Abstract
Background: The purpose of this study is to examine the incidence of nerve injury, clinical variables associated with nerve palsy, and predictive factors of nerve laceration after gunshot wounds to the upper extremity. Methods: Forty-one patients from a level I trauma center with gunshot wounds to the upper extremity who underwent surgical exploration between 2007 and 2014 were identified retrospectively. Patients with proximal ipsilateral injuries, inadequate documentation, imaging, or with a pre-existing neurologic deficit were excluded. Patient demographics, clinical sensory and motor examination, the presence of retained bullet fragments, fracture, vascular injury, and compartment syndrome were recorded. Univariate analysis was performed to determine significant predictors of intraoperative nerve laceration. Significance was set at P < .05. Results: Fifty-nine nerves were explored in 41 patients. There were higher frequencies of fractures, retained fragments, vascular injury, and compartment syndrome in patients with nerve palsies, although none were associated with nerve laceration. Patients with palsies on presentation were significantly more likely to have a nerve laceration found intraoperatively. Conclusions: Gunshot wounds to the upper extremity with focal nerve deficits remain a difficult problem for orthopedic surgeons. The present study provides evidence that may help guide operative decision making in treatment of these injuries.
Keywords: gunshot, upper extremity, nerve, palsy, laceration
Introduction
Gunshot wounds (GSWs) to the upper extremity can result in permanent nerve damage causing substantial morbidity and loss of function.1-3 The precise mechanism of nerve injury is multifactorial and thought to be caused by a combination of direct and indirect (heat, shock wave) trauma, making selection of treatment often difficult.4,5 Unfortunately, there is no reliable method for distinguishing between neuropraxia, axonotmesis, and neurotmesis through clinical examination, and while neuropraxia is thought to be common, some large studies have reported a high incidence of nerve laceration after GSWs.2,5
Although lacerated nerves must be treated surgically to stand a chance at recovery, it is unclear what the optimal treatment is for nerves in continuity.6 Some authors have found that simple decompression or internal neurolysis leads to good clinical outcomes.6-8 In addition, even if a nerve is in continuity, there can be dysfunction due to glial scar and neuroma formation after the initial injury, further complicating treatment decisions. Historically, many of these palsies have been observed without operative intervention as 70% of nerves in continuity regained function after 3 to 9 months of observation.9 However, delayed exploration of completely lacerated nerves may result in inferior clinical outcomes, resulting in some surgeons recommending early exploration.10-12 Unfortunately, clinically distinguishing lacerated nerves from those in continuity remains a continuous challenge since both nerves in continuity and lacerated nerves can present with deficits.
Additional studies are needed to better understand factors associated with nerve injury after GSWs to better guide surgical treatment. The purpose of this study is to examine the incidence of nerve injury, clinical variables associated with nerve palsy, and predictive factors of nerve laceration after GSWs to the upper extremity to better guide preoperative surgical decision making. We hypothesize that the presence of nerve palsy on clinical exam will be predictive of intraoperative nerve laceration.
Patients and Methods
After institutional review board approval was obtained, all patients aged 18 years and older with operatively treated GSWs between the diaphyseal humerus and distal radius that presented to a single level I trauma center between July 2007 and June 2015 were screened for inclusion in our study. Data were extracted using the hospital’s electronic medical record. Patients with ipsilateral proximal injuries, pre-existing neurologic deficits, or inadequate documentation or imaging were excluded. Orthopedic clinical notes were reviewed to determine the presence or absence of a focal nerve deficit distal to the site of injury as well as the specific nerve thought to be in dysfunction. Nerves considered in the analysis included the radial, median, ulnar, superficial radial nerve (SRN), posterior interosseous nerve (PIN), and anterior interosseous nerve (AIN). For sensory nerves, patients were considered to have a sensory dysfunction if found to have decreased or absent sensation corresponding to a known distribution. For motor nerves, patients were considered to have motor dysfunction if weakness was found in thumb abduction for the median nerve, thumb interphalangeal joint (IPJ) extension for the PIN/radial nerves, thumb IPJ flexion for the AIN, or digital abduction for the ulnar nerve. After identification of the initial cohort, operative notes were reviewed to determine which patients underwent nerve exploration and had documentation of the condition of the nerve near the blast zone (i.e., intact, bruised/contused, lacerated). Patient demographic information as well as the presence of retained bullet fragments, associated fracture with AO/OTA classification, vascular injury, compartment syndrome, and length of hospitalization were recorded. Wilcoxon-Mann-Whitney test was used to compare interval variables, and χ2 analysis or fisher exact test was used to compare categorical variables. Significance was set at P < .05.
Results
One hundred fifty patients were initially identified with GSWs to the upper extremity. After applying exclusion criteria, the final cohort consisted of 41 patients with 59 nerve explorations (Figure 1). Patient demographics were similar between those with and without palsies (Table 1). There were no significant differences in fracture rates or classification, retained fragments, vascular injury, or compartment syndrome between patients with and without palsies (Table 1). Patients with palsies had significantly longer hospital stays (12 days vs 5 days, P = .014).
Figure 1.
Flowchart of patient selection.
Table 1.
Patient Characteristics and Findings.
| Total | Palsy | No palsy | P value | |
|---|---|---|---|---|
| Total (%) | 43 | 29 (67) | 14 (33) | |
| Mean age, y (range) | 29 (18 to 54) | 28 (19 to 54) | 31 (18 to 44) | .357 |
| Female, n (%) | 4 (9.6) | 2 (50) | 2 (50) | .393 |
| Mean length of stay, d (range) | 10 (0 to 32) | 12 (0 to 32) | 5.2 (0 to 13) | .014 |
| Fracture, n (%) | 30 (70) | 18 (60) | 12 (40) | .108 |
| AO/OTA, n | .668 | |||
| 12/13 | 8 | 6 | 4 | |
| 21/22/23 | 22 | 12 | 8 | |
| Fragments, n (%) | 30 (70) | 22 (73) | 9 (27) | .419 |
| Vascular injury, n (%) | 10 (23) | 8 (80) | 2 (20) | .287 |
| Compartment syndrome, n (%) | 6 | 4 (67) | 2 (33) | .649 |
Of the 59 explored nerves, 35 (59%) were intact, 14 (24%) were contused, and 10 (17%) were lacerated (Table 2). On documented clinical exam, there were 13 ulnar palsies, 8 median, 7 radial, 3 PIN, 5 SRN, and 1 AIN palsy. Ten of 37 (27%) patients with palsies were found to have lacerated nerves and no nerve lacerations were found in patients without palsies. Having a palsy on presentation was significantly associated with an intraoperative nerve laceration (P = .006). Similarly, 12 of 37 (32%) patients with palsies were found to have nerve contusion on exploration, in comparison with 9 of 22 (9%) patients without palsies.
Table 2.
Operative Findings.
| Nerve | Palsy | Operative findings |
||
|---|---|---|---|---|
| Intact | Contusion | Laceration | ||
| Radial | Yes | 2 | 3 | 2 |
| No | 2 | 2 | 0 | |
| PIN | Yes | 0 | 1 | 2 |
| No | 3 | 0 | 0 | |
| SRN | Yes | 4 | 1 | 0 |
| No | 6 | 0 | 0 | |
| Median | Yes | 4 | 3 | 1 |
| No | 2 | 0 | 0 | |
| AIN | Yes | 1 | 0 | 0 |
| No | 0 | 0 | 0 | |
| Ulnar | Yes | 4 | 4 | 5 |
| No | 7 | 0 | 0 | |
| Total | Yes | 15 | 12 | 10 |
| No | 20 | 2 | 0 | |
| Total | 35 (59%) | 14 (24%) | 10 (17%) | |
Note. PIN = posterior interosseous nerve; SRN = superficial radial nerve; AIN = anterior interosseous nerve.
Case Example
Figure 2 shows a photograph of a 20-year-old male’s right hand and forearm. The patient sustained a low-velocity GSW entering volar in zone V and exiting along the distal ulnar border. The patient presented with numbness in an ulnar nerve distribution in his hand with loss of digital abduction and adduction. This “ulnar claw” is depicted in Figure 3. Upon operative exploration, the ulnar nerve was found pierced by the bullet, leaving a hole through the middle (Figure 4). The patient was treated with resection of the ulnar nerve segment and interposition repair with sural nerve autograft.
Figure 2.
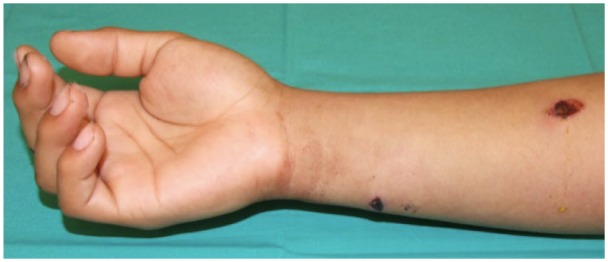
Photograph of the right hand and forearm showing the entrance wound on the volar, proximal forearm and the exit wound on the distal, ulnar forearm.
Figure 3.

Photograph of the right hand of a patient showing resting posture.
Figure 4.

Intraoperative photograph of volar forearm of patient showing defect (black arrow) in the ulnar nerve where bullet passed through the nerve midsubstance.
Discussion
GSWs continue to contribute to the spectrum of orthopedic pathology seen commonly around the United States. One recent database publication found that 23,152 patients experienced extremity firearm trauma between 2007 and 2012, of which 6,987 had documented neurovascular injury.1 The Centers for Disease Control and Prevention (CDC)13 reported 84,258 (26.65 per 100,000) non-fatal firearm injuries in 2013, an increase from 23.2 per 100,000 in 2007. This increasing trend is likely to result in a continuing rise of neurovascular injuries secondary to GSWs. In the present study, we aimed to address the incidence of nerve injury, clinical variables associated with nerve dysfunction and predictive factors of nerve laceration after GSWs to the upper extremity to better guide operative decision making.
Reported rates of nerve injury after upper extremity GSWs are highly variable. Elstrom et al noted a 45% rate of peripheral nerve injury after GSWs about the radius and ulna, whereas Kouyoumdjian et al reported rates between 15% and 21% in the upper extremity, depending on the nerve.14,15 These incidences of nerve injury are lower than reported in the present study; however, our 63% incidence is likely an overestimation as we only included operatively treated patients and some of these patients may have been explored primarily due to concern for nerve injury. In addition, the definition of nerve injury is highly variable and its cause is multifactorial, likely resulting in the wide distribution of incidences.
Traumatic injury to surrounding nerves resulting in clinical palsy after a GSW to the upper extremity can be secondary to direct trauma from the bullet, or indirect trauma such as thermal damage, laceration secondary to fracture fragment displacement, or compression secondary to swelling or subacute scar formation among many other variables. Neither retained fragments nor fractures were predictive of nerve injury either, even though these may signify higher energy dissipation into the soft tissues and may cause damage through multiple mechanisms. The association between retained fragments and nerve injury is currently unknown; however, our results suggest that the fragments themselves may not cause nerve injury. The initial shock wave, cavitation, and through-and-through bullet wounds may be more common causes of nerve injury.2 Elstrom et al examined 29 patients with forearm fractures secondary to GSWs and found that 13 (45%) patients presented with palsies, compared with 18 (60%) patients presently.14 Berg et al performed a large database study and found that 48% of GSWs to the upper extremity resulted in a fracture.1 Unlike our study, they found that fractures increased the likelihood of nerve injury 2.6 times in all upper extremity nerves excluding the axillary nerve. Our study found higher rates of fracture, but did not include the same anatomic distribution of injuries or have as many patients, which may explain the differing results.
Vascular injury was also not found to be significantly associated with palsy in our study, although 80% of patients with vascular injury did have a palsy and 28% of patients with a palsy had a vascular injury. Previous studies have found vascular injuries to be associated with nerve injury after GSWs, which can be explained by the closed proximity of neurovascular structures. Rodrigues et al found a 28% incidence of vascular injury in patients who sustained a nerve injury from a GSW, and Sitzmann et al found a 54% rate of nerve involvement with arterial injuries to the upper extremity.16,17 With a larger number of patients, we suspect this would reach significance in our study as well.
We found a 63% rate of nerve dysfunction after upper extremity GSWs, and nerve deficits were significantly associated with intraoperative nerve contusion or laceration. Twenty-seven percent of all palsies were associated with a corresponding nerve laceration upon operative exploration. Our results suggest that in patients with an arm or forearm GSW and a nerve deficit, operative exploration should be strongly considered due to the high likelihood of nerve laceration, although this continues to remain a debated topic without clear consensus.
Previous studies have noted high rates of return of function, which likely contributed to the tendency to monitor rather than explore nerves acutely.9,18 However, some studies have demonstrated improved clinical outcomes following early exploration and repair. Secer et al found that patients had better outcomes when they required shorter graft lengths and were operated on soon after injury (0-4 months).5,10 Proponents of early exploration cite the avoidance of dissection through dense scar tissue as well as the ability to identify the nerve and suture it to a local structure to prevent retraction.2,19 In the setting of nerve contusion, there may also be an advantage to performing an epineurial release to avoid damage caused by intraneural edema. In addition, early exploration may allow for repair in the setting of decreased nerve retraction, possibly allowing for a primary, tension-free, repair or use of nerve allograft or conduit to span a short gap.19 The presence of greater retraction in the setting of delayed exploration may mandate the use of nerve autograft and the associated morbidity associated with the donor site. Basic science studies have shown that in the acute setting after nerve injury, there is increased growth factor expression, less fibrosis, and less impairment to axonal growth, but a correlation with clinical outcomes has yet to be established.11,20 On the contrary, as GSWs can often cause blast injury, delayed exploration may be beneficial to allow declaration of the zone of injury prior to repair.19 Delayed exploration may also avoid the morbidity of a second delayed surgery if nerve repair/reconstruction is not feasible during the initial exploration due to surrounding infection or concerns about the surrounding soft tissue viability. Further clinical studies are needed to determine whether early exploration and repair/reconstruction results in superior clinical outcomes versus delayed repair/reconstruction and the avoidance of future nerve or tendon transfers.
Our data provide evidence that supports nerve exploration in patients with nerve dysfunction after upper extremity GSWs due to the high incidence of nerve laceration, which requires surgical repair/construction. We did not have enough patients to perform subanalyses to determine whether specific nerve deficits were predictive of nerve laceration, however, as more patients present with these injuries this may be possible. Strengths of this study include the long time period studied and heterogeneous population at a high-volume, level I trauma center. With a large number of patients (60%) both with and without deficits with intact nerve explorations, we also feel we achieved an unbiased sample of patients, rather than selecting only for those patients with lacerated nerves.
The incidence of traumatic nerve laceration after a GSW to the upper extremity is likely higher than previously believed and the delayed exploration of patients with partial or complete nerve laceration may result in inferior clinical outcomes and increased morbidity. Early exploration may allow identification of the lacerated nerve, possible early repair/reconstruction, and help guide future management and monitoring. Based on the results of this study, we recommend early nerve exploration in patients with a clinical palsy after a GSW to the upper extremity, particularly in patients going to the operating room for other indications such as irrigation and debridement, fracture fixation, or vascular repair.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: Institutional review board approval was obtained prior to performing this retrospective study. No patient identifying information has been included in the manuscript.
Statement of Informed Consent: Informed consent was not obtained for this retrospective study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Berg R, Okoye O, Inaba K, et al. Extremity firearm trauma: the impact of injury pattern on clinical outcomes. Am Surg. 2012;78:1383-1387. [PubMed] [Google Scholar]
- 2. Dicpinigaitis P, Koval K, Tejwani N, Egol K. Gunshot wounds to the extremities. Bull NYU Hosp Jt Dis. 2006;64(3):139-155. [PubMed] [Google Scholar]
- 3. Moed B, Fakhouri A. Compartment syndrome after low-velocity gunshot wounds to the forearm. J Orthop Trauma. 1991;5(2):134-137. [DOI] [PubMed] [Google Scholar]
- 4. Bowyer G, Rossier N. Management of gunshot wounds of the limbs. J Bone Joint Surg Br. 1997;79(6):1031-1036. [DOI] [PubMed] [Google Scholar]
- 5. Secer H, Daneyemez M, Tehli O, Gonul E, Izci Y. The clinical, electrophysiological, and surgical characteristics of peripheral nerve injuries caused by gunshot wounds in adults: a 40-year experience. Surg Neurol. 2008;69:143-152. [DOI] [PubMed] [Google Scholar]
- 6. Daneyemez M, Solmaz I, Izci Y. Prognostic factors for the surgical management of peripheral nerve lesions. Tohoku J Exp Med. 2005;205:269-275. [DOI] [PubMed] [Google Scholar]
- 7. Kim D, Kam A, Chandika P, Tiel R, Kline D. Surgical management and outcomes in patients with median nerve lesions. J Neurosurg. 2001;95:584-594. [DOI] [PubMed] [Google Scholar]
- 8. Kim D, Kam A, Chandika P, Tiel R, Kline D. Surgical management and outcome in patients with radial nerve lesions. J Neurosurg. 2001;95:573-583. [DOI] [PubMed] [Google Scholar]
- 9. Omer G. Injuries to nerves of the upper extremity. J Bone Joint Surg Am. 1974;56:1615-1624. [PubMed] [Google Scholar]
- 10. Sunderland S. Nerve injuries and their repair: a critical appraisal. New York, NY: Churchill Livingstone; 1991. [Google Scholar]
- 11. Saito H, Kange M, Dahlin L. Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci. 2008;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trumble TE, McCallister MW. Repair of peripheral nerve defects in the upper extremity. Hand Clin. 2000;16(1):37-52. [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control. “WISQARS nonfatal injuries.” 2014. www.cdc.gov/injury/wisqars/. Accessed October 5, 2014.
- 14. Elstrom J, Pankovich A, Egwele R. Extra-articular low-velocity gunshot fractures of the radius and ulna. J Bone Joint Surg Am. 1978;60:335-341. [PubMed] [Google Scholar]
- 15. Kouyoumdjian J. Peripheral nerve injuries: a retrospective survey of 456 cases. Muscle Nerve. 2006;34:785-788. [DOI] [PubMed] [Google Scholar]
- 16. Rodrigues R, Sammer D, Chung K. Treatment of complex below-the-elbow gunshot wounds. Ann Plast Surg. 2006; 56:122-127. [DOI] [PubMed] [Google Scholar]
- 17. Sitzmann J, Ernst C. Management of arm arterial injuries. Surgery. 1984;96:895-901. [PubMed] [Google Scholar]
- 18. Omer G. Results of untreated peripheral nerve injuries. Clin Orthop Relat Res. 1982;163:15-19. [PubMed] [Google Scholar]
- 19. Houdek MT, Shin AY. Management and complications of traumatic peripheral nerve injuries. Hand Clin. 2015;31(2):151-163. [DOI] [PubMed] [Google Scholar]
- 20. Jonsson S, Wiberg R, McGrath A, et al. Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery. PLoS ONE. 2013;8(2):e56584. [DOI] [PMC free article] [PubMed] [Google Scholar]



