Abstract
Objective
Low glycemic index (GI) foods have been suggested to minimize large fluctuations in blood glucose levels and reduce food intake. However, the majority of studies have been conducted on Caucasian populations with limited data on Asians. The objective of this study was to investigate how the provision of a low GI breakfast and afternoon snack affected daily blood glucose profiles and food intake.
Materials and methods
In a randomized, controlled crossover non blind design, 11 healthy Chinese male adults (body mass index 22.4 ± 1.3 kg m−2) attended two sessions where they consumed either a high or low GI breakfast and afternoon snack, and a standardized buffet lunch. Daily changes in glycemic response (GR) were measured using the Medtronic MiniMed (Northridge, CA) iPro™2 continuous glucose monitoring system (CGMS). The GR was further calculated to obtain the incremental area under the curve (IAUC). Glycemic variability was calculated as mean amplitude of glycemic excursion (MAGE) and energy intake (kcal) was measured quantitatively at the buffet lunch.
Results
Compared to the high GI intervention, the low GI intervention significantly reduced the GR following breakfast (p = 0.02), lunch (p = 0.02) and dinner (p = 0.05). The low GI treatment showed a reduction in daily AUC (p = 0.03). There was a significant reduction in IAUC after a low GI breakfast compared to the high GI breakfast (p = 0.03). The low GI breakfast resulted in a significantly lower food intake at lunch and a resulting decreased energy intake of 285 kcal (p = 0.02). The MAGE was significantly lower during the entire low GI treatment (p = 0.03).
Conclusions
Consumption of a low GI breakfast and afternoon snack was capable of attenuating 24-h blood glucose profiles, minimize glycemic excursions and reduce food intake in healthy Asian males. This simple dietary intervention may be an acceptable approach in improving overall glycemia and energy balance in Asians.
Clinical trial registration number
Keywords: Glycemic index, Glycemic response, Diet, Continuous glucose monitoring, Asian
Abbreviations: GI, glycemic index; GR, glycemic response; CGMS, continuous glucose monitoring system; BMI, body mass index; SD, standard deviation; IAUC, incremental area under the curve; MAGE, mean amplitude of glycemic excursion; kcal, kilocalories
Highlights
-
•
The impact of low and high glycemic index foods on 24 h blood glucose profile.
-
•
The CGMS provides detailed information on how diets affect longer term glycemia.
-
•
Low GI breakfast and afternoon snack minimize large blood glucose fluctuations.
-
•
Low GI foods reduce glycemic variability and total energy intake.
Introduction
Asia has the unenviable reputation as being the epicenter for type 2 diabetes. The Asian phenotype has been shown to be most susceptible to diabetes than Caucasians [1]. More significantly, the transition from prediabetes to diabetes is more dramatic and severe in Asians [2].
There is substantial evidence suggesting that consumption of low glycemic index (GI) foods minimize blood glucose fluctuations, and help in the prevention and management of diabetes and prediabetes 3, 4, 5, 6, 7, 8, 9. Given the rising incidence of prediabetes and diabetes in Asia, dietary interventions to complement pharmacological management of diabetes are increasingly being encouraged [10]. The majority of studies on GI and Glycemic Response (GR) have been conducted on Caucasian populations 11, 12, 13. Asians has been shown to have a greater GR to the same food compared to Caucasians 14, 15, 16. The present study investigated how manipulating the GI of one main meal (breakfast) and one snack meal (afternoon) affected 24 h blood glucose profiles and food intake in Asian Chinese adults.
The continuous glucose monitoring system (CGMS) is a relatively new and innovative methodology for measuring glucose levels. The present study, for the first time, comparatively looked at how providing either a high or low GI breakfast and mid-afternoon snack impacted on 24 h glucose profiles and food intake in Chinese adult males. The novelty of the study is that it demonstrates how two relatively small interventions to the daily diet could affect 24 h glucose levels in Asians within a free-living context.
Research design and methods
Thirteen healthy Chinese adults were enrolled using a variety of methods which included flyers and online advertisements. Prior to inclusion in the study, potential participants were briefed on all aspects of the study and were given the opportunity to ask questions. A written Informed Consent was obtained from participants. This was followed by a health screening to assess if participants met the inclusion/exclusion criteria. The health screening performed included anthropometric measurements (Table 1) and a health questionnaire (giving details of food allergies/intolerance, metabolic diseases, special dietary needs, and smoking habits). Those who fulfilled all the inclusion criteria (Gender, male; age, 21–40 years; body mass index calculated from weight and height measurement, 17–24 kg m−2; blood pressure (BP) – systolic BP between 110 and 120 mm Hg and diastolic BP between 75 and 85 mm Hg; fasting blood glucose, <6 mmol/l; not allergic/intolerant to any of the test foods; does not intentionally restrict food intake, not on prescription medication; non-smoking; no genetic or metabolic diseases) were enrolled in the study. Baseline anthropometric and biochemistry data of the study participants are shown in Table 1. Physical activity was quantified using the questionnaire by Baecke et al. [17]. Eating behavior was quantified using a Dutch eating behavior questionnaire by Van Strien et al. [18].
Table 1.
Anthropometric characteristics of study participants (n = 11)a
| Characteristic | Statistic |
|---|---|
| Age (years) | 22.9 ± 1.4 |
| BMI (kg/m2) | 22.4 ± 1.3 |
| Waist circumference (cm) | 76.7 ± 3.6 |
| Fasting blood glucose (mmol/l) | 4.5 ± 0.5 |
| HbA1c (%)b | 5.3 ± 1.4 |
| Blood pressure | |
| Systolic (mm Hg) | 120.3 ± 8.2 |
| Diastolic (mm Hg) | 72.9 ± 1.4 |
Data presented as mean ± SD.
HbA1c normal range 2.5–14.0%.
The study was conducted at the Clinical Nutrition Research Centre (CNRC), Singapore. Ethical approval was obtained from the National Health Group Domain Specific Review Board (NHG DSRB).
A crossover design of 11 participants would be sufficient to detect a 15% change in area under the 24 h glucose curve using a power of 85% and significance level of 0.05 11, 13.
Study design
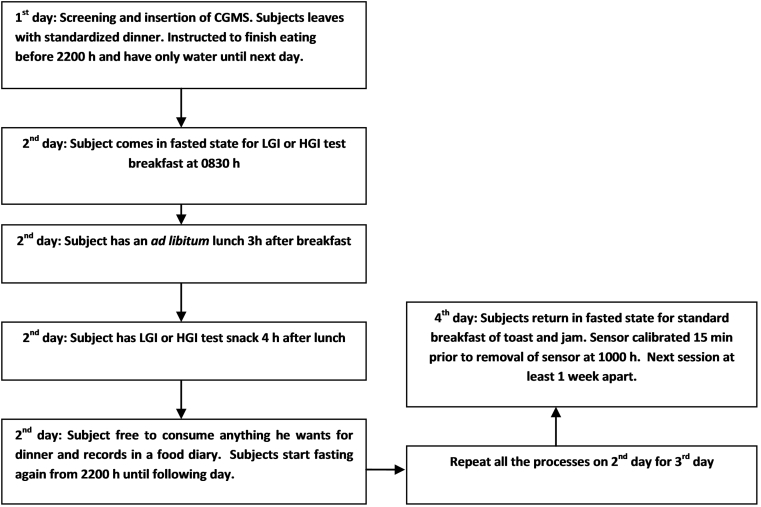
The study had a randomized, controlled crossover non-blind design. Volunteers attended two test sessions separated by a week as a wash-out period. Each session spanned four consecutive days during which two complete 24 h periods of glucose measures were captured with CGMS devices. At each session participants consumed either a low glycemic index (LGI) or high glycemic index (HGI) breakfast and snack for two consecutive 24 h periods. Food diaries were given to capture their food consumption and a log sheet to assess their physical activity for rest of the day. A schematic study flow is presented in Fig. 1.
Fig. 1.
Schematic presentation of study protocol. There will be two days of consecutive test meals which will be the 1st 24 h and 2nd 24 h. The 1st 24 h (0600 h–0600 h) will be regarded as day 1 and the 2nd 24 h (0600 h–0600 h) regarded as day 2.
The iPro™2 continuous glucose monitoring (CGM) system (iPro™2 Professional CGM-Medtronic MiniMed, Northbridge, CA, USA) was used in this study. The insertion was performed on the first day at 1400 h and the sensor was removed on the fourth day of the study at 1000 h. Data was collated and processed using an online software (Medtronic Diabetes CareLink iPro; https://carelink.minimed.eu). The data reported in this paper represent interstitial glucose readings recorded every 5 min between 0600 h and 0600 h for two consecutive days (total 48 h). At each test session the CGMS sensor was calibrated before every meal and before sleeping using the OneTouch®Ultra®2 blood glucose meter (Life Scan, Inc., Milpitas, CA, USA).
The standardized dinner options provided on the first day is shown in Table 2. The low and high GI test foods were selected to obtain as wide a difference in GI values. The test breakfast consisted of either a high or low GI rice (New Moon Premium glutinous rice GI: 92, Tek Seng Rice Mill Co. Ltd, Thailand and Dream™ long grain parboiled basmati rice GI:55, Diabetic Specialties Pte Ltd, Singapore). Both rice types were given in portions containing 75 g of available carbohydrates (which corresponded to 92.7 g and 98 g for the high and low GI rice respectively). The nutritional composition of the test foods is provided in Table 3a. The high and low GI rice was cooked in 139 ml and 179 ml of water respectively, with 2 g of chicken stock (Knorr chicken stock, Malaysia). Rice was cooked for 10 min in an electric rice cooker. The snack was a high or low GI bread served in portions containing 50 g of available carbohydrates with 10 g strawberry spread (high GI white bread GI: 79, Gardenia Brand, Singapore; low GI multigrain bread GI: 44, Sunshine Brand, Singapore; Bonne Maman strawberry preserve, France). Subjects were requested to consume both meals with table water (180 ml) within 15 min. Two types of stir fried noodles (egg noodles and rice vermicelli) were standardized for recipe method, and for the energy and macronutrient content (Table 3b, Table 3c). The noodles, jellies and table water were served as a cafeteria style ad libitum buffet. Food intake was quantitatively measured in grams and converted to kcal, taking into account plate waste.
Table 2.
Standardized dinner meal choices (1, 2, 3)
| Choice | Food | Portion | Energy (kcal) | Protein (g) | Total fat (g) | Carbohydrate (g) | Sugars (g) | Fiber (g) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Main course | ||||||||||
| 1 | Teriyaki chicken with rice (CP) | 1 packet (320 g) | 510 | 19 | 10 | 85 | 5 | 0 | ||
| 2 | Grilled Teriyaki Salmon with Japanese rice (CP) | 1 packet (320 g) | 470 | 19 | 8 | 76 | 2 | 5 | ||
| 3 | Chicken Green Curry with rice (CP) | 1 packet (320 g) | 432 | 14.4 | 4.8 | 82.6 | 1.6 | 4.5 | ||
| Drinks | ||||||||||
| 3 | Ice Lemon Tea (F&N) | I can (300 ml) | 129 | 0 | 0 | 32.1 | ||||
| 1 | 2 | 100 Plus (F&N) | 1 can (324 ml) | 88 | 0 | 0 | 22.1 | 22.1 | ||
| Desserts | ||||||||||
| 1 | 2 | 3 | Jelly (Wang Coco) | 1 cup (108 g) | 85 | 1.4 | 0 | 19.2 | ||
| 2 | 3 | Rice Crackers (Bin Bin) | 1 packet (7.5 g) | 36 | 0.4 | 1.3 | 5.6 | 0 | ||
| 1 | 2 | 3 | Japanese Sweet (Hi-Chew) | 1 sweet (5 g) | 21 | 0.08 | 0.47 | 4.08 | 3.13 | |
Participants have a choice between 1, 2 and 3 for their dinner. Brand names are in parentheses.
Table 3a.
Nutritional composition of test foods
| Test food | Composition based on per 100 g |
||||
|---|---|---|---|---|---|
| Energy (kcal) | Carbohydrate (g) | Protein (g) | Fat (g) | Dietary fiber (g) | |
| Multigrain bread | 259 | 32.7 | 13.5 | 8.2 | 10.4 |
| White bread | 263 | 54.7 | 9.9 | 1.8 | 2.5 |
| Parboiled basmati rice | 350 | 76.6 | 9.4 | 0.7 | 1.4 |
| Glutinous rice | 354 | 80.9 | 7.2 | 0.2 | 0.2 |
Table 3b.
Lunch buffet: Ingredients, recipe and nutritional information
| Yellow noodle (LG Brand) | Salt (Flying Man) | Shrimp sauce (Dancing Chef) |
|---|---|---|
| Bee Hoon (Red Moon) | Jelly (Wang Coco) | Special sauce (Dancing Chef) |
| Sunflower oil (Sunbeam) | Prawns (King Fisher) | Sweet and Spicy sauce (prima) |
| Tomato sauce (Heinz) | Carrots, bak choy | Mee goreng sauce (Hai's) |
Standardized recipe
| ||
Brand names are in parentheses. Carrots and bak choy were bought fresh from the same supermarket.
Table 3c.
Nutritional information of each noodle disha
| Qty (g) | Energy (kcal) | Protein (g) | Fat (g) | Carbohydrate (g) | Fiber (g) | |
|---|---|---|---|---|---|---|
| Noodle 1 | ||||||
| Yellow noodles | 200 | 720.0 | 30.6 | 0.0 | 149.0 | 9.6 |
| Prawns | 100 | 79.0 | 18.3 | 0.6 | 0.0 | 0.0 |
| Bak choy | 150 | 19.5 | 2.3 | 0.3 | 3.3 | 1.5 |
| Carrot | 150 | 45.0 | 1.1 | 0.8 | 9.0 | 3.6 |
| mee goreng paste | 65 | 254.6 | 4.2 | 19.3 | 16.0 | 0.7 |
| Oil | 45 | 404.6 | 0.0 | 45.0 | 0.0 | 0.0 |
| Salt | 5 | |||||
| Total | 715 | 1523 | 56 | 66 | 177 | 15 |
| Noodle 2 | ||||||
| Yellow noodles | 200 | 720.0 | 30.6 | 0.0 | 149.0 | 9.6 |
| Prawns | 100 | 79.0 | 18.3 | 0.6 | 0.0 | 0.0 |
| Bak choy | 150 | 19.5 | 2.3 | 0.3 | 3.3 | 1.5 |
| Carrot | 150 | 45.0 | 1.1 | 0.8 | 9.0 | 3.6 |
| Shrimp paste | 100 | 242.6 | 8.2 | 15.4 | 17.8 | 0.4 |
| Oil | 45 | 404.6 | 0.0 | 45.0 | 0.0 | 0.0 |
| Salt | 0 | |||||
| Total | 745 | 1511 | 60 | 62 | 179 | 15 |
| Noodle 3 | ||||||
| Bee hoon (rice vermicelli) | 200 | 718.0 | 11 | 1.2 | 165.6 | 4.2 |
| Prawns | 100 | 79.0 | 18.3 | 0.6 | 0.0 | 0.0 |
| Bak choy | 150 | 19.5 | 2.3 | 0.3 | 3.3 | 1.5 |
| Carrot | 150 | 45.0 | 1.1 | 0.8 | 9.0 | 3.6 |
| Special sauce mix | 100 | 236.4 | 2.5 | 3.2 | 49.4 | 0.5 |
| Oil | 45 | 404.6 | 0.0 | 45.0 | 0.0 | 0.0 |
| Salt | 3 | |||||
| Total | 748 | 1502 | 35 | 51 | 227 | 10 |
| Noodle 4 | ||||||
| Bee hoon (rice vermicelli) | 200 | 718.0 | 11 | 1.2 | 165.6 | 4.2 |
| Prawns | 100 | 79.0 | 18.3 | 0.6 | 0.0 | 0.0 |
| Bak choy | 150 | 19.5 | 2.3 | 0.3 | 3.3 | 1.5 |
| Carrot | 150 | 45.0 | 1.1 | 0.8 | 9.0 | 3.6 |
| Sweet and spicy sauce | 80 | 162.4 | 2.1 | 7.6 | 16.8 | 0.6 |
| Oil | 45 | 404.6 | 0.0 | 45.0 | 0.0 | 0.0 |
| Tomato sauce | 10 | 53.0 | 0 | 0 | 11.3 | 0 |
| Salt | 5 | |||||
| Total | 725 | 1428 | 35 | 55 | 195 | 10 |
| Jelly with Nata De Coco | 1 | 90 | 0.3 | 0 | 23 | 1 |
Lunch buffet was one yellow noodle dish, and one bee hoon vermicelli dish, provided with a jug of table water (1500 ml) and 10 cup jellies. Subjects consumed how much they wanted until comfortably full. The carrots and bak choy nutritional information was obtained from a local food composition table.
Calculations and statistical analyses
All statistical analyses were performed using R (version 3.0.3) [19]. Values were presented as mean ± SD unless otherwise stated. Prior to statistical analysis, the normality of the data was assured using the Shapiro–Wilk test.
The primary outcome of this study was the effects of the LGI and HGI diets on the incremental change in glucose (i.e. the GR) over two consecutive 24 h and for five distinct periods of the day (breakfast, lunch, snack, dinner and overnight fasting i.e. 2200–0600 h). The GR was calculated by using the first hour average of CGM interstitial glucose readings under the fasting state as baseline value. The average baseline value was then used to convert every 5 min reading of 23 subsequent hours of CGMS interstitial glucose data. The GR data from the CGMS was converted to the “change in glucose” concentration (i.e. the incremental change in glucose) for every 5 min reading for the 23 subsequent hours of CGMS data. The other primary outcome measure was the total glucose response expressed as the incremental area under the curve (i.e. the GR IAUC) calculated using the trapezoidal rule 20, 21. The GR values were important for further analyses such as the GR IAUC calculations, CGMS glucose curve construction and statistics. The secondary outcome measures were the total daily AUC, the glycemic variability measured as the mean amplitude of glycemic excursion (MAGE) and the energy intake consumed at the ad libitum lunch. The MAGE was used in the present study to assess glucose fluctuations during the day [22]. The energy intake was calculated by measuring food intake and converting this to energy using standard Singapore food composition guide tables (Food Composition Guide Singapore, Health Promotion Board, Singapore). Paired t-test was performed to test the differences in the GR between LGI and HGI over 48 h, and the GR differences between day 1(1st 24 h) and day 2 (2nd 24 h). These comparisons were also performed for the GR IAUC, total daily AUC and the MAGE. Energy intake data between the LGI and HGI treatments was compared using a paired t-test. Alpha (α) was set at 0.05 for statistical analyses.
Results
Eleven out of thirteen study participants completed the study. The experimental protocol was completed by eleven out of thirteen study participants. Two subjects dropped out on the first day of the study as they felt unwell during insertion of the CGMS. Therefore no data was available for subsequent analysis. All eleven participants had complete data for both the LGI and HGI diets.
The overall glycemic response over the whole measuring period (48 h) is presented in Table 4. The fasting glucose concentrations were not significantly different prior to feeding of the two diets on the first test day (p = 0.10; LGI: 4.6 ± 0.1; HGI: 4.4 ± 0.2 mmol/l). The absolute mean glucose concentration did not reach statistical significance (p = 0.09). But there was a significantly higher GR observed with the HGI treatment compared to the LGI treatment (p = 0.02). The GR IAUC was also significantly higher for the HGI treatment than the LGI treatment (p = 0.01).
Table 4.
Overall glycemic response over the whole measuring period (48 h)
| Parameter |
|||
|---|---|---|---|
| Absolute mean glucose (mmol/l) | GR (mmol/l) | GR IAUC (mmol/l min) | |
| Low GI | 5.05 ± 0.5 | 0.24 ± 0.1 | 1089.91 ± 127.8 |
| High GI | 5.17 ± 0.7 | 0.59 ± 0.1 | 1868.72 ± 244.4 |
| p-Values | 0.086 | 0.022 | 0.012 |
Values presented as mean ± SD.
GR: glycemic response; IAUC: Incremental area under the curve.
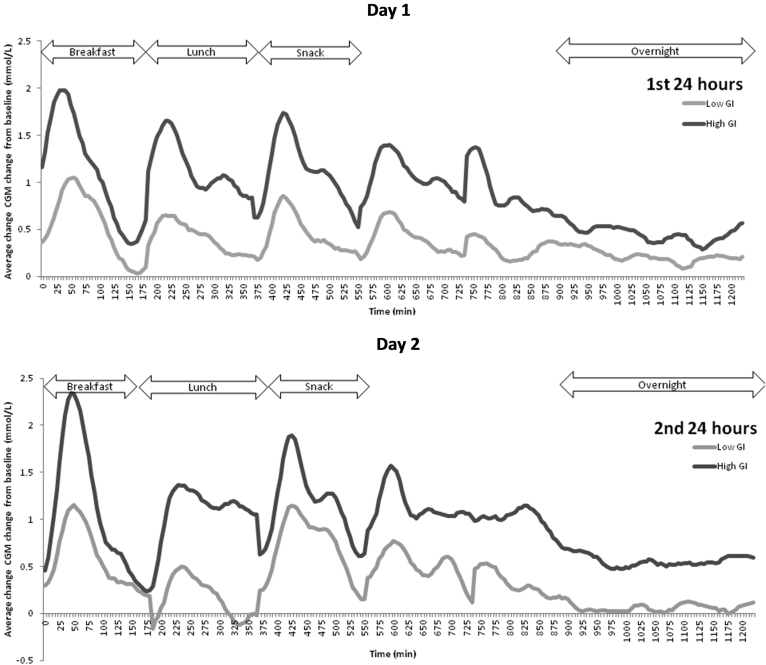
The glycemic profiles for the LGI and HGI diets are graphically presented in Fig. 2. The calculated GR, GR IAUC and MAGE results are presented in Table 5. Generally the HGI intervention produced a sustained higher GR throughout the day (Fig. 2).
Fig. 2.
Average change in interstitial glucose concentrations from baseline of healthy Chinese male participants on a low GI or high GI breakfast and snack for each day (n = 11). CGM, continuous glucose monitoring. The dinner time range is between the snack and the overnight fast which had varied timings (range from 7 pm to 9 pm).
Table 5.
Mean glycemic outcome variables of the 11 participants for whom a complete set of continuous glucose monitoring data were obtained
| Outcome measure | LGI |
HGI |
LGI compared to HGI (overall 48 h) |
||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | p-Value | Day 1 | Day 2 | p-Value | p-Value | |
| Breakfast (mmol/l)a | 0.5 ± 0.5 | 0.3 ± 0.2 | 0.15 | 1.1 ± 0.9 | 0.6 ± 0.5 | 0.03 | 0.02 |
| Lunch (mmol/l)a | 0.5 ± 0.3 | 0.4 ± 0.1 | 0.30 | 1.2 ± 0.5 | 0.6 ± 0.3 | 0.06 | 0.02 |
| Snack (mmol/l)a | 0.5 ± 0.4 | 0.5 ± 0.2 | 0.99 | 1.1 ± 0.5 | 0.7 ± 0.3 | 0.16 | 0.05 |
| Dinner (mmol/l)a | 0.5 ± 0.3 | 0.3 ± 0.2 | 0.59 | 1.1 ± 0.5 | 0.7 ± 0.3 | 0.11 | 0.05 |
| Overnight (mmol/l)a | 0.3 ± 0.3 | −0.1 ± 0.4 | 0.16 | 0.6 ± 0.4 | 0.2 ± 0.3 | 0.08 | 0.08 |
| MAGE (mmol/l)b | 1.2 ± 0.7 | 1.3 ± 0.4 | 0.39 | 1.9 ± 0.7 | 1.8 ± 1.0 | 0.50 | 0.03 |
| Daily total AUC (mmol/l min) | 605.7 ± 330.0 | 401.5 ± 215.0 | 0.03 | 1099.7 ± 700.9 | 655.7 ± 492.1 | 0.06 | 0.03 |
| Breakfast IAUC (mmol/l min) | 117.2 ± 52.0 | 142.7 ± 82.3 | 0.34 | 215.4 ± 85.3 | 204.7 ± 29.0 | 0.62 | 0.03 |
| Lunch IAUC (mmol/l min) | 103.0 ± 64.3 | 139.5 ± 82.6 | 0.03 | 214.1 ± 119.7 | 206.1 ± 107.5 | 0.79 | 0.08 |
| Snack IAUC (mmol/l min) | 112.4 ± 66.3 | 151.8 ± 77.4 | 0.02 | 211.7 ± 146.7 | 219.7 ± 126.4 | 0.80 | 0.10 |
| Dinner IAUC (mmol/l min) | 115.0 ± 68.5 | 132.6 ± 123.3 | 0.63 | 204.1 ± 144.3 | 225.7 ± 165.2 | 0.63 | 0.08 |
| Overnight IAUC (mmol/l min) | 70.3 ± 53.7 | 206.1 ± 213.2 | 0.05 | 93.8 ± 83.9 | 356.8 ± 263.6 | 0.003 | 0.14 |
Values are expressed as mean ± SD.LGI: low glycemic index; HGI: high glycemic index.
Incremental change in glucose values.
MAGE: Mean Amplitude of Glycemic Excursion.
Following an HGI breakfast, the GR was greater on day 2 than day 1 (p = 0.03). In the LGI intervention, the GR following breakfast and lunch was significantly lower (p = 0.02 and 0.02 respectively) compared to the HGI intervention over 48 h. The GR following the snack and dinner were near significant (p = 0.05). Although the overnight glucose concentrations were not significantly different between the LGI and HGI treatments, the former showed a trend towards a lower GR than the latter. Compared to the HGI intervention, the LGI intervention showed a non-significant (p = 0.15) trend towards a lower GR on day 2 (HGI: 0.2 ± 0.3 mmol/l, LGI: −0.1 ± 0.4 mmol/l).
Compared to the HGI breakfast, the LGI breakfast produced a significantly lower GR IAUC over the 48 h (p = 0.03). The GR IAUC was significantly lower on day 1 than day 2 for the LGI snack (p = 0.02). Although the LGI snack showed no significant difference compared to the HGI snack for the 48 h IAUC (p = 0.10), there was an observed reduction in the GR IAUC. The dinner and overnight GR IAUC failed to reach significance for the 48 h (p > 0.05). The HGI treatment showed a marked increase in GR IAUC overnight on day 2 compared to day 1 (p = 0.003). There was a significantly higher fasting glucose at 0600 h on day 2 for the HGI than the LGI (p = 0.04) (Fig. 2).
The daily total AUC was significantly higher for the HGI compared to the LGI intervention over the 48 h period (p = 0.03). The daily total AUC also reduced on day 2 of the LGI treatment (p = 0.03). The MAGE values did not significantly differ between day 1 and day 2 for the LGI and HGI interventions. However, the HGI treatment produced a significantly greater MAGE than the LGI treatment over the 48 h period (p = 0.03).
The energy intake data showed a significantly reduced intake of energy at the lunch buffet after an LGI breakfast than the HGI breakfast (t(10) = -2.662, p = 0.02). The HGI session showed a higher mean energy intake of (1041.6 ± 258.9 kcal) compared to the LGI (840.7 ± 404.8 kcal).
Discussion
The CGMS was utilized to provide more detailed information on glycemic excursions throughout the day in an Asian population. To our knowledge a limited number of studies have measured continuous blood glucose profiles in healthy, non-diabetic subjects 11, 12, 13, 23 with even fewer studies performed on Asians 10, 24. The results from our present study showed how the consumption of a low GI breakfast and snack down regulated the blood glucose profiles in healthy Asians over the subsequent period of the day.
Within the first 3 h after an HGI glutinous rice breakfast, the GR was two-fold higher than the LGI parboiled basmati rice breakfast. There was a significant reduction in the 48 h GR IAUC after an LGI breakfast which suggested that an LGI breakfast helped to attenuate glycemia over the remaining day. There was a reduced GR and GR IAUC to the standardized lunch after an LGI breakfast compared to the HGI breakfast, indicating that the former may have elicited a second meal effect and improved the glycemic response to the subsequent lunch meal. Some studies have shown that LGI foods ingested at breakfast improved glycemia following a subsequent standardized lunch meal, a phenomenon named the “second-meal effect” 25, 26, 27. Consuming foods that elicit a second meal effect may help towards the maintenance of low blood glucose concentrations in the short-medium term and thereby reduce demands on the insulin mediated blood glucose regulatory systems.
There was a significant reduction in energy intake at lunch following the LGI breakfast suggesting its ability to reduce energy intake at the subsequent meal. Studies have shown that consuming HGI foods induced a greater voluntary food intake relative to the consumption of LGI foods and the results of the present study agreed with other observations 8, 28, 29. It has been suggested that LGI foods promote satiety by maintaining a steady GR and by preventing large excursions and resulting hypoglycemia [4]. Consuming an LGI multigrain bread as an afternoon snack appeared to modestly improve 48 h blood glucose profiles compared to an HGI white bread. Previous research has shown that the substitution of high GI bread with an LGI bread at breakfast, lunch and dinner can favorably alter glucose profiles [12]. In the present study the LGI snack appeared to attenuate the GR following dinner compared to the HGI snack. This was an interesting observation especially as the volunteers were allowed to eat anything they wished for dinner and this potentially demonstrated an LGI food mediated second meal effect even in free-living and free-eating conditions. There was a consistent trend where a lower GR during the night was observed following an LGI breakfast and snack, and a greater attenuation on day 2. This may have important clinical relevance as nocturnal hyperglycemia is a common occurrence in diabetics [30]. Normalizing nocturnal glycemia will also help to reduce fasting glucose concentrations and potentially reduce cardiovascular disease (CVD) risk in healthy people [31]. It has been recognized that fasting blood glucose may be an independent risk factor for CVD [32]. The MAGE assessed over 48 h, was significantly greater on the HGI treatment compared to the LGI treatment. Glycemic variability is of significant clinical concern due to its negative effects on oxidative stress [33], insulin regulatory mechanisms and food intake.
In the present study food diaries were not analyzed. We were therefore unable to ascertain whether volunteers ate more or less energy at subsequent meals following the snack event for the two treatments. Moreover, we were unable to evaluate the weighted GI of the ad libitum lunch and dinner in the present study. Strengths of our study included the use of only male subjects of comparable age and BMI to minimize biological variability. Males have also been known to be better subjects in satiety studies as they are not influenced by confounders such as the menstrual cycle [34]. The use of the CGMS has enabled us to illustrate elegantly the “second-meal” effect. The importance of modulating the glucose response during the “second meal” effect has now been recognized as an important precursor for improving glucose tolerance 27, 35. A significant strength and novelty of the study was that it collected both CGMS and food intake data, a combination which to our knowledge has not been done before in Asians. Another strength of the study was that it combined both controlled (first part of the day until the snack) and free-living conditions (after the snack until they come back the next morning), and looked at glycemic variations throughout the day over two complete days.
There is mounting evidence to suggest that Asians are more susceptible to developing diabetes for the same level of adiposity, BMI and waist circumference compared to Caucasians [36]. Indeed, it has also been recognized that even certain lean Asian subjects are more susceptible to insulin resistance compared to Caucasians and are prone to transit from prediabetes to diabetes more rapidly [37]. This study demonstrated that the ingestion of an LGI breakfast and afternoon snack attenuated the blood glucose response over the entire day and reduced glycemic variability. An LGI breakfast also produced a second-meal effect and reduced energy intake. Substituting breakfast and snack with low GI alternatives may be an effective means of improving overall glycemia and energy balance in Asians, and may help to reduce the risk of developing type 2 diabetes in this vulnerable group.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We warmly thank the volunteers for taking the time to participate in this study. The study was supported by the Singapore Institute for Clinical Sciences.
References
- 1.Yoon K.-H., Lee J.-H., Kim J.-W., Cho J.H., Choi Y.-H., Ko S.-H. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368(9548):1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 2.Xu Y., Wang L., He J., Bi Y., Li M., Wang T. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 3.Riccardi G., Rivellese A.A., Giacco R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr. 2008;87(1):269S–274S. doi: 10.1093/ajcn/87.1.269S. [DOI] [PubMed] [Google Scholar]
- 4.Brand-Miller J.C., Holt S.H.A., Pawlak D.B., McMillan J. Glycemic index and obesity. Am J Clin Nutr. 2002;76(1):281S–285S. doi: 10.1093/ajcn/76/1.281S. [DOI] [PubMed] [Google Scholar]
- 5.Brand-Miller J., Hayne S., Petocz P., Colagiuri S. Low-glycemic index diets in the management of diabetes A meta-analysis of randomized controlled trials. Diabetes Care. 2003;26(8):2261–2267. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 6.Ludwig D.S. Dietary glycemic index and obesity. J Nutr. 2000;130(2):280S–283S. doi: 10.1093/jn/130.2.280S. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins D.J.A., Kendall C.W.C., Augustin L.S.A., Franceschi S., Hamidi M., Marchie A. Glycemic index: overview of implications in health and disease. Am J Clin Nutr. 2002;76(1):266S–273S. doi: 10.1093/ajcn/76/1.266S. [DOI] [PubMed] [Google Scholar]
- 8.Warren J.M., Henry C.J.K., Simonite V. Low glycemic index breakfasts and reduced food intake in preadolescent children. Pediatrics. 2003;112(5):e414. doi: 10.1542/peds.112.5.e414. [DOI] [PubMed] [Google Scholar]
- 9.Lennerz B.S., Alsop D.C., Holsen L.M., Stern E., Rojas R., Ebbeling C.B. Effects of dietary glycemic index on brain regions related to reward and craving in men. Am J Clin Nutr. 2013;98(3):641–647. doi: 10.3945/ajcn.113.064113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohan V., Spiegelman D., Sudha V., Gayathri R., Hong B., Praseena K. Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: a randomized controlled trial. Diabetes Technol Ther. 2014;16(5):317–325. doi: 10.1089/dia.2013.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henry C.J.K., Newens K.J., Lightowler H.J. Low-glycaemic index sweetener-based beverages reduce 24-h glucose profiles in healthy adults. JAMA. 2009;22(1):77–80. doi: 10.1111/j.1365-277X.2008.00930.x. [DOI] [PubMed] [Google Scholar]
- 12.Henry C.J.K., Lightowler H.J., Tydeman E.A., Skeath R. Use of low-glycaemic index bread to reduce 24-h blood glucose: implications for dietary advice to non-diabetic and diabetic subjects. Int J Food Sci Nutr. 2006;57(3–4):273–278. doi: 10.1080/09637480600931626. [DOI] [PubMed] [Google Scholar]
- 13.Brynes A.E., Adamson J., Dornhorst A., Frost G.S. The beneficial effect of a diet with low glycaemic index on 24 h glucose profiles in healthy young people as assessed by continuous glucose monitoring. Br J Nutr. 2005;93(02):179–182. doi: 10.1079/bjn20041318. [DOI] [PubMed] [Google Scholar]
- 14.Venn B.J., Williams S.M., Mann J.I. Comparison of postprandial glycaemia in Asians and Caucasians. Diabet Med. 2010;27(10):1205–1208. doi: 10.1111/j.1464-5491.2010.03069.x. [DOI] [PubMed] [Google Scholar]
- 15.Henry C.J.K., Lightowler H.J., Newens K., Sudha V., Radhika G., Sathya R.M. Glycaemic index of common foods tested in the UK and India. Br J Nutr. 2008;99:840–845. doi: 10.1017/S0007114507831801. [DOI] [PubMed] [Google Scholar]
- 16.Dickinson S., Colagiuri S., Faramus E., Petocz P., Brand-Miller J.C. Postprandial hyperglycemia and insulin sensitivity differ among lean young adults of different ethnicities. J Nutr. 2002;132(9):2574–2579. doi: 10.1093/jn/132.9.2574. [DOI] [PubMed] [Google Scholar]
- 17.Baecke J.A., Burema J., Frijters J.E. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 18.Van Strien T., Frijters J.E.R., Bergers G., Defares P.B. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5(2):295–315. [Google Scholar]
- 19.R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2008. http://www.R-project.org [Updated Last Updated Date] [Google Scholar]
- 20.Allison D.B., Paultre F., Maggio C., Mezzitis N., Pi-Sunyer F.X. The use of areas under curves in diabetes research. Diabetes Care. 1995;18(2):245–250. doi: 10.2337/diacare.18.2.245. [DOI] [PubMed] [Google Scholar]
- 21.Brouns F., Bjorck I., Frayn K.N., Gibbs A.L., Lang V., Slama G. Glycaemic index methodology. Nutr Res Rev. 2005;18(01):145–171. doi: 10.1079/NRR2005100. [DOI] [PubMed] [Google Scholar]
- 22.Service F.J., Molnar G.D., Rosevear J.W., Ackerman E., Gatewood L.C., Taylor W.F. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 23.Powers M.A., Cuddihy R.M., Wesley D., Morgan B. Continuous glucose monitoring reveals different glycemic responses of moderate-vs high-carbohydrate lunch meals in people with type 2 diabetes. J Am Diet Assoc. 2010;110(12):1912–1915. doi: 10.1016/j.jada.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Wang J.S., Tu S.T., Lee I.T., Lin S.D., Lin S.Y., Su S.L. Contribution of postprandial glucose to excess hyperglycaemia in Asian type 2 diabetic patients using continuous glucose monitoring. Diabetes Metab Res Rev. 2011;27(1):79–84. doi: 10.1002/dmrr.1149. [DOI] [PubMed] [Google Scholar]
- 25.Liljeberg H.G.M., Åkerberg A.K.E., Björck I.M.E. Effect of the glycemic index and content of indigestible carbohydrates of cereal-based breakfast meals on glucose tolerance at lunch in healthy subjects. Am J Clin Nutr. 1999;69(4):647–655. doi: 10.1093/ajcn/69.4.647. [DOI] [PubMed] [Google Scholar]
- 26.Wolever T.M., Bolognesi C. Prediction of glucose and insulin responses of normal subjects after consuming mixed meals varying in energy, protein, fat, carbohydrate and glycemic index. J Nutr. 1996;126(11):2807–2812. doi: 10.1093/jn/126.11.2807. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins D.J., Wolever T.M., Taylor R.H., Griffiths C., Krzeminska K., Lawrie J.A. Slow release dietary carbohydrate improves second meal tolerance. Am J Clin Nutr. 1982;35(6):1339–1346. doi: 10.1093/ajcn/35.6.1339. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig D.S., Majzoub J.A., Al-Zahrani A., Dallal G.E., Blanco I., Roberts S.B. High glycemic index foods, overeating, and obesity. Pediatrics. 1999;103(3):e26. doi: 10.1542/peds.103.3.e26. [DOI] [PubMed] [Google Scholar]
- 29.Spitzer L., Rodin J. Effects of fructose and glucose preloads on subsequent food intake. Appetite. 1987;8(2):135–145. doi: 10.1016/s0195-6663(87)80006-5. [DOI] [PubMed] [Google Scholar]
- 30.Buckingham B., Block J., Burdick J., Kalajian A., Kollman C., Choy M. Response to nocturnal alarms using a real-time glucose sensor. Diabetes Technol Ther. 2005;7(3):440–447. doi: 10.1089/dia.2005.7.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kristo A.S., Matthan N.R., Lichtenstein A.H. Effect of diets differing in glycemic index and glycemic load on cardiovascular risk factors: review of randomized controlled-feeding trials. Nutrients. 2013;5(4):1071–1080. doi: 10.3390/nu5041071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawes C.M., Parag V., Bennett D.A., Suh I., Lam T.H., Whitlock G. Blood glucose and risk of cardiovascular disease in the Asia Pacific region. Diabetes Care. 2004;27(12):2836–2842. doi: 10.2337/diacare.27.12.2836. [DOI] [PubMed] [Google Scholar]
- 33.Sun J., Xu Y., Sun S., Sun Y., Wang X. Intermittent high glucose enhances cell proliferation and VEGF expression in retinal endothelial cells: the role of mitochondrial reactive oxygen species. Mol Cell Biochem. 2010;343(1–2):27–35. doi: 10.1007/s11010-010-0495-5. [DOI] [PubMed] [Google Scholar]
- 34.Ranawana D.V., Henry C.J.K. Are caloric beverages compensated for in the short-term by young adults? An investigation with particular focus on gender differences. Appetite. 2010;55(1):137–146. doi: 10.1016/j.appet.2010.05.046. [DOI] [PubMed] [Google Scholar]
- 35.Wolever T.M., Jenkins D.J., Ocana A.M., Rao V.A., Collier G.R. Second-meal effect: low-glycemic-index foods eaten at dinner improve subsequent breakfast glycemic response. Am J Clin Nutr. 1988;48(4):1041–1047. doi: 10.1093/ajcn/48.4.1041. [DOI] [PubMed] [Google Scholar]
- 36.Misra A., Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93(11 Suppl. 1):s9–s30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 37.King G.L., McNeely M.J., Thorpe L.E., Mau M.L.M., Ko J., Liu L.L. Understanding and addressing unique needs of diabetes in Asian Americans, Native Hawaiians, and Pacific Islanders. Diabetes Care. 2012;35(5):1181–1188. doi: 10.2337/dc12-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]