its relevance to volar digital sensibility. J Hand Surg Am. 2002;27(1):143-146. [DOI] [PubMed] [Google Scholar]
- 14. Guo D, Guo D, Guo J, Malone DG, Wei N, McCool LC. A cadaveric study for the improvement of thread carpal tunnel release. The Journal of Hand Surgery. 2016; http://dx.doi.org/10.1016/j.jhsa.2016.07.098 [DOI] [PubMed]
- 15. Guo D, Tang Y, Sun T, Guo J, Guo D. Non-scalpel technique for minimally invasive surgery: percutaneously looped thread transection of the transverse carpal ligament. Hand (N Y). 2015;10(1):40-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hong JT, Lee SW, Han SH, et al. Anatomy of neurovascular structures around the carpal tunnel during dynamic wrist motion for endoscopic carpal tunnel release. Neurosurgery. 2006;58(suppl):ONS127-133. [DOI] [PubMed] [Google Scholar]
- 17. Katz JN, Gelberman RH, Wright EA, Abrahamsson SO, Lew RA. A preliminary scoring system for assessing the outcome of carpal tunnel release. J Hand Surg Am. 1994;19(4):531-538. [DOI] [PubMed] [Google Scholar]
- 18. Kolic Z, Micovic V, Zamolo G, Golubovic V, Uravic M, Stancic M F. The anatomy of the Berrettini branch: implications for endoscopic carpal tunnel release. Neurosurg Focus. 1997;3(1):E9.15099046 [Google Scholar]
- 19. Levine DW, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75(11):1585-1592. [DOI] [PubMed] [Google Scholar]
- 20. Lewis C, Mauffrey C, Newman S, Lambert A, Hull P. Current concepts in carpal tunnel syndrome: a review of the literature. Eur J Orthop Surg Traumatol. 2010;20:445-452. [Google Scholar]
- 21. Ludlow KS, Merla JL, Cox JA, Hurst LN. Pillar pain as a postoperative complication of carpal tunnel release: a review of the literature. J Hand Ther. 1997;10(4):277-282. [DOI] [PubMed] [Google Scholar]
- 22. MalcicGürbüz J, Özdoĝmuş Ö, Ĉavdar S. Unusual anatomic variation of palmar sensory branches of the ulnar nerve: a case report. J Hand Surg Am. 2002;27(1):147-149. [DOI] [PubMed] [Google Scholar]
- 23. Mannerfelt L. Studies on the hand in ulnar nerve paralysis. A clinical-experimental investigation in normal and anomalous innervation. Acta Orthop Scand. 1966;37(suppl 87):31-76. [DOI] [PubMed] [Google Scholar]
- 24. Meals RA, Shaner M. Variations in digital sensory patterns: a study of the ulnar nerve-median nerve palmar communicating branch. J Hand Surg Am. 1983;8:411-414. [DOI] [PubMed] [Google Scholar]
- 25. Menon J. Endoscopic carpal tunnel release: preliminary report. Arthroscopy. 1994;10(1):31-38. [DOI] [PubMed] [Google Scholar]
- 26. Mirza MA, King ET, Tanveer S. Palmar uniportal extrabursal endoscopic carpal tunnel release. Arthroscopy. 1995;11(1): 82-90. [DOI] [PubMed] [Google Scholar]
- 27. Morrell NT, Harris A, Skjong C, Akelman E. Carpal tunnel release: do we understand the biomechanical consequences? J Wrist Surg. 2014;3(4):235-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oertel J, Schroeder HW, Gaab MR. Dual-portal endoscopic release of the transverse ligament in carpal tunnel syndrome: results of 411 procedures with special reference to technique, efficacy, and complications. Neurosurgery. 2006;59(2):333-340. [DOI] [PubMed] [Google Scholar]
- 29. Olave E, Del Sol M, Gabrielli C, Mandiola E, Rodrigues CFS. Biometric study of the relationships between palmar neurovascular structures, the flexor retinaculum and the distal wrist crease. J Anat. 2001;198(6):737-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. RojoManaute JM, CapaGrasa A, RodríguezMaruri GE, Moran LM, Martínez MV, Martín JV. Ultra-minimally invasive sonographically guided carpal tunnel release: anatomic study of a new technique. J Ultrasound Med. 2013;32(1):131-142. [DOI] [PubMed] [Google Scholar]
- 31. Seitz WH, Jr, Lall A. Open carpal tunnel release with median neurolysis and Z-plasty reconstruction of the transverse carpal ligament. Curr Orthop Pract. 2013;24(1):53-57. [Google Scholar]
- 32. Stancić MF, Mićović V, Potocnjak M. The anatomy of the Berrettini branch: implications for carpal tunnel release. J Neurosurg. 1999;91:1027-1030. [DOI] [PubMed] [Google Scholar]
- 33. Sunderland SIRS. Nerves and Nerve Injuries. 2nd ed. Edinburgh, Scotland: Churchill Livingstone; 1978. [Google Scholar]
- 34. Trumble TE, Diao E, Abrams RA, GilbertAnderson MM. Single-portal endoscopic carpal tunnel release compared with open release. J Bone Joint Surg Am. 2002;84(7):1107-1115. [DOI] [PubMed] [Google Scholar]
- 35. Wilson JN. Profiles of the carpal canal. J Bone Joint Surg Am. 1954;36A:127-132. [PubMed] [Google Scholar]


