Abstract
Objective
The Durham Diabetes Coalition (DDC) was established in response to escalating rates of disability and death related to type 2 diabetes mellitus, particularly among racial/ethnic minorities and persons of low socioeconomic status in Durham County, North Carolina. We describe a community-based demonstration project, informed by a geographic health information system (GHIS), that aims to improve health and healthcare delivery for Durham County residents with diabetes.
Materials and Methods
A prospective, population-based study is assessing a community intervention that leverages a GHIS to inform community-based diabetes care programs. The GHIS integrates clinical, social, and environmental data to identify, stratify by risk, and assist selection of interventions at the individual, neighborhood, and population levels.
Results
The DDC is using a multifaceted approach facilitated by GHIS to identify the specific risk profiles of patients and neighborhoods across Durham County. A total of 22,982 patients with diabetes in Durham County were identified using a computable phenotype. These patients tended to be older, female, African American, and not covered by private health insurance, compared with the 166,041 persons without diabetes. Predictive models inform decision-making to facilitate care and track outcomes. Interventions include: 1) neighborhood interventions to improve the context of care; 2) intensive team-based care for persons in the top decile of risk for death or hospitalization within the coming year; 3) low-intensity telephone coaching to improve adherence to evidence-based treatments; 4) county-wide communication strategies; and 5) systematic quality improvement in clinical care.
Conclusions
To improve health outcomes and reduce costs associated with type 2 diabetes, the DDC is matching resources with the specific needs of individuals and communities based on their risk characteristics.
Keywords: Diabetes mellitus type 2, Population diabetes, Community health, Barriers to diabetes care, Diabetes complications, Cardiovascular risk and diabetes
Abbreviations: CAB, community advisory board; CAARE, Case management of AIDS and Addiction through Resources and Education; DDC, Durham Diabetes Coalition; DIO, diabetes information and communication officer; DSR, Decision Support Repository; eMERGE, Electronic Medical Records and Genomics; GHIS, geographic health information system; ICD-9, International Classification of Diseases, Ninth Revision; NHB, non-Hispanic black; NHW, non-Hispanic white; SUPREME-DM, Surveillance, Prevention, and Management of Diabetes Mellitus
Introduction
More than 26 million Americans have diabetes mellitus, a burden of disease that results in estimated costs of $US 245 billion annually 1, 2, 3. Numerous advances in therapeutic and behavioral interventions over the past 20 years have improved the prevention, detection, and treatment of diabetes 4, 5, 6, 7, 8, 9, 10, 11, but the application of these interventions has not been sufficiently widespread or equitably distributed to all vulnerable populations. In addition, these challenges are exacerbated by an epidemic of obesity that has escalated the trajectory of diabetes diagnoses, disability, and death. Disadvantaged populations bear a disproportionate burden of type 2 diabetes and its complications 3, 7, 12, 13. Within the United States, an estimated 12.6% of non-Hispanic blacks (NHBs) and 11.8% of Hispanics/Latinos over the age of 20 are diagnosed with type 2 diabetes, compared with just 7.1% of non-Hispanic white (NHW) Americans over age 20.
Geographic disparities also exist: the prevalence of type 2 diabetes mirrors the rise in obesity and is most heavily concentrated in the southeastern United States—a geographic area that also shares the highest national prevalence of stroke, hypertension, and physical inactivity. Further, even after accounting for changing diagnostic criteria, diabetes prevalence is increasing. For example, diabetes diagnoses among North Carolinians grew from <2% in the 1960s to >9% in 2009. The Centers for Disease Control and Prevention estimates that 9.8% of the 280,000 residents of Durham County, North Carolina have been diagnosed with diabetes and that another 2.2% have undiagnosed diabetes. This is in addition to already high rates of obesity and overweight (29.6% and 34%, respectively) among adults in the region [3].
Recognizing that physical, social, and environmental factors shape the diabetes epidemic, and that reducing poor health outcomes and potentially avoidable costs related to poorly controlled type 2 diabetes will require innovative models of care and patient engagement, a group of Durham County formed the Durham Diabetes Coalition (DDC) in 2011. The goals of the DDC are 1) to improve population-level diabetes management, health outcomes, and quality of life for diagnosed and undiagnosed adults living with type 2 diabetes; and 2) to reduce disparities (including those based on race, age, sex, socioeconomic status, and insurance status) in diabetes management, health outcomes, and quality of life for adults living with type 2 diabetes.
In this paper, we describe the strategies employed by the DDC, including the project's design, the initial phase of characterizing and understanding the local diabetes problem, interventions resulting from lessons learned during the initial phase, and strengths and weaknesses affecting implementation of the project and its assessment.
Materials and methods
Study area
Durham County is located in the Central Piedmont region of North Carolina and is demographically distinct from the rest of the state. Compared with North Carolina overall and with the United States, Durham County has a larger proportion of NHBs. Educational attainment is also higher: 86.9% of Durham County adults aged ≥25 years have at least a high school diploma (vs. 84.5% for North Carolina; 85.7% for the United States) and 44.7% hold a bachelor's degree or higher (vs. 26.8% for North Carolina; 28.5% for the United States) 14, 15. From 2008 to 2012, median household income in Durham County was $50,997, exceeding the state median of $46,450. However, educational attainment and socioeconomic status are not evenly distributed in Durham County; during the same interval, 18% of Durham residents lived below the poverty level 14, 15 vs. 16.8% for North Carolina and 14.9% for the United States.
Engaged organizations
The DDC was founded to significantly reduce death and disability from type 2 diabetes countywide by 2017. The coalition comprises members from the Duke University Health System (Duke Medicine), Durham County Department of Public Health, National Center for Geospatial Medicine at the University of Michigan, Lincoln Community Health Center (LCHC; a Federally Qualified Health Center), and a community advisory board representing 23 public, political, and private community agencies. The DDC approach integrates detailed geographic information systems with existing electronic health record (EHR) data to create a geographic health information system (GHIS) that is combined with patient care and community-based approaches using proven constructs from chronic disease care models. This approach simultaneously addresses individual and community health in our pursuit of the triple aim of improved health, improved health care, and reduced costs [16].
Durham is home to Duke University and its affiliated health system, including an academic hospital, a community hospital, and a large number of outpatient clinics. Based on utilization data from Duke Medicine and U.S. Census data on population in Durham County, an estimated 80% of Durham County residents received care from a Duke Medicine provider at some point during the interval of 2007–2011. Lincoln Community Health Center and the Durham County Department of Public Health also provide care to Durham County residents, and the three institutions together touch almost every resident of Durham County. An important element of the DDC is the weaving together of data sources, analytics, and strategies across these entities that account for the overwhelming majority of healthcare delivery in Durham County.
Clinical data
The primary source of clinical data for this project is the Duke Enterprise Data Warehouse (DEDW). The DEDW integrates EHRs containing clinical data (laboratory, diagnostic, clinical notes, tests, etc.) as well as administrative and financial data from clinical encounters across the health system, including more than 25 major clinical and financial systems within the institution. Social and environmental data come from a wide variety of sources, detailed below.
Geographic health information systems
Geographic health information systems (GHISs) 17, 18, 19 are used to build large-scale spatial data architectures that connect data from EHRs with social and environmental data from the communities where patients reside. These systems use geographic information such as patient addresses or neighborhood locations to connect previously unrelated datasets. The resulting spatial data architecture allows us to understand patients both on the basis of their medical records and the context of their surroundings, thus affording a data-driven capacity to identify geographic areas in which individuals are at particular risk of poor outcomes. These geographic areas can be as small or as large as is useful to the organizations working within them.
In this project, patient addresses taken from EHRs are geocoded by associating medical record information with a particular physical location—specifically, an individual tax parcel in Durham County. Because we have extensive experience working with health records and with NC and Durham County tax parcel data, we were able to achieve an overall geocoding match rate of 84%. After excluding non-geocodable data such as incomplete addresses and post office boxes, our match rate is 88%. Although we typically achieve match rates well above 90% with North Carolina administrative datasets, the quality and completeness of EHR address information prevented us from attaining similar match rates for this project.
To obtain high-quality matches, we perform a three-batch matching process using Esri ArcGIS 10.1 software (Redlands, CA). First, raw addresses from the EHR are matched against StreetMap North America data with a match score requirement of 100/100 for Batch 1. Remaining unmatched addresses are standardized (for example, “Street,” “Str”, and “STR” are all standardized to “ST”) and then re-run against the StreetMap North America data with a match score of 90/100 for Batch 2. Remaining unmatched addresses are matched manually at the geocoder's discretion for Batch 3. For the present dataset, we achieved a match rate of 70.2% with Batch 1, 83% after incorporating Batch 2, and 84% after incorporating Batch 3.
The DEDW assigns each patient a unique identifier. Probabilistic matching is done and individuals with multiple unique identifiers are de-duplicated. Once geocoding is completed, we connect medical record data to other variables including neighborhood characteristics, census data, environmental data, community resources, social stressors (e.g., poor housing quality or crime), access to green spaces and areas for outdoor or indoor recreation and exercise, and community and clinic-based health care resources, all while leveraging rapidly evolving technologies and methods in GIS that allow for better geocoding [20]. We tracked whether addresses were matched in Batches 1, 2, or 3, allowing us to perform sensitivity analyses on all subsequent models and test whether match strength in geocoded data has an influence on model results and performance.
Such linkages can lead to greater understanding of racial disparities in health care outcomes [17]. Similarly, linking patient data to neighborhood-level characteristics can describe patient populations over space and time, potentially informing alternative approaches to health care. However, linking EHR data to neighborhood characteristic data presents a host of challenges. Our research team has previously linked clinical data on pregnancy and early childhood to neighborhood data in the same geography, and to adult disease in a more extended geography, and are using the same methods for this project 17, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31. Most importantly, this type of spatial data architecture enables risk stratification of individuals based on the integration of medical, social, and environmental risk. This in turn affords the opportunity to examine the spatial distribution of risk relative to venues for community and clinic-based interventions and to use this knowledge in developing individualized treatment plans.
Defining type 2 diabetes using a “computable phenotype”
In order to assess the impact of type 2 diabetes within Durham County, we first identified affected patients through secondary data analysis of the DEDW. Such identification requires a definition or phenotype that can be computed to correctly label patients or encounters with diabetes. To date, there are few published descriptions of other institutions' phenotypic definitions for diabetes registries. The Electronic Medical Records and Genomics (eMERGE) Consortium is focused on the genetics of complex phenotypes, and its definition was specifically created to identify only patients with type 2 diabetes for research purposes. It is therefore constructed to exclude patients with type 1 diabetes mellitus codes [32] and to return a low false-positive rate for type 2 diabetes diagnoses. The Surveillance, Prevention, and Management of Diabetes Mellitus (SUPREME-DM) project was established among institutions with an interest in population health, similar to the goals of the DDC. However, this group has a different EHR than that used by Duke and uses different coding practices, requiring us to build and validate our own phenotype [33].
The DDC began with a basic definition of diabetes based on International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes. This definition was supplemented by additional criteria to identify patients who lacked a formal coded diagnosis but whose clinical measures indicate that they have diabetes, as other groups have done 32, 33. The DDC EHR diabetes phenotype definition includes the adult Durham population of patients who met one or more of the following criteria during a Duke Medicine encounter between 2007 and 2011:
-
•
One or more instances of the specified ICD-9-Clinical Modification (CM) diagnosis codes (250.xx [with the exclusion of 250.x1 and 250.x3], 249.xx, 357.2, 362.0x, 366.41) on any type of encounter (inpatient, outpatient, emergency department);
-
•
One or more active medications associated with diabetes mellitus treatment reported during outpatient medication reconciliation (acarbose, acetohexamide, amylin agonists, chlorpropamide, glucagon-like peptide-1 agonists, insulin, metformin, miglitol, nateglinide, pioglitazone, repaglinide, rosiglitazone, sulfonylureas, tolazamide, troglitazone); and/or
-
•
Two or more abnormal diabetes labs within a 365-day period (hemoglobin A1c ≥ 6.5%; fasting glucose ≥126 mg/dL; random glucose ≥200 mg/dL).
This definition was deliberately constructed to include persons with undiagnosed or potential diabetes, persons with well-controlled diabetes on single oral therapy, or those who see specialty providers who may not code all medical illnesses. A more detailed discussion of this process has been published [34].
According to the U.S. Census, Durham County's 2010 population was 267,587, of whom 77.9% (208,450) were aged >18 years. The DEDW contains data on 189,023 adult patients living Durham County. Thus, we have data on >90% of adult Durham county residents. We are almost certainly missing some patients with early, noncomplicated, or undiagnosed diabetes, but patients requiring inpatient or emergency evaluation are seen at Duke Medicine for a supermajority of the population. Similarly, the vast majority of outpatients are seen through Duke Medicine, LCHC, or the public health system. The LCHC data will eventually be incorporated into the system so that a comprehensive superset of data is available across the county.
In addition to missing patients who may not seek health care, Durham residents who both receive all of their health care outside of the county and do not see providers employed by Duke are not accounted for in our data set. We also lack follow-up data for patient who move out of Durham County and no longer see Duke Medicine providers. As the project evolves and regional data exchange occurs, we expect to be able to quantify the degree of missingness in our numbers, including follow-up of patients who have not had contact with the system within the previous year.
Identifying high-risk areas and individuals
Given that we are able to identify patients with our clinical definition of type 2 diabetes from our computable phenotype, we have deployed GHIS tools to perform a series of tasks enabling a detailed depiction of health status and an approach to directing interventions at the individual, neighborhood, and county levels. These tasks include: 1) developing and refining risk algorithms; 2) identifying high-risk individuals and sub-county geographies where high-risk individuals cluster; and 3) identifying gaps in care, driven by knowledge of the spatial distributions of available social and health care resources. The GHIS allows us to identify patients on the basis of risk level matched with intensity of services, providers, and locations.
A logistic regression equation has been developed using existing clinical data to predict risk for serious outcomes, defined as hospital/emergency department admission or death. The initial algorithm predicts poor outcomes in calendar year 2011 based on 2010 EHR data, and then validates the model prediction using 2012 EHR data. Candidate variables were selected from proven risk factors for poor outcomes in patients with diabetes reported in the literature and suggested by expert clinician input. We then constructed a logistic regression model using an AIC-based backward stepwise regression procedure. Of note, neighborhood data are not used to construct the risk algorithm, but instead are used to design subsequent intervention strategies. These initial efforts were reported at the American Diabetes Association annual meeting in 2014 [31].
Because this work is conceived as a county-wide quality improvement project, we will continuously improve the model by including increasingly refined clinical, neighborhood, and social data as they become available. We also plan to develop predictive models for specific diabetes-related outcomes that may have different predictors, such as amputation, stroke, and myocardial infarction.
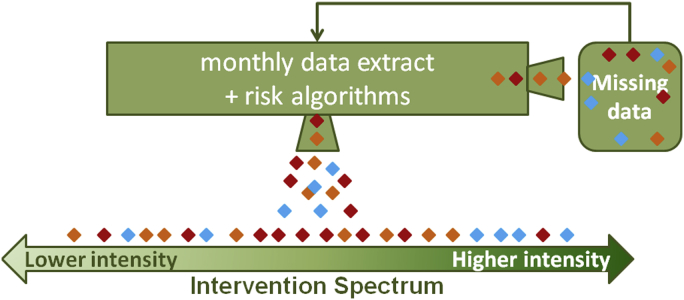
Fig. 1 demonstrates how the risk algorithm works in practice. A regular data extract is coupled with the risk algorithm. Persons with type 2 diabetes are assigned a composite risk score that places them on the intervention spectrum, from relatively low risk and lower-intensity, community-based interventions to relatively high risk and higher-intensity, individually-based interventions. Each diamond in Fig. 1 represents a patient, and each color represents a different geographic area. Multiple individuals at high risk (requiring a more intense intervention) characterize the blue neighborhood, which would then serve as a logical target area for more intense interventions. Assessments that combine risk assessment with geographic distribution can be performed on the entire patient population.
Figure 1.
Data and analysis work flow. This schematic demonstrates the application of the risk algorithm in practice. A regular data extract is coupled with the risk algorithm and persons with type 2 diabetes are assigned a composite risk score that places them on the intervention spectrum, from relatively low risk and lower-intensity, community-based interventions to relatively high risk and higher-intensity, individually-based interventions. Each diamond represents a patient; each color represents a different geographic area. In this representation, multiple persons at high risk characterize the blue neighborhood.
Interventions
Our initial focus has been on patients in the high-risk group, defined as the upper 10% of risk for death or hospitalization in the coming year. These patients receive an intensive, individually tailored intervention that includes access to in-home intervention via community health workers, or home visits by a health care team. Moderate-risk patients receive individual telephone-based interventions that support behavior change, focused community-based programs, and provider-based health care. This stratification of interventions allows limited resources to be targeted for maximal impact in the community.
To date, the DDC has identified 5 intervention strategies for this project: 1) targeted intensive neighborhood interventions; 2) intensive clinical care intervention to reach high-risk patients with potential barriers to traditional clinical care; 3) low-intensity telephone coaching to support moderate-risk patients in sustaining change in health behavior; 4) community interventions applicable to all Durham County residents; and 5) systematic change to clinical care systems to improve adherence to rigorous evidence-based diabetes care across Durham County.
Metrics and evaluation
Study data will enable multilevel examination across a range of patient outcomes and experiences. Specific baseline indicators of the prevalence of diabetes complications such as microvascular and macrovascular comorbidities have been assessed at the onset of the project and the system is updated periodically (Supplemental Table S1). With each health-system encounter, new outcomes and events are added to the DEDW, and these patterns can be examined in aggregate and by sub-geographies. Guideline-based treatment patterns for risk factors such as glycemic control, hypertension, hypercholesterolemia, and tobacco abuse are assessed to evaluate quality of care and identify pertinent quality-improvement initiatives. Patient-reported outcomes, including measures of depression, self-care, and medication adherence, are continuously evaluated in the high-risk group and iteratively used to drive treatment plans for high- and moderate-risk patients.
Health services utilization for diabetes-related events (such as diabetes-related emergency department and hospital admissions) and care episodes associated with quality care (such as primary care visits, ophthalmology screens, and dietician visits) are recorded, and these elements will be evaluated with the presumption that better adherence to proven beneficial care will improve outcomes, although the direct reduction in clinical events is unlikely to be measurable, because it can take years for a risk factor to change in ways that are reflected in a reduction of major events such as death and myocardial infarction. The system will be able to assign costs to these interventions and interactions with the health system, thereby enabling modeling of the value of the interventions. All-cause and diabetes- and cardiovascular-specific mortality will also be reported.
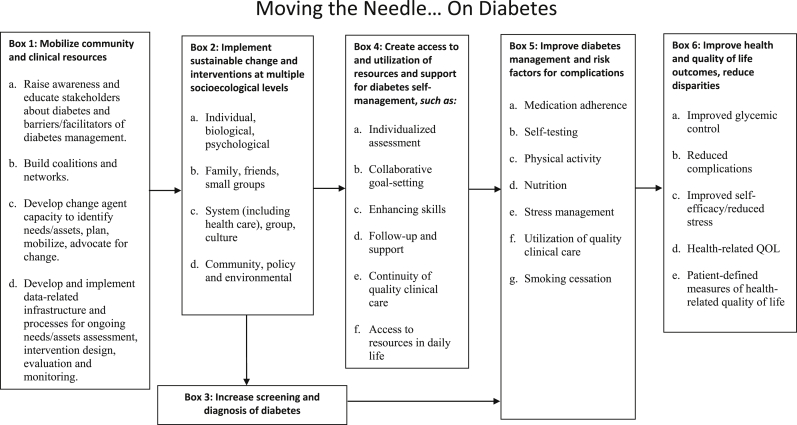
The DDC will evaluate outcomes and the processes that led to those outcomes as described in the logic model (Appendix), which depicts the relationships between the planned work and the intended results [35]. DDC interventions are designed to contribute to both individual and population-based measures of health improvement, such as improved self-management of diabetes, improved access to care, and sustainable change in health and healthcare delivery in Durham communities.
The DDC program evaluation will take place on 3 levels: 1) as determined by individual risk, with a focus on high-risk patients receiving the intensive clinical intervention; 2) the whole-population level for determining the effects of the program on diabetes-related health outcomes in Durham County; and 3) the sub-county geography level to examine the impact of interventions across demographically diverse areas.
Results
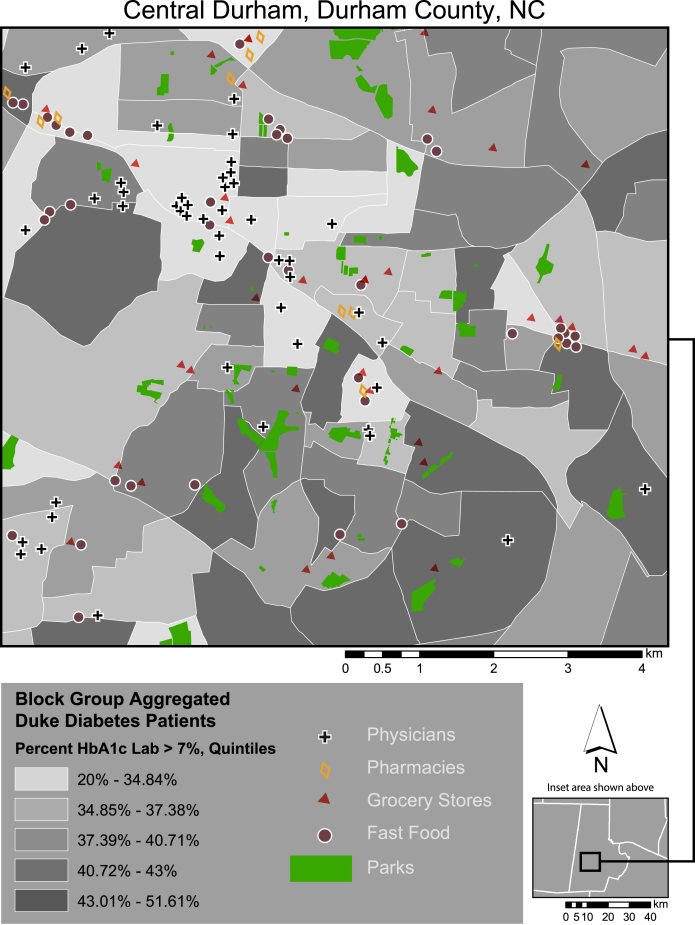
Using the computable phenotype described above, 22,982 unique patients with type 2 diabetes have been identified from the DEDW as living in Durham County (Table 1). Persons with diabetes are older, more often female, less often insured with private insurance, and more often African American. Through the use of the GHIS, these data have been mapped and geographically linked with other key social and environmental factors (Fig. 2).
Table 1.
Prevalence of diabetes in Durham County, North Carolina
| All |
Without diabetes |
With diabetes |
||||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Total | 189,023 | 166,041 | 87.8 | 22,982 | 12.2 | |
| Age (in years) | ||||||
| 18–21 | 11,522 | 6.1 | 11,327 | 6.8 | 195 | 0.9 |
| 22–29 | 35,166 | 18.6 | 34,418 | 20.7 | 748 | 3.3 |
| 30–39 | 41,944 | 22.2 | 40,037 | 24.1 | 1907 | 8.3 |
| 40–49 | 31,362 | 16.6 | 28,174 | 17.0 | 3188 | 13.9 |
| 50–64 | 40,021 | 21.2 | 32,195 | 19.4 | 7826 | 34.1 |
| 65+ | 29,008 | 15.4 | 19,890 | 12.0 | 9118 | 39.7 |
| Race/ethnicity | ||||||
| Non-Hispanic white | 83,483 | 44.2 | 74,586 | 44.9 | 8897 | 38.7 |
| Non-Hispanic black | 67,371 | 35.6 | 55,365 | 33.3 | 12,006 | 52.2 |
| Hispanic | 12,771 | 6.8 | 11,953 | 7.2 | 818 | 3.6 |
| Asian | 5731 | 3.0 | 5399 | 3.3 | 332 | 1.4 |
| Other | 6254 | 3.3 | 5793 | 3.5 | 461 | 2.0 |
| Not reported | 13,413 | 7.1 | 12,945 | 7.8 | 468 | 2.0 |
| Sex | ||||||
| Female | 108,204 | 57.2 | 95,072 | 57.3 | 13,132 | 57.1 |
| Male | 80,731 | 42.7 | 70,882 | 42.7 | 9849 | 42.9 |
| Not reported | 88 | 0.1 | 87 | 0.1 | 1 | 0.0 |
| Insurance status | ||||||
| Private | 114,515 | 60.6 | 105,236 | 63.4 | 9279 | 40.4 |
| Medicaid/Medicare | 41,401 | 21.9 | 30,093 | 18.1 | 11,308 | 49.2 |
| Self-pay | 27,782 | 14.7 | 25,485 | 15.4 | 2297 | 10.0 |
| Not reported | 5325 | 2.8 | 5227 | 3.2 | 98 | 0.4 |
Figure 2.
Durham resources and diabetes control. This map of the central area of the city of Durham in Durham County displays patient data that have been mapped and geographically linked with key social and environmental factors through the application of the geographic health information system.
Characterizing the burden of diabetes
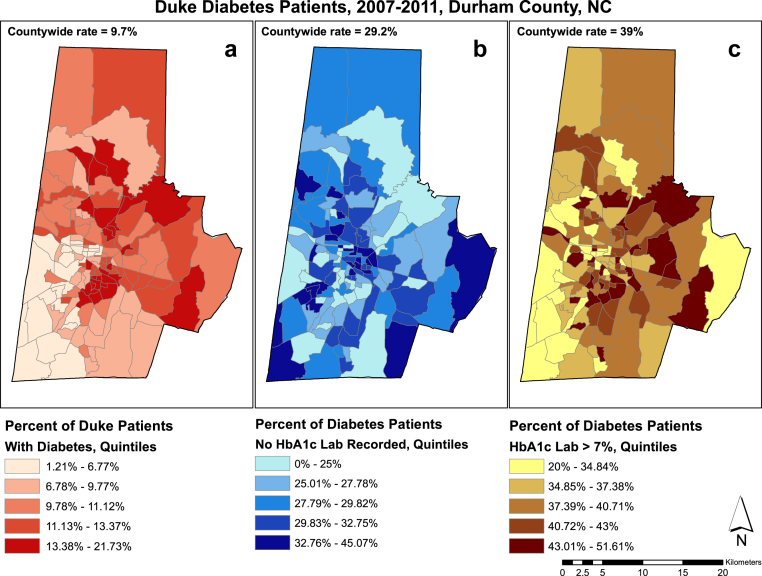
Fig. 3 displays the percentage of patients with type 2 diabetes who reside in Durham County and are present in the DEDW (a), the percentage of diabetes patients for whom no hemoglobin (Hb) A1c measurement was available (b), and the percentage of diabetes patients whose HbA1c measurements were outside of goal range (>7%; c). These maps reveal spatial patterns in the prevalence of diabetes, degree of success in achieving best practices (e.g., HbA1c measurements are recommended every 6 months), and degree of glycemic control in Durham County.
Figure 3.
Duke diabetes patients, 2007–2011. This figure displays the percentage of patients with type 2 diabetes who reside in Durham County and are present in the Duke Enterprise Data Warehouse (a), the percentage of diabetes patients for whom no hemoglobin (Hb) A1c measurement was available (b), and the percentage of diabetes patients whose HbA1c measurements were outside of goal range (>7%) (c).
Fig. 3 maps a series of key community resources onto the pattern of diabetes control from Fig. 2. This sample map highlights the social context of health disparities. For example, Fig. 3 demonstrates that some of the highest concentrations of diabetes occur in neighborhoods without grocery stores or medical clinics.
Targeted interventions
General concepts of targeting
Targeted interventions occur at both individual and neighborhood levels. We assumed that persons identified as high-risk would benefit from more intensive interventions. Many of these patients have not benefitted from proven interventions due to barriers to care such as lack of access to food (or to healthful food), lack of transportation, and inability to pay for all medications. Connecting patients to medical, social, and financial resources is an essential part of our intervention for these high-risk patients.
Although neighborhood data were not used to construct the first version of the risk algorithm and subsequently assign patients to intervention groups, spatial information is used to make home-visit strategies more efficient and to connect high-risk individuals with local resources. In addition, the moderate-risk intervention includes an analysis of how patients in this group cluster geographically, and how those clusters relate to local resources. This in turn is used to iteratively refine the moderate-risk intervention.
Neighborhood interventions
Neighborhood interventions are being piloted in 3 neighborhoods in Durham County, chosen with the assistance of the DDC's community advisory board. These communities have been selected using GHIS maps that analyze type 2 diabetes prevalence and complications, socioeconomic factors, existing neighborhood resources, and level of potential project impact. The Board helps ensure that the DDC selects interventions that are culturally appropriate, sustainable, and meet community needs.
Community health integrators (trained professional health educators) are deployed to each targeted neighborhood to assess and mobilize the community by building relationships with residents, neighborhood leaders, and other stakeholders such as churches, nonprofits, and community groups. Community interventions are informed by feedback from these stakeholders and reflect each neighborhood's unique needs. Some community interventions are furnishing Durham County residents with the tools and resources they need to manage their diabetes and are fostering healthier lifestyle habits through evidence-based initiatives such as the Diabetes Self-Management Program and the Chronic Disease Self-Management Program. Other interventions are changing the actual environment by creating “Healthy Mile Trails” on sidewalks.
Community activities also provide community members with skill building and health-education services such as Shopping Matters® grocery store tours, walking groups, diabetes presentations, webinars, and a monthly educational community newsletter. In order to sustain interventions and create permanent environmental changes, lay leaders will be trained to work with neighborhood organizations to write policies that promote health.
A health-department–based diabetes information and communication officer (DIO) uses GHIS data and community input to develop outreach campaigns supporting and advertising neighborhood and county interventions. The DIO provides the public with information about DDC interventions and services, diabetes-related health information, and local diabetes resources, including a DDC website, DDC-specific social media sites (e.g., Facebook, Twitter, YouTube), a television show aired on the local government access cable channel, and various countywide media campaigns that will encourage improved health behaviors for Durham County residents. The DIOs are also responsible for creating a DDC brand to foster name recognition in the community. The DIOs also work closely with health educators to tailor health messages and brand all DDC outreach materials.
These efforts enable continuous quality improvement and mid-course corrections based on both real-time GHIS data and community feedback on the short- and long-term impact of interventions.
Intensive clinical care intervention
An intensive, in-home clinical care program is offered for patients identified by the risk algorithm as being within the top 10% in terms of risk for death or hospitalization in the coming year. Each clinical team is supervised by a clinical endocrinologist and includes nurse practitioners, a community health worker, a registered dietician, and a licensed clinical social worker. The clinical intervention is based on the “Just for Us” clinical program [36] managed by the Duke Division of Community Health and Lincoln Community Health Center, which offers in-home medical services to older adults and adults with disabilities in Durham's public and subsidized housing facilities.
The high-risk clinical team communicates with primary care physicians to ensure that all care providers are aware of team goals and each patient's progress. The high-risk clinical team also helps coordinate care for moderate-risk patients by communicating with their existing medical home providers and using centralized telephone coaching modules [37] implemented by the team of community health workers at the Durham County Department of Public Health and Healing with CAARE, Inc. The clinical intervention team tracks patient progress through ongoing monitoring of patient data at 6-month intervals. The intervention suite provides a coordinated approach to achieving sustainable improvements in self-management in the high-risk population, preventing unnecessary hospitalization and emergency department use.
County-wide community interventions
Successful interventions for managing chronic illness integrate community resources and policies with health system organizational structure 38, 39 and depend upon shared systems for exchanging clinical information, decision support, design of care delivery, and self-management support. By integrating resources, communities and health systems can foster communication and productive interactions between patients and providers, leading to improved clinical outcomes. The GHIS expands our ability to direct resources where they are needed by providing a more complete picture of risk factors within a neighborhood context and targeting health messages to the community using map displays. We are leveraging the existing structures and missions of the Durham County Department of Public Health, houses of worship, and outreach programs of medical centers such as Duke, LCHC, Healing with CAARE, Inc., the American Diabetes Association, the North Carolina county extension office, and numerous other community organizations. Using this network, we are conducting health fairs and media events and promoting policy decisions that improve the social and physical environment to make health management easier for patients living with diabetes in Durham County.
Systematic changes to clinical care
In order to improve rigorous, evidence-based diabetes care throughout Durham County, we are working with Durham County providers to implement guidelines-based care for all patients with type 2 diabetes through EHR systems using care protocols, best practice alerts, and risk category alerts based on individual and neighborhood characteristics. The initial suite of care protocols emphasizes 1) glycemic control through nutrition, physical activity, and medication management; 2) treatment of hypertension, including appropriate use of blood pressure medications; 3) treatment of hypercholesterolemia and cardiovascular risk, including use of statins when appropriate; and 4) telephone coaching to improve adherence to evidence-based treatments. In its full form, this will be accomplished by implementing diabetes care protocols and quality score cards across locations, training the workforce in patient-centered care and motivational interviewing, as well as creating virtual visits to review medications, glucose and blood pressure readings, and achievement of patient goals 40, 41.
Limitations
The DDC is a quality improvement project implemented at the population level of a county. As such, it is continuously evolving as improvements are made to the data systems linking elements of the health delivery systems and as interventions are adapted based on data and community feedback. Because we lack a randomized comparison group, we cannot draw definitive causal inferences regarding trends over time in diabetes outcomes and costs. We are currently developing approaches for assessing outcomes that include causal inference modeling and non-randomized comparison to other counties.
Movement of populations into and out of both Durham County and the Duke Medicine health system presents challenges to our ability to determine the effectiveness of this project. In addition, continuous changes to the racial, ethnic, and socioeconomic makeup of communities may correspondingly alter risk profiles for individuals and neighborhoods.
Although we have identified individuals and neighborhoods across the entire spectrum of risk for negative outcomes from diabetes, determining which interventions would be most effective at reducing risk remains challenging. The results of population interventions have been mixed 42, 43, 44, 45, 46 and there is no single proven approach, although some concepts seem to be central to health improvement. Our intervention complements traditional medical approaches by addressing substance abuse, violence, psychiatric illness, inability to pay for medications, lack of access to food, and homelessness. However, we acknowledge that there are currently few examples of successful interventions across multiple dimensions. Nonetheless, we believe identifying patients and neighborhoods with gaps in care and developing interventions to address these gaps will be the best way to improve outcomes.
Diabetes control, and therefore diabetes complications, are affected by the ability to self-manage diabetes through a healthful lifestyle; thus, access to places to walk, exercise, and find healthful food are essential. Some studies have suggested that coordinated care for high-risk patients is not always successful 42, 43, 44. For this reason, we have combined coordinated care with geospatial analysis that can ultimately inform individual, population, and governmental policies.
Conclusions
The DDC is pursuing a strategy to improve diabetes outcomes at the individual, neighborhood, and population levels using interventions consonant with the “triple aim” of improved experience of care, improved health outcomes, and lower utilization 16, 45. By enabling risk stratification of patients and neighborhoods, GHISs are being used to assist decision-making and evaluation of interventions and clinical care. GHISs are also used to efficiently focus limited resources by identifying communities in need of higher-intensity interventions and providing continuous monitoring of individuals and populations. By coordinating and integrating clinical care within the community, we aim to improve health care delivery and health outcomes for both individuals and populations while simultaneously reducing preventable complications, procedures, and admissions. This system will provide the platform for monitoring and evaluating the initial implementation of coordinated care for high-risk groups and community-wide interventions. Lessons learned will contribute to knowledge needed that we hope will create a sustainable and replicable platform to be implemented in the southeastern United States and other areas disproportionately affected by diabetes.
Key points
-
•
The diabetes epidemic is a physically and financially costly burden.
-
•
Known therapies to improve diabetes outcomes have not been equally applied to all populations.
-
•
Matching resources to individuals and communities based on risk may improve health outcomes.
-
•
Geographic disparities in diabetes prevalence and complications exist.
-
•
A geographic health information system can inform community-based diabetes care programs.
-
•
Social and environmental factors affect diabetes care and outcomes as much as medical factors.
-
•
Combining community and clinical approaches of varying intensities is being used to impact death and disability from diabetes.
Conflict of interest statement
Susan E. Spratt reports receiving funds from the Exeter Group and Sanofi-Aventis for continuing medical education events in diabetes. She also reports owning stock in Merck, Pfizer, Johnson & Johnson, Procter & Gamble, and Medtronic. Bryan C. Batch has no disclosures to report. Lisa P. Davis has no disclosures to report. Ashley A. Dunham has no disclosures to report. Michele Easterling has no disclosures to report. Mark N. Feinglos reports receiving clinical research funding from Sanofi-Aventis, Eli Lilly & Co, Novo Nordisk, Merck, Amylin Pharmaceuticals, AstraZeneca, Procter & Gamble, BMS, and GSK. Bradi B. Granger has no disclosures to report. Gayle Harris has no disclosures to report. Michelle J. Lyn has no disclosures to report. Pamela J. Maxson has no disclosures to report. Bimal R. Shah's disclosures available at https://dcri.org/about-us/conflict-of-interest. Benjamin Strauss has no disclosures to report. Tainayah Thomas has no disclosures to report. Robert M. Califf's disclosures are available at https://dcri.org/about-us/conflict-of-interest. Marie Lynn Miranda has no disclosures to report.
Acknowledgments
The authors acknowledge the efforts of Claire Osgood and Shelley Rusincovitch for data management; Howard Shang, Stephanie Brinson, and the Duke Health Technology Solutions Information Management team for consultation on project implementation and use of the DSR infrastructure.
Author contributions: SES participated in planning and implementing the project, analyzing the data, and writing the paper. BCB participated in implementing the project, analyzing the data, and writing the paper. LPD participated in implementing the project, analyzing the data, and writing the paper. AAD participated in implementing the project, analyzing the data, and writing the paper. ME participated in planning and implementing the project, analyzing the data, and evaluating team efforts. MNF participated in planning and implementing the project, analyzing the data, and writing the paper. BBG participated in planning and implementing the project, analyzing the data, and writing the paper. GH participated in planning and implementing the project, analyzing the data, and editing the paper. MJL participated in planning and implementing the project and editing the paper. PJM participated in planning and implementing the project, analyzing the data, and writing and editing the paper. BRS participated in implementing the project, analyzing the data, and writing the paper. BS participated in planning and implementing the project, analyzing the data, and editing the paper. TT participated in implementing the project, analyzing the data, evaluating team efforts, and writing the paper. RMC participated in planning and implementing the project, analyzing the data, and writing and editing the paper. MLM participated in planning and implementing the project, analyzing the data, and writing and editing the paper.
Guarantor: Susan E. Spratt takes responsibility for the accuracy of this paper and guarantees the data.
Footnotes
Funding: This project is funded by the Bristol-Myers Squibb Foundation and the Center for Medicare & Medicaid Innovation (CMS grant number: 1C1CMS331018).
Appendix.
Supplemental Table S1.
Outcome measures
| Domain | Outcome measure | Process measure | Population prevalence | Intervention cohort incidence |
|---|---|---|---|---|
| Microvascular complications | Kidney disease – classification
|
Kidney disease – process/quality care
|
X | X |
| Dialysis | X | X | ||
| Peripheral neuropathy | Process/quality care
|
X | X | |
Retinopathy – classification
|
Retinopathy – process/quality care
|
X | X | |
Wound/skin ulcer treatment – classification
|
Wound/skin ulcer treatment – process
|
X | X | |
| Macrovascular complications | Amputation procedures – classification
|
Amputation procedures – process/quality care
|
X | X |
| Acute MI events | Guideline medication use | X | X | |
| Stroke events | Guideline medication use | X | X | |
| Coronary revascularization procedures | Guideline medication use | X | X | |
| Heart failure | Guideline medication use | X | X | |
| Diabetes control | Hemoglobin A1c:
|
Hemoglobin A1c – process/quality care
|
X | X |
| Glucose |
|
X | X | |
| Hypoglycemia events | X | X | ||
| Hyperglycemia events | X | X | ||
| Hyperosmolar | X | X | ||
| Ketoacidosis | X | X | ||
| Risk factors | Obesity | Weight, diet, activity monitoring | X | X |
Hypertension
|
Hypertension
|
X | X | |
Hyperlipidemia
|
Hyperlipidemia
|
X | X | |
| Smoking status (Fagerstrom test for Nicotine Dependence) | Smoking cessation program attendance | X | ||
| Exercise status (Stanford scale) | Activity monitoring | X | ||
| Patient-reported outcomes | Global health scale score (PROMIS-9) | Survey completion rate | X | |
| Patient depression score (PHQ-2) | Survey completion rate | X | ||
| mDiabetes care profile score (patient perception of self-management skills) | Survey completion rate | X | ||
| Medication adherence score (Morisky) | Survey completion rate | X | ||
| Health services utilization | Emergency department encounters (DM-related and non-DM related | X | X | |
| Inpatient encounters (DM-related, cardiovascular-related, and other) | X | X | ||
Outpatient encounters
|
X | X | ||
| Length of stay for inpatient admissions | X | X | ||
| Billing charges for health services | X | |||
| Prediction of adverse outcomes | SEDI risk algorithm scoring | X | X | |
| Death | Mortality status | X | X |
BP, blood pressure; CDE, Certified Diabetes Educator; CKD, chronic kidney disease; DM, diabetes mellitus; MI, myocardial infarction; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; RD, Registered Dietitian; SEDI, Southeast Diabetes Initiative.
Appendix. Durham diabetes control logic model
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046. doi: 10.2337/dc12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . U.S. Department of Health and Human Services, Center for Disease Control and Prevention; Atlanta, GA: 2011. National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States, 2011.http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf Available at: Accessed 26.09.14. [Google Scholar]
- 4.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–865. [PubMed] [Google Scholar]
- 5.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 6.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–389. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowie C.C., Rust K.F., Ford E.S., Eberhardt M.S., Byrd-Holt D.D., Li C. Full accounting of diabetes and pre-diabetes in the U.S. population in 1988–1994 and 2005-2006. Diabetes Care. 2009;32:287–294. doi: 10.2337/dc08-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holman R.R., Paul S.K., Bethel M.A., Matthews David R., Neil H.A.W. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 9.Martin C.L., Albers J., Herman W.H., Cleary P., Waberski B., Greene D.A. Neuropathy among the diabetes control and complications trial cohort 8 years after trial completion. Diabetes Care. 2006;29:340–344. doi: 10.2337/diacare.29.02.06.dc05-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohkubo Y., Kishikawa H., Araki E., Miyata T., Isami S., Motoyoshi S. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995;28:103–117. doi: 10.1016/0168-8227(95)01064-k. [DOI] [PubMed] [Google Scholar]
- 11.Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dartmouth Atlas of Health Care. 2014. http://www.dartmouthatlas.org/ Available at: Accessed 26.09.14. [Google Scholar]
- 13.Smedley B.D., Stith A.Y., Nelson A.R., editors. Unequal treatment: confronting racial and ethnic disparities in health care. Institute of Medicine. National Academies Press; Washington, DC: 2002. http://www.nap.edu/openbook.php?record_id=10260&page=R1 Available at: Accessed 26.09.14. [PubMed] [Google Scholar]
- 14.U.S. Census Bureau . 2014. State and County QuickFacts.http://quickfacts.census.gov/qfd/index.html# Available at: Accessed 26.09.14. [Google Scholar]
- 15.Partnership for a Healthy Durham . 2008. Durham county state of the county health report. [Google Scholar]
- 16.Berwick D.M., Nolan T.W., Whittington J. The triple aim: care, health, and cost. Health Aff (Millwood) 2008;27:759–769. doi: 10.1377/hlthaff.27.3.759. [DOI] [PubMed] [Google Scholar]
- 17.Miranda M.L., Ferranti J., Strauss B., Neelon B., Califf R.M. Geographic health information systems: a platform to support the ‘triple aim’. Health Aff (Millwood) 2013;32:1608–1615. doi: 10.1377/hlthaff.2012.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards T.B., Croner C.M., Rushton G., Brown C.K., Fowler L. Information technology: geographic information systems and public health: mapping the future. Public Health Rep. 1999;114:359–373. doi: 10.1093/phr/114.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Croner C.M., Sperling J., Broome F.R. Geographic information systems (GIS): new perspectives in understanding human health and environmental relationships. Stat Med. 1996;15:1961–1977. doi: 10.1002/(sici)1097-0258(19960930)15:18<1961::aid-sim408>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Edwards S.E., Strauss B., Miranda M.L. Geocoding large population-level administrative datasets at highly resolved spatial scales. Trans GIS. 2014;18:586–603. doi: 10.1111/tgis.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gray S.C., Edwards S.E., Schultz B.D., Miranda M.L. Assessing the impact of race, social factors, and air pollution on birth outcomes: a population-based study. Environ Health. 2014;13:4. doi: 10.1186/1476-069X-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anthopolos R., Kaufman J.S., Messer L.C., Miranda M.L. Racial residential segregation and preterm birth: built environment as a mediator. Epidemiology. 2014;25:397–405. doi: 10.1097/EDE.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 23.Gray S., Edwards S.E., Miranda M.L. Race, socioeconomic status, and air pollution exposure in North Carolina. Environ Res. 2013;126:152–158. doi: 10.1016/j.envres.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Messer L., Maxson P., Miranda M.L. The urban built environment and associations with women's psychosocial health. J Urban Health. 2013;90:857–871. doi: 10.1007/s11524-012-9743-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miranda M.L., Edwards S.E., Chang H.H., Auten R.L. Proximity to roadways and pregnancy outcomes. J Expo Sci Environ Epidemiol. 2013;23:32–38. doi: 10.1038/jes.2012.78. [DOI] [PubMed] [Google Scholar]
- 26.Miranda M.L., Edwards S.E., Anthopolos R., Dolinsky D.H., Kemper A.R. The built environment and childhood obesity in Durham, NC. Clin Pediatr (Phila) 2012;51:750–758. doi: 10.1177/0009922812446010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda M.L., Messer L., Kroeger G. Associations between the quality of the residential built environment and pregnancy outcomes among women in North Carolina. Environ Health Perspect. 2012;120:471–477. doi: 10.1289/ehp.1103578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthopolos R., James S.A., Gelfand A.E., Miranda M.L. A spatial measure of neighborhood-level racial isolation applied to low birthweight, preterm birth, and birthweight in North Carolina. Spat Spatiotemporal Epidemiol. 2011;2:235–246. doi: 10.1016/j.sste.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Strauss B.W., Valentiner E.M., Bhattacharya S., Smerek M.M., Dunham A.A., Newby L.K. Improving population representation through geographic health information systems: mapping the MURDOCK study. Am J Transl Res. 2014;6:402–412. [PMC free article] [PubMed] [Google Scholar]
- 30.Ward-Caviness C, Kraus W.E, Blach C, Haynes C, Dowdy E, Miranda M.L. et al. Association of traffic-related air pollution with fasting plasma glucose and metabolic risk factors for cardiovascular disease. Environ Health Perspect (in press). [DOI] [PMC free article] [PubMed]
- 31.Spratt S.E., Shriver J., Maxon P., Batch B., Shah B., Califf R. American Diabetes Association; June 2014. A risk model to identify patients with diabetes at highest risk for death or hospitalization: combining geography and risk to guide resources and improve outcomes. [Abstract] [Google Scholar]
- 32.Kho A.N., Hayes M.G., Rasmussen-Torvik L., Pacheco J.A., Thompson W.K., Armstrong L.L. Use of diverse electronic medical record systems to identify genetic risk for type 2 diabetes within a genome-wide association study. J Am Med Inf Assoc. 2012;19:212–218. doi: 10.1136/amiajnl-2011-000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols G.A., Desai J., Elston L.J., Lawrence J.M., O'Connor P.J., Pathak R.D. Construction of a multisite DataLink using electronic health records for the identification, surveillance, prevention, and management of diabetes mellitus: the SUPREME-DM project. Prev Chronic Dis. 2012;9:E110. doi: 10.5888/pcd9.110311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richesson R.L., Hammond W.E., Nahm M., Wixted D., Simon G.E., Robinson J.G. Electronic health records based phenotyping in next-generation clinical trials: a perspective from the NIH Health Care Systems Collaboratory. J Am Med Inf Assoc. 2013;20:e226–e231. doi: 10.1136/amiajnl-2013-001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.W.K. Kellogg Foundation . 2004. W.K. Kellogg Foundation Logic Model Development Guide.http://www.wkkf.org/resource-directory/resource/2006/02/wk-kellogg-foundation-logic-model-development-guide Available at: Accessed 26.09.14. [Google Scholar]
- 36.Duke Division of Community Health . 2014. Just for us: providing medical care to Durham's older adults.http://communityhealth.mc.duke.edu/clinics/just-us Available at: Accessed 26.09.14. [Google Scholar]
- 37.Lowry K.P., Dudley T.K., Oddone E.Z., Bosworth H.B. Intentional and unintentional nonadherence to antihypertensive medication. Ann Pharmacother. 2005;39:1198–1203. doi: 10.1345/aph.1E594. [DOI] [PubMed] [Google Scholar]
- 38.Agency for Healthcare Research and Quality website. U.S. Department of Health and Human Services. 2011 Report to Congress: National Strategy for Quality Improvement in Health Care. Available at: http://www.ahrq.gov/workingforquality/nqs/nqs2011annlrpt.htm; [Accessed 26.09.14].
- 39.Congressional Budget Office . 2012. Issue Brief. Lessons from Medicare's demonstration projects on disease management, care coordination, and value-based payment.http://www.cbo.gov/sites/default/files/cbofiles/attachments/01-18-12-MedicareDemoBrief.pdf Available at: Accessed 26.09.14. [Google Scholar]
- 40.Agency for Healthcare Research and Quality website . March 12, 2007. AHRQ quality indicators. Guide to prevention quality indicators: hospital admission for ambulatory care sensitive conditions. Version 3.1.http://www.qualityindicators.ahrq.gov/Downloads/Modules/PQI/V31/pqi_guide_v31.pdf Available at: Accessed 26.09.14. [Google Scholar]
- 41.Agency for Healthcare Research and Quality website . 2010. AHRQ quality indicators. Prevention quality indicators appendices. Version 4.2. [Google Scholar]
- 42.Curry N., Harris M., Gunn L.H., Pappas Y., Blunt I., Soljak M. Integrated care pilot in north-west London: a mixed methods evaluation. Int J Integr Care. 2013;13:e027. doi: 10.5334/ijic.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson L. Congressional Budget Office; Washington, DC: 2012. Lessons from Medicare's demonstration projects on disease management and care coordination.http://www.cbo.gov/sites/default/files/cbofiles/attachments/WP2012-01_Nelson_Medicare_DMCC_Demonstrations.pdf Available at: Accessed 26.09.14. [Google Scholar]
- 44.Joynt K.E., Gawande A.A., Orav E.J., Jha A.K. Contribution of preventable acute care spending to total spending for high-cost Medicare patients. JAMA. 2013;309:2572–2578. doi: 10.1001/jama.2013.7103. [DOI] [PubMed] [Google Scholar]
- 45.Califf R.M., Sanderson I., Miranda M.L. The future of cardiovascular clinical research: informatics, clinical investigators, and community engagement. JAMA. 2012;308:1747–1748. doi: 10.1001/jama.2012.28745. [DOI] [PubMed] [Google Scholar]
- 46.Chan W.C., Jackson G., Wright C.S., Orr-Walker B., Drury P.L., Boswell D.R. The future of population registers: linking routine health datasets to assess a population's current glycaemic status for quality improvement. BMJ Open. 2014;4:e003975. doi: 10.1136/bmjopen-2013-003975. [DOI] [PMC free article] [PubMed] [Google Scholar]