Abstract
Objective
The prevalence of diabetes in sub-Saharan Africa is rising, but its relationship to depression is not well-characterized. This report describes depressive symptom prevalence and associations with adherence and outcomes among patients with diabetes in a rural, resource-constrained setting.
Methods
In the Webuye, Kenya diabetes clinic, we conducted a chart review, analyzing data including medication adherence, hemoglobin A1c (HbA1c), clinic attendance, and PHQ-2 depression screening results.
Results
Among 253 patients, 20.9% screened positive for depression. Prevalence in females was higher than in males; 27% vs 15% (p = 0.023). Glycemic control trends were better in those screening negative; at 24 months post-enrollment mean HbA1c was 7.5 for those screening negative and 9.5 for those screening positive (p = 0.0025). There was a nonsignificant (p = 0.269) trend toward loss to follow-up among those screening positive.
Conclusions
These findings suggest that depression is common among people with diabetes in rural western Kenya, which may profoundly impact diabetes control and treatment adherence.
Keywords: Diabetes, Depression, Resource-constrained, Kenya, Sub-Saharan Africa
Highlights
-
•
Patients attending a rural diabetes clinic were screened for depression.
-
•
Screen negative patients had better glycemic control trends.
-
•
Screen positive patients showed higher trends of loss to follow-up.
-
•
There is need for psychological care among patients with diabetes.
Introduction
Depression and diabetes mellitus are two chronic disease states that have a profoundly negative impact on quality of life and overall life expectancy [1]. They frequently co-occur, and relationships between both conditions are bidirectional [2]. Some data suggest that the burden and emotional impact of managing a chronic disease like diabetes may lead to development of mental health problems such as depression [3]. Conversely, people with depression may develop behavioral factors that lead to an overall lack of self-care, thereby increasing the risk of developing diabetes [4]. There is more than a threefold increase in the prevalence of depression in people with type 1 diabetes, and nearly a two fold increase in people with type 2 diabetes. However, there is very limited data on the prevalence of comorbid depression and diabetes in sub-Saharan Africa [5].
Both hypotheses present serious implications in the management of these comorbid disease states. If diabetes is caused or exacerbated by depression, then proper management of diabetes cannot exist without addressing depressive symptoms. Patients with diabetes experiencing significant depressive symptoms are less likely to engage in diet and exercise recommendations than are those without depressive symptoms, and have lower medication adherence than non-depressed counterparts [6]. Comorbid depression profoundly impacts diabetes self-management and treatment adherence, and is associated with significant risk of complications including stroke and myocardial infarction. Overall, comorbid depression in individuals with diabetes is associated with a 1.5-fold increase in mortality risk as compared with those without depression [7].
As such, co-management of diabetes and depression is critical; in high income countries programs have been established to co-manage depression and diabetes, but such programs are virtually nonexistent in sub-Saharan Africa (SSA) [8]. Globally, mental illnesses such as depression have become an increasingly prevalent component of the overall burden of disease [9]. Depression is the second leading cause of disability in young to middle aged adults in low and middle income countries, surpassed only by HIV/AIDS [10]. Despite this high burden and increasing global health care strain, limited infrastructure exists to confront these issues. In Kenya, approximately 0.1% of the national health care budget is directed toward mental health care [11]. Generally, there is underdiagnosis of psychiatric illnesses among patients attending medical facilities in Kenya. This calls for increased awareness among health care workers, as well as the use of screening tools that are quick and easy to use in busy clinical settings [12]. This study seeks to identify the comorbidity of depression in a rural population of patients receiving care within a public sector hospital. The Webuye diabetes clinic is located in Bungoma County, western Kenya. Webuye includes both rural and semi-urban areas, with a population of around 230,252 people. It is approximately 380 km west of the capital city, Nairobi. The diabetes clinic is affiliated with Academic Model Providing Access to Healthcare (AMPATH), and serves over 650 patients [13]. AMPATH, a partnership between Indiana University School of Medicine, Moi University School of Medicine, and Moi Teaching and Referral Hospital in Eldoret, Kenya, has developed one of Africa's largest and most effective HIV/AIDS prevention and treatment programs [14]. AMPATH has since expanded into one of the first comprehensive chronic disease management programs in SSA.
This study was approved by the Kenyan based Moi University Institutional Research and Ethics Committee and the Institutional Review Board of the North American investigators.
Methods
This study describes prevalence of depressive symptoms as measured by questions from the ultrabrief depression screening tool, the Patient Health Questionnaire 2 (PHQ-2) [15], and describes associations between depressive symptoms and adherence to medications and clinic visits in a cohort of patients with diabetes in a rural, resource-constrained clinic setting in western Kenya. In this retrospective chart review, baseline demographic information was collected including age, age at diabetes diagnosis, gender, substance use, food security, travel time to the clinic, income, presence of comorbid diseases including HIV, tuberculosis, or hypertension, and family history of diabetes or cardiovascular disease. Responses to two questions from the PHQ-2, “During the last month have you been feeling down, depressed or hopeless?” and “During the last month have you often been bothered by having little interest or pleasure in doing things?” were recorded. Patients were considered to screen positive for depression if they answered ‘yes’ to either question. Longitudinal diabetes management data were also collected, including current diabetes medication regimen and most recent HbA1c.
Wilcoxon rank-sum and Mann–Whitney tests were used for statistical analysis of non-parametric data, and Fisher's exact test was used for dichotomous data. A p-value of <0.05 was accepted as statistically significant. These tests were used to identify clinical and demographic differences between patients with and without a positive screen for depressive symptoms.
Results
Data from 253 patients were collected, of which 55% were female. Mean age was 57.6 years (depression screen-positive 58.1 years, depression screen-negative 55.8 years, p = 0.303) with a mean age of 50.9 at diabetes diagnosis (depression screen-positive 50.9 years, depression screen-negative 47.9 years; p = 0.160). Overall, 20.9% screened positive for depression (Table 1), with a statistically significant difference between the proportion of women (27%) and men (15%) screening positive (p = 0.023). There were no significant differences between those with and without depressive symptoms with respect to HIV status, presence of tuberculosis, comorbid hypertension, family history of cardiovascular disease or diabetes, alcohol use, or current diabetes medications.
Table 1.
Factors associated with positive depression screen among patients with diabetes
| Variable (n) | Depression screen positive n (%) | p |
|---|---|---|
| Gender | 0.023* | |
| Male (113) | 17 (15.0) | |
| Female (140) | 38 (27.1) | |
| TB history | 0.623 | |
| Positive (6) | 1 (16.7) | |
| Negative (243) | 52 (21.4) | |
| Aware of HIV status | 0.565 | |
| Yes (89) | 19 (21.4) | |
| No (159) | 34 (21.4) | |
| If aware, positive HIV result | 0.654 | |
| Yes (4) | 1 (25.0) | |
| No (79) | 18 (22.8) | |
| Hypertension | 0.422 | |
| Yes (84) | 17 (20.2) | |
| No (157) | 35 (22.3) | |
| Smoking history | 0.183 | |
| Yes (7) | 0 (0.0) | |
| No (242) | 53 (21.9) | |
| Chew tobacco, miraa, or khat | 1.000 | |
| Yes (3) | 0 (0.0) | |
| No (138) | 28 (20.3) | |
| Alcohol use | 0.311 | |
| Yes (26) | 4 (15.4) | |
| No (223) | 49 (22.0) | |
| Family history of DM | 0.445 | |
| Yes (84) | 17 (20.2) | |
| No (164) | 36 (22.0) | |
| Has a caretaker | 0.380 | |
| Yes (164) | 36 (22.0) | |
| No (78) | 15 (19.2) | |
| Food insecure | 0.058 | |
| Yes (83) | 23 (27.7) | |
| No (166) | 30 (18.1) | |
| Loss to follow up (>12 months) | 0.269 | |
| Yes (22) | 15 (68.2) | |
| No (231) | 185 (80.1) | |
| Travel time to clinic (>30 min) | 0.601 | |
| Yes (48) | 32 (66.7) | |
| No (191) | 152 (79.6) | |
*p < 0.05 statistically significant.
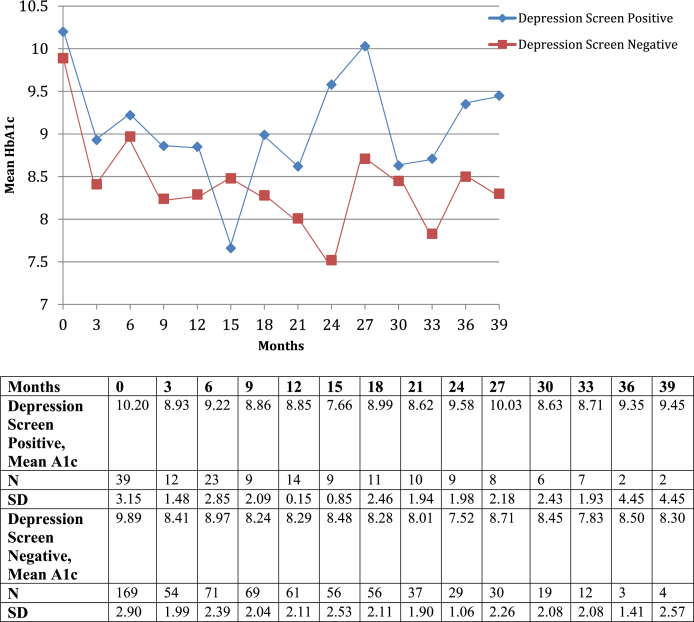
Regarding diabetes management, 13.2% of patients who screened positive for depression were lost to follow-up at 12 months following initial survey, as compared with 7.5% of patients who screened negative and 8.7% of all diabetes clinic patients (p = 0.269). Significant differences in HbA1c between the two groups were not detected until 24 months following initial survey; however, there was a consistent trend toward higher Hba1cs amongst patients screening positive for depression as demonstrated in Fig. 1. Baseline HbA1c for depression screen-negative patients was 9.9 compared to 10.2 for those screening positive. After 24 months, patients who obtained regular HbA1cs demonstrated a significant difference, as patients without depressive symptoms at baseline (N = 29) averaged an HbA1c of 7.5 and those who screened positive (N = 9) averaged 9.5 (p < 0.05).
Figure 1.
HbA1c Trends between depression screen positive and depression screen negative patients with diabetes.
Discussion
These findings suggest that depressive symptoms are common among patients with diabetes in rural western Kenya. In North American and European populations, comorbid depression has consistently been found to impact diabetes adherence, severity, and outcomes, and depression treatment interventions among patients with diabetes improve medication adherence and diabetes outcomes [16]. A higher proportion of women were screen positive for depression compared to men and this is thought to be due in part to a reporting bias as male patients tended to be more reluctant to report symptoms consistent with depression. The validity of this finding of a statistically significant difference between men and women needs to be further evaluated before conclusive findings are reported on this gender disparity. Though unable to demonstrate significant differences in diabetes severity as indicated by most recent HbA1c, our findings suggest that those with depressive symptoms trend toward a higher mean HbA1c and more loss to follow up than do those without depressive symptoms.
This study has several limitations. Firstly, the retrospective nature and small sample size limits the power to detect true associations with HbA1c or loss to follow up amongst patients with and without depressive symptoms. Further, the PHQ-2 has not been widely validated in SSA populations and may therefore not be a precise indicator of whether individuals screening positive would meet diagnostic criteria for major depressive disorder. However, these questions are quick, easy to implement in busy clinic settings, and positive response to one or both questions indicates distress that likely impacts individuals' diabetes disease severity and ability to engage in treatment.
Further research is needed to investigate prevalence of depression and relationships between depression, adherence to medication and clinic visits, and outcomes in this population. Larger studies of depression among people with diabetes and other chronic diseases in SSA are indicated to better understand relationships between these comorbid illnesses, as well as the growing discrepancy between the burden of these conditions and the infrastructure in place to treat them. As chronic disease management programs are rolled out in western Kenya and other resource-constrained settings, it is important to identify and address depression and other mental illnesses, not only as significant sources of distress and disability in their own right, but as potential barriers to adherence so that treatment interventions may be implemented efficiently and effectively.
Conflict of interest statement
Sonak Pastakia has received fees for serving as a speaker and consultant for Abbott within the last three years. None of the other authors have any disclosures to make of conflicts of interest to report.
Acknowledgments
This research has been supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through USAID under the terms of Cooperative Agreement No. AID-623-A-12-0001. It is made possible through joint support of the United States Agency for International Development (USAID). The contents of this research article are the sole responsibility of AMPATH and do not necessarily reflect the views of USAID or the United States Government.
This publication was made possible by the Indiana Clinical and Translational Sciences Institute, funded in part by grant # UL1 TR001108 from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award.
We would like to thank Abbott Fund for providing glucose testing supplies and funding to support this effort. We would also like to thank Lilly for their donation of insulin and seed funding to support our overarching diabetes work.
References
- 1.O'Connor P.J., Crain A.L., Rush W.A., Hanson A.M., Fischer L.R., Kluznik J.C. Does diabetes double the risk of depression? Ann Fam Med. 2009;7:328–335. doi: 10.1370/afm.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Renn B.N., Feliciano L., Segal D.L. The bidirectional relationship of depression and diabetes: a systematic review. Clin Psychol Rev. 2011;31:1239–1246. doi: 10.1016/j.cpr.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Knol M.J., Heerdink E.R., Egberts A.C.G., Geerlings M.I., Gorter K.J., Numans M.E. Depressive symptoms in subjects with diagnosed and undiagnosed type 2 diabetes. Psychosom Med. 2007;69:300–305. doi: 10.1097/PSY.0b013e31805f48b9. [DOI] [PubMed] [Google Scholar]
- 4.de Groot M., Anderson R., Freedland K.E., Clouse R.E., Lustman P.J. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63:619–630. doi: 10.1097/00006842-200107000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Knol M.J., Twisk J.W.R., Beekman A.T.F., Heine R.J., Snoek F.J., Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus: a metaanalysis. Diabetologia. 2006;49:837–845. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez J.S., Safren S.A., Delahanty L.M., Cagliero E., Wexler D.J., Meigs J.B. Symptoms of depression prospectively predict poorer self-care in patients with type 2 diabetes. Diabet Med. 2008 Sep;25(9):1102–1107. doi: 10.1111/j.1464-5491.2008.02535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park M., Katon W.J., Wolf F.M. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen Hosp Psychiatry. 2013;35(3):217–225. doi: 10.1016/j.genhosppsych.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson L.C., Amick H.R., Gaynes B.N., Brownley K.A., Thaker S., Viswanathan M. Practice-based interventions addressing concomitant depression and chronic medical conditions in the primary care setting: a systematic review and meta-analysis. J Prim Care Community Health. 2013 Oct;4(4):294–306. doi: 10.1177/2150131913484040. [DOI] [PubMed] [Google Scholar]
- 9.Mathers C.D., Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel V. Mental health in low and middle income countries. Br Med Bull. 2007;81-82:81–96. doi: 10.1093/bmb/ldm010. [DOI] [PubMed] [Google Scholar]
- 11.Kenya National Commission on Human Rights . Kenya; Nairobi: 2011. Silenced minds: the systemic neglect of the mental health system in Kenya.http://www.knchr.org/Portals/0/Reports/THE_%20MENTAL_HEALTH_REPORT.pdf website. [accessed 26.08.12] [Google Scholar]
- 12.Ndetei D.M., Khasakhala L.I., Kuria M.W., Mutiso V.N., Ongecha-Owuor F.A., Kokonya D.A. The prevalence of mental disorders in adults in different level general medical facilities in kenya: a cross-sectional study. Ann Gen Psychiatry. 2009;8:1. doi: 10.1186/1744-859X-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pastakia S.D., Karwa R., Kahn C.B., Nyabundi J.S. The evolution of diabetes care in the rural, resource-constrained setting of western Kenya. Ann Pharmacother. 2011;45:721–726. doi: 10.1345/aph.1P779. [DOI] [PubMed] [Google Scholar]
- 14.Einterz R.M., Kimaiyo S., Mengech H.N.K., Khwa-Otsyula B.O., Esamai F., Quigley F. Responding to the HIV pandemic: the power of an academic medical partnership. Acad Med. 2007;82:812–818. doi: 10.1097/ACM.0b013e3180cc29f1. [DOI] [PubMed] [Google Scholar]
- 15.Kroenke K., Spitzer R.L., Williams J.B. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz S.M., Gonzalez J.S., Wilkinson J.L., Safren S.A. A review of treating depression in diabetes: emerging findings. Psychosomatics. 2011;52:1–18. doi: 10.1016/j.psym.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]