Abstract
Background: Giant cell tumor (GCT) of bone is a benign, though locally aggressive tumor, classically described as an eccentric lytic lesion, often with cortical expansion and destruction. It typically involves the metaphysis or epiphysis of long bones in skeletally mature patients, with a slight female predominance. The incidence in the small bones of the hand has been reported to be 2% to 5%. Methods: Treatment options have evolved in recent years, and currently include intralesional curettage with or without adjuvant therapy, wide resection, and occasionally amputation. Results: In this report, we present a long-term follow-up (10 years) of a patient with GCT involving a metacarpal, who was initially reconstructed with a metacarpal head allograft, which was eventually revised to a metacarpophalangeal (MCP) total joint arthroplasty. Conclusions: To our knowledge, this is the only report of pyrocarbon being used for tumor reconstruction and the only report of late MCP allograft salvage.
Keywords: giant cell tumor, metacarpal, MCP arthroplasty, pyrocarbon, hand
Introduction
Giant cell tumor (GCT) of bone is a benign, though locally aggressive tumor. It typically involves the metaphysis or epiphysis of long bones in skeletally mature patients, with a slight female predominance.17 The incidence in the small bones of the hand has been reported to be 2% to 5%.3 Those that do occur in the hand most often involve the metacarpals and phalanges.15 Although occasionally patients suffer a pathologic fracture, most present with pain, swelling, and stiffness of the involved digit.
Treatment options have evolved in recent years, and currently include intralesional curettage with or without adjuvant therapy, wide resection, and occasionally amputation. Multiple studies have shown that recurrence rates are largely influenced by treatment type, with the highest recurrence rates associated with curettage alone.19 However, there remains a paucity of studies examining the reconstruction after tumor resection. Specifically, when there is articular involvement of the small joints of the hand, there is little information on the results of joint reconstruction techniques, including total joint replacement, allograft reconstruction, or joint arthrodesis augmented with bone graft. In this report, we present a long-term follow-up (10 years) of a patient with GCT involving a metacarpal, who was initially reconstructed with a metacarpal head allograft, which was eventually revised to an MCP total joint arthroplasty.
Case
A 57-year-old right-hand dominant female nurse presented with a 3-year history of pain along the dorsum of the right ring finger. These symptoms were limiting her daily activities, specifically playing the harp. Her medical history was notable for cervical cancer. She was otherwise healthy and had no history of trauma. Of note, the patient had been evaluated for similar symptoms 3 years prior by another physician. She was diagnosed with synovitis, and conservative measures, including splinting, were undertaken. Radiographs from that time appeared normal (Figure 1).
Figure 1.
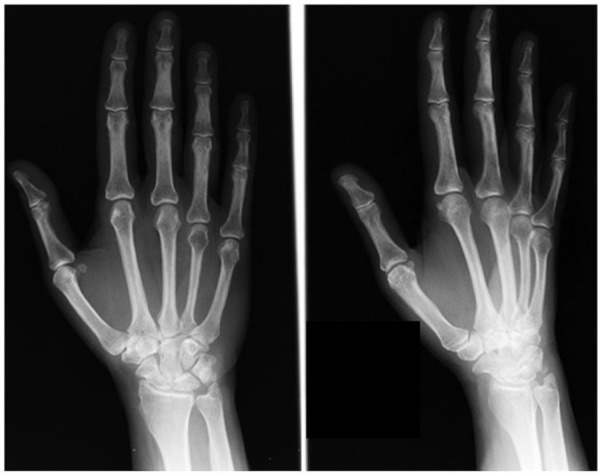
Radiographs prior to presentation (February 5, 2003).
Physical exam revealed a mass along the dorsum of the ring finger metacarpal, which was tender to palpation. A 30° extension deficit at the MCP joint was noted. Range of motion (ROM) of the proximal interphalangeal (PIP) and distal interphalangeal (DIP) joints of the ring finger was normal, as was ROM of the other digits.
Radiographs from the time of our initial evaluation (Figure 2) showed a lytic expansile lesion involving the distal half of the metacarpal, extending to the articular surface. A magnetic resonance imaging (MRI) scan was then performed (Figures 3a-3c), showing the cortex was thin but intact, with no transcortical extension. Given the articular involvement and extensive bony destruction, en bloc resection of the mass and allograft reconstruction of the metacarpal head were performed. The tumor was removed through a dorsal approach. The tumor was resected with a 1-cm bony margin proximally. The specimen was sent to Pathology, and the diagnosis was confirmed to be a GCT of bone (Figures 4a and 4b). The fresh-frozen allograft metacarpal was then used to reconstruct the defect. Permanent sutures were used to repair the collateral ligaments. The volar plate was reconstructed, in addition to the transverse metacarpal ligament. At the conclusion of the case, the patient had approximately 15° of hyperextension and 60° of flexion of the MCP joint. Postoperative radiographs are shown in Figure 5.
Figure 2.
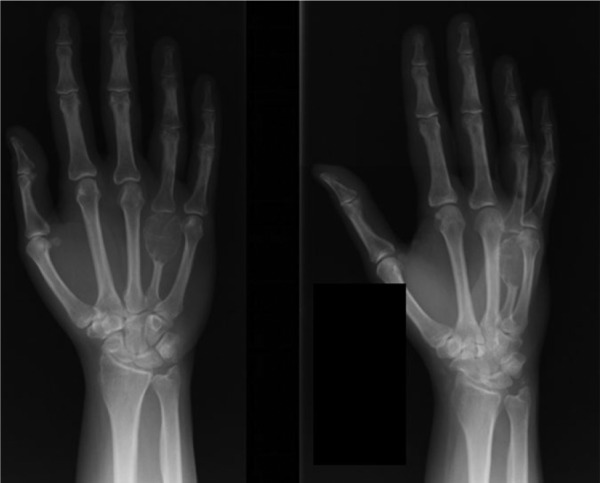
Radiographs from the time of our initial evaluation showed a lytic expansile lesion involving the distal half of the metacarpal, extending to the articular surface (May 16, 2006).
Figure 3.
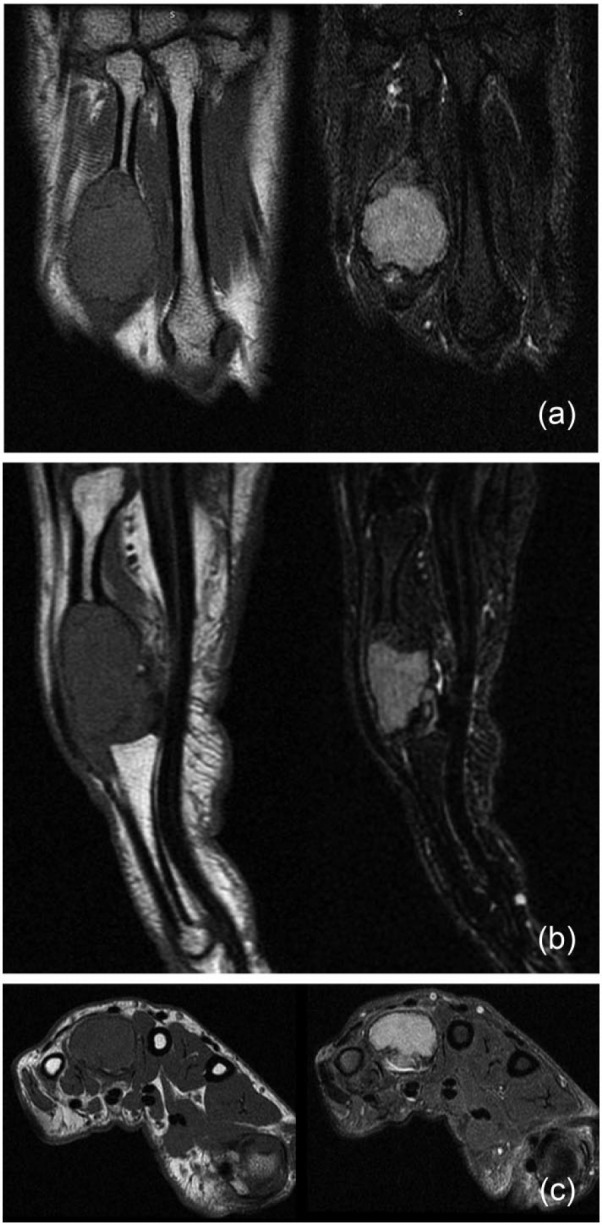
(a, b, c) A magnetic resonance imaging scan was performed, showing the cortex was thin but intact, with no transcortical extension (June 1, 2006).
Figure 4.
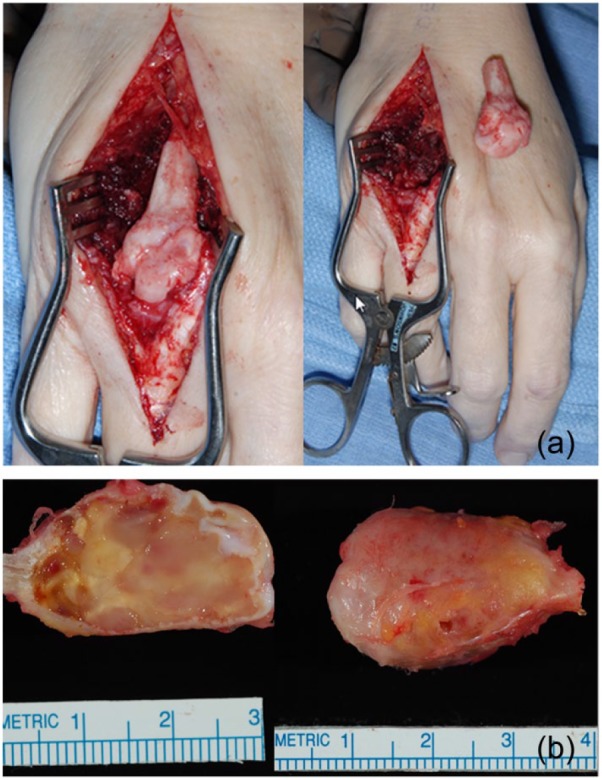
(a) The tumor was resected with a 1-cm bony margin proximally (July 5, 2006). (b) The specimen was sent to Pathology, and the diagnosis was confirmed to be a giant cell tumor of bone (July 5, 2006).
Figure 5.
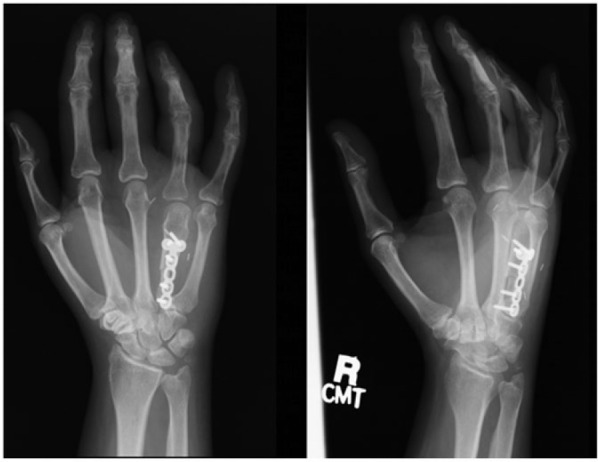
The fresh-frozen allograft metacarpal was used to reconstruct the defect. Postoperative radiographs are shown here (July 17, 2006).
The patient had an uneventful recovery and did well for several years. Four years postoperatively, she began having pain with repetitive gripping. Her ROM had also gradually decreased. Physical exam now revealed a 10° extension deficit and 80° of flexion at the MCP joint. Radiographs showed MCP joint space narrowing (Figure 6). For these reasons, an MCP total joint arthroplasty using a pyrocarbon implant was recommended. The same dorsal approach was used as in the original surgery. The allograft metacarpal head had undergone significant osteolysis and bone resorption. The metacarpal head and proximal phalanx were then prepared for the pyrocarbon implant via measured resection, sequential broaching to size 10 implants, and trial reduction. Slight instability was noted at the ulnar collateral ligament; therefore, a Vicryl suture was used to augment the collateral ligament. The final implants were placed after satisfactory stability and motion were achieved with the trial components and ulnar collateral reconstruction. At the conclusion of the case, full extension and 65° of flexion were observed. Postoperative radiographs are shown in Figure 7.
Figure 6.
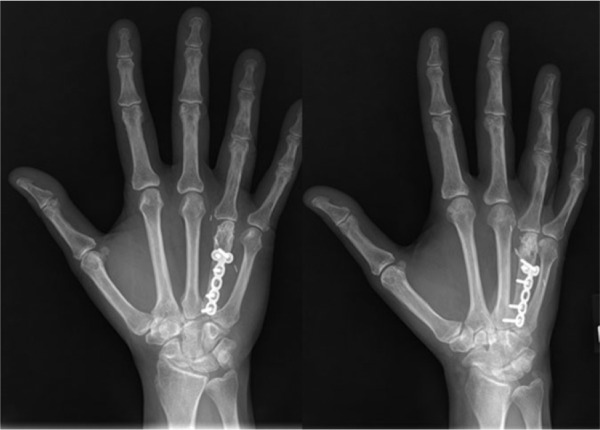
Four years postoperatively, the patient began having pain with repetitive gripping. Radiographs of metacarpophalangeal joint space narrowing are shown here (May 26, 2010).
Figure 7.
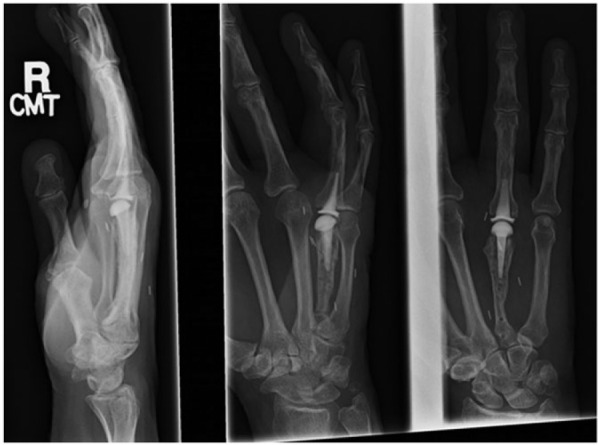
Metacarpophalangeal arthroplasty using a pyrocarbon implant was performed. Postoperative radiographs are shown here (October 15, 2010).
At most recent follow-up, 5.5 years following MCP arthroplasty, the patient was doing quite well. ROM showed 10° of extension lag with 105° of flexion at the MCP joint, and 10° of extension lag with 90° of flexion at the PIP joint (Figure 8a). Radiographs showed stable implant fixation without any signs of subsidence, migration, or loosening, as well as no evidence of recurrence of the GCT (Figure 8b).
Figure 8.
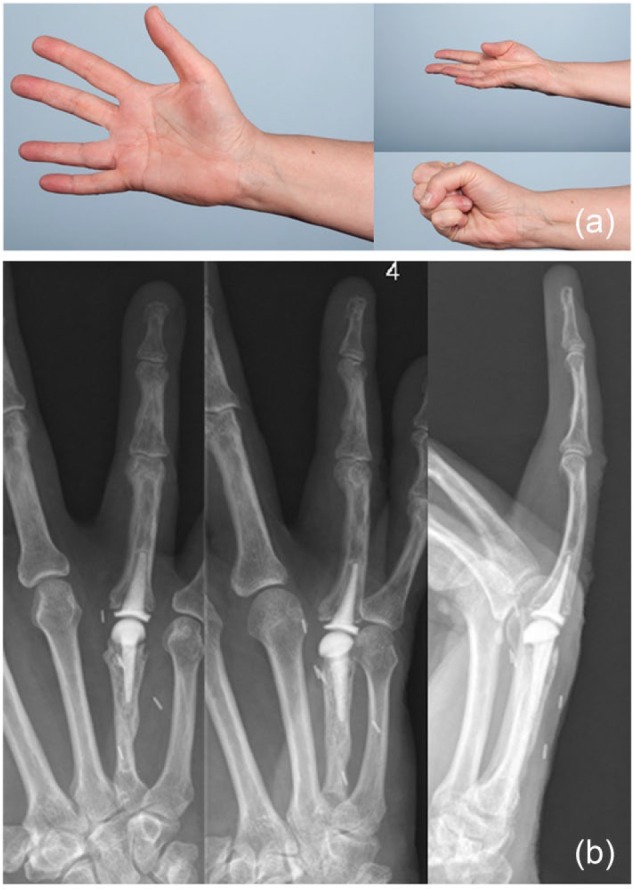
(a) At most recent follow-up, 5.5 years following metacarpophalangeal (MCP) arthroplasty, the patient was doing quite well. Range of motion showed 10° of extension lag with 105° of flexion at the MCP joint, and 10° of extension lag with 90° of flexion at the proximal interphalangeal joint. (b) Radiographs showed stable implant fixation without any signs of subsidence, migration, or loosening, as well as no evidence of recurrence of the giant cell tumor (January 25, 2016).
Discussion
GCTs are classically described as eccentric lytic lesions, often with cortical expansion/destruction. Workup includes biopsy, as well as chest radiograph and bone scan, to evaluate for aggressive tumors that can be multicentric and metastasize to the lungs. Treatment of the tumor includes intralesional curettage with adjuvant treatment (polymethyl methacrylate, phenol, burr, +/− bone graft), local versus wide excision and reconstruction, and amputation.1,2 Wide resection is indicated for cases with extensive bone destruction or articular involvement, as seen in our patient. Recurrence rates following wide excision range from 7% to 15%.14,19 Given the high recurrence rate after isolated curettage (up to 72%),19 curettage alone is not typically recommended. Local adjuncts, such as PMMA, phenol, hydrogen peroxide, and cryotherapy, are used to decrease the risk of recurrence while possibly preserving the adjacent joint.11 However, the extensive resection and need for local adjuvants often make joint preservation difficult in periarticular tumors. Given the critical role of the MCP joint in overall hand function, tumors involving this joint can be especially challenging to reconstruct and preserve function.
When the treatment of the GCTs requires resection of the MCP joint, reconstruction options include fresh-frozen osteoarticular allograft, vascularized or nonvascularized autograft3,13,15 or joint transfer,12 or MCP total joint arthroplasty. Although fusion and amputation are other potential options to consider, loss of MCP joint motion or the entire ray has marked consequences on hand function. The reconstruction options vary based upon the extent of the bone loss after the resection and the state of the soft tissue MCP joint stabilizers. Patients with extensive bone loss often require larger reconstructions involving osteoarticular allografts. These have the advantage of restoring a large amount of bone loss, while providing allograft bone to anchor the reconstruction of the ligament and soft tissue stabilizers. Conversely, the native bone and soft tissues are not thought to fully incorporate into the allograft, with a future risk of resorption, fracture, or hardware failure.4,5,8,9 Furthermore, the articular component of the osteoarticular allograft likely leads to a higher rate of joint degeneration than the native joint, as seen in our patient. MCP joint arthroplasty is another option for postresection reconstruction, either in revision cases of osteoarticular allografts (as in our case), in combination with allografts as an allograft prosthetic composite, or in cases of smaller tumors where metaphyseal preservation is possible to maintain joint stability. Given the potential compromise to the collateral ligaments and other soft tissue stabilizers, when possible, the constrained silicone prosthesis is a viable option in the setting of preserved metaphyseal bone. When collateral ligament reconstruction is possible, either to an allograft or to native bone, unconstrained prosthesis including the pyrocarbon arthroplasty or surface replacement arthroplasty (SRA) is a viable option.7,10,16,18,20-25 This is an important consideration in younger patients given the high rate of fracture with silicone implants.6,20
In our report, the patient initially had a satisfactory result after reconstruction with a fresh-frozen osteoarticular metacarpal head allograft. However, the patient began to experience pain after several years secondary to MCP joint degeneration, and required revision to a pyrocarbon total joint arthroplasty at 4 years postoperatively. The allograft provided the bone stock and soft tissue attachments needed to utilize a nonconstrained implant. This total joint arthroplasty has done well at 5.5 years of follow-up, with reasonable pain-free MCP joint motion and stability, without any compromise of implant stability. To our knowledge, this is the only report of pyrocarbon being used for tumor reconstruction and the only report of late MCP allograft salvage. This suggests that MCP SRA may be possible following allograft reconstruction. Further studies should examine the long-term effects of joint stability and implant subsidence.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all patients for being included in the study.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Steven L. Moran is a consultant for Integra.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Arroud M, Afifi MA, Chbani L, Riffi AA, Bouabdallah Y. Giant-cell tumor of the fourth metacarpal bone in children: case report. J Pediatr Orthop B. 2010;19(1):86-89. [DOI] [PubMed] [Google Scholar]
- 2. Athanasian EA, Wold LE, Amadio PC. Giant cell tumors of the bones of the hand. J Hand Surg Am. 1997;22(1):91-98. [DOI] [PubMed] [Google Scholar]
- 3. Baki ME, Guvercin Y, Yildiz M, Aynaci O, Yildiz K. Giant cell tumor of the metacarpal bone in children: free osteoarticular metatarsal transfer: case report. J Pediatr Orthop B. 2015;24(1):79-81. [DOI] [PubMed] [Google Scholar]
- 4. Chacon A, Virani N, Shannon R, Levy JC, Pupello D, Frankle M. Revision arthroplasty with use of a reverse shoulder prosthesis-allograft composite. J Bone Joint Surg Am. 2009;91(1):119-127. [DOI] [PubMed] [Google Scholar]
- 5. Cheng EY, Gebhardt MC. Allograft reconstructions of the shoulder after bone tumor resections. Orthop Clin North Am. 1991;22(1):37-48. [PubMed] [Google Scholar]
- 6. Chung KC, Burns PB, Kim HM, Burke FD, Wilgis EF, Fox DA. Long-term follow-up for rheumatoid arthritis patients in a multi-center outcomes study of silicone metacarpophalangeal joint arthroplasty. Arthritis Care Res. 2012;64(9):1292-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cook SD, Beckenbaugh RD, Redondo J, Popich LS, Klawitter JJ, Linscheid RL. Long-term follow-up of pyrolytic carbon metacarpophalangeal implants. J Bone Joint Surg Am. 1999;81(5):635-648. [DOI] [PubMed] [Google Scholar]
- 8. Gebhardt MC, Roth YF, Mankin HJ. Osteoarticular allografts for reconstruction in the proximal part of the humerus after excision of a musculoskeletal tumor. J Bone Joint Surg Am. 1990;72(3):334-345. [PubMed] [Google Scholar]
- 9. Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81(8):1138-1146. [DOI] [PubMed] [Google Scholar]
- 10. Houdek MT, Wagner ER, Rizzo M, Moran SL. Metacarpophalangeal joint arthroplasty in the setting of trauma. J Hand Surg Am. 2015;40(12):2416-2420. [DOI] [PubMed] [Google Scholar]
- 11. Klenke FM, Wenger DE, Inwards CY, Rose PS, Sim FH. Recurrent giant cell tumor of long bones: analysis of surgical management. Clin Orthop Relat Res. 2011;469(4):1181-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotwal PP, Nagaraj C, Gupta V. Vascularised joint transfer in the management of recurrent giant cell tumour of the second metacarpal. J Hand Surg Eur Vol. 2008;33(3):314-316. [DOI] [PubMed] [Google Scholar]
- 13. Manfrini M, Stagni C, Ceruso M, Mercuri M. Fibular autograft and silicone implant arthroplasty following resection of giant cell tumor of the metacarpal: a case report with 8 years follow-up. Orthopedics. 2008;31(1):96. [DOI] [PubMed] [Google Scholar]
- 14. McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68(2):235-242. [PubMed] [Google Scholar]
- 15. Naam NH, Jones SL, Floyd J, Memisoglu EI. Multicentric giant cell tumor of the fourth and fifth metacarpals with lung metastases. Hand. 2014;9(3):389-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neral MK, Pittner DE, Spiess AM, Imbriglia JE. Silicone arthroplasty for nonrheumatic metacarpophalangeal joint arthritis. J Hand Surg Am. 2013;38(12):2412-2418. [DOI] [PubMed] [Google Scholar]
- 17. Novais EN, Shin AY, Bishop AT, Shives TC. Multicentric giant cell tumor of the upper extremities: 16 years of ongoing disease. J Hand Surg Am. 2011;36(10):1610-1613. [DOI] [PubMed] [Google Scholar]
- 18. Nunez VA, Citron ND. Short-term results of the Ascension pyrolytic carbon metacarpophalangeal joint replacement arthroplasty for osteoarthritis. Chir Main. 2005;24(3-4):161-164. [DOI] [PubMed] [Google Scholar]
- 19. Oliveira VC, van der Heijden L, van der Geest IC, et al. Giant cell tumours of the small bones of the hands and feet: long-term results of 30 patients and a systematic literature review. Bone Joint J. 2013;95(6):838-845. [DOI] [PubMed] [Google Scholar]
- 20. Rizzo M. Metacarpophalangeal joint arthritis. J Hand Surg Am. 2011;36(2):345-353. [DOI] [PubMed] [Google Scholar]
- 21. Simpson-White RW, Chojnowski AJ. Pyrocarbon metacarpophalangeal joint replacement in primary osteoarthritis. J Hand Surg Eur Vol. 2014;39(6):575-581. [DOI] [PubMed] [Google Scholar]
- 22. Syed MA, Smith A, Benjamin-Laing H. Pyrocarbon implant fracture after metacarpophalangeal joint arthroplasty: an unusual cause for early revision. J Hand Surg Eur Vol. 2010;35(6):505-506. [DOI] [PubMed] [Google Scholar]
- 23. Wagner ER, Demark RV, III, Wilson GA, Kor DJ, Moran SL, Rizzo M. Intraoperative periprosthetic fractures associated with metacarpophalangeal joint arthroplasty. J Hand Surg Am. 2015;40(5):945-950. [DOI] [PubMed] [Google Scholar]
- 24. Waljee JF, Chung KC. Objective functional outcomes and patient satisfaction after silicone metacarpophalangeal arthroplasty for rheumatoid arthritis. J Hand Surg Am. 2012;37(1):47-54. [DOI] [PubMed] [Google Scholar]
- 25. Wall LB, Stern PJ. Clinical and radiographic outcomes of metacarpophalangeal joint pyrolytic carbon arthroplasty for osteoarthritis. J Hand Surg Am. 2013;38(3):537-543. [DOI] [PubMed] [Google Scholar]


