Abstract
Purpose
The purpose of the study is to assess whether proper Injection Technique (IT) is associated with improved glucose control over a three month period.
Methods
Patients (N = 346) with diabetes from 18 ambulatory centers throughout northern Italy who had been injecting insulin ≥ four years answered a questionnaire about their IT. The nurse then examined the patient's injection sites for the presence of lipohypertrophy (LH), followed by an individualized training session in which sub-optimal IT practices highlighted in the questionnaire were addressed. All patients were taught to rotate sites correctly to avoid LH and were begun on 4 mm pen needles to avoid intramuscular (IM) injections. They were instructed not to reuse needles.
Results
Nearly 49% of patients were found to have LH at study entry. After three months, patients had mean reductions in HbA1c of 0.58% (0.50%–0.66%, 95% CI), in fasting blood glucose of 14 mg/dL (10.2–17.8 mg/dL, 95% CI) and in total daily insulin dose of 2.0 IU (1.4–2.5 IU, 95% CI) all with p < 0.05. Follow-up questionnaires showed significant numbers of patients recognized the importance of IT and were performing their injections more correctly. The majority found the 4 mm needle convenient and comfortable.
Conclusions
Targeted individualized training in IT, including the switch to a 4 mm needle, is associated with improved glucose control, greater satisfaction with therapy, better and simpler injection practices and possibly lower consumption of insulin after only a three month period.
Keywords: Insulin injection, Lipohypertrophy, Site rotation, Injection education
Introduction
Most physician visits with insulin-injecting patients involve discussions about glucose control and dose adjustments, but very little time is spent on improving Injection Technique (IT). However IT may in certain cases be just as important to diabetes management as the type of insulin or dosage used.
This study was the outgrowth of a survey performed in 21 hospitals in northern Italy in 2011 by the ANIED Group – Associazione Nazionale Infermieri in Endocrinologia e Diabetologia (National Association of Nurses in Diabetology and Endocrinology; see Acknowledgments section) [1]. That study, termed the Swansdown Survey and done in the same centers as our study, involved 472 injecting patients and consisted of training to improve their injecting technique. Patients were using a variety of needle lengths (12.7 mm [1.2%], 8 mm [37.7], 6 mm [35.4%], 5 mm [18.4%], 4 mm [7.6%], unknown [5%]). No needle length changes were proscribed by the study. Patients were followed up at 3 and 6 months for effects of the training on their glucose control and injection technique knowledge. No significant changes were found in HbA1c or fasting glucose levels, but understanding of injections was improved. The Survey had initially revealed that the greatest educational needs were in older patients who had been injecting insulin for over ten years and who had outdated practices. By study conclusion a majority of patients showed improved understanding of injecting devices (including shorter, finer-gauge needles), the care and maintenance of injection sites, the means for avoiding complications such as lipohypertrophy (LH) and the necessity for rotating injection sites. As a result of this survey, ANIED felt this training should include both general education in optimal IT as well as individualized and targeted attention to specific needs as revealed by patient questionnaires and hands-on nurse examinations.
Studies have been published in recent years supporting the safety, efficacy and patient preference for the 4 mm pen needle (PN) 2, 3, 4, 5, 6, 7, 8, 9. These needles have been shown to provide equivalent glucose control compared to longer needles while reducing both the risk of IM injections as well as injection pain. For these reasons it was felt that the 4 mm PN played an integral role in optimizing IT and should be the needle of choice for all injecting patients in the participating centers. Since the switch to this needle was planned anyway, we decided to study several glucose control and IT parameters at the same time. We evaluated the effects of an integrated IT educational approach which included general and targeted training, advice on rotation of sites and needle reuse as well as a switch to the shortest PN (4 mm).
Methods
Patients with diabetes from 18 ambulatory centers throughout the Piedmont region of northern Italy (Table 1) who had been injecting insulin for at least four years were queried by their nurse/educator using a questionnaire (Table 2) about their IT. The nurse then examined the patient's injection sites for the presence of abnormalities including LH. A general IT education session lasting approximately 15 min was given using the BD Educational Starter Kit® (Becton Dickinson, Inc., Franklin Lakes, NJ, USA). The kit contains site rotation grids, an educational injection technique leaflet and a blood-glucose log-book. The ‘look and feel’ of LH was taught using a BD ‘Lipobox’®, which provided visual and tactile practice on typical LH lesions. This general training was followed by an individualized training session in which sub-optimal IT practices highlighted in the patient's questionnaire were addressed. All patients were taught how to rotate sites correctly to avoid LH and all were begun on the BD Micro-Fine™ 4 mm 32G pen needle (Becton Dickinson, Inc., Franklin Lakes, NJ, USA) to avoid intramuscular (IM) injections. They were also instructed not to reuse the needles. Furthermore each patient received an information card in which ANIED underlines the importance that IT has in achieving optimal glucose control.
Table 1.
Participating centers and subjects
| Province | City | Center | Total PTS | PTS with HbA1c at exit |
|---|---|---|---|---|
| AL | Novi Ligure | CAD Osp. San Giacomo | 105 | 98 |
| Acqui Terme | CAD Osp. Civile Monsignor Galliano | |||
| Tortona | CAD Osp. SS Antonio e Margherita | |||
| Alessandria | CAD A.S.O. SS Antonio Biagio e Cesare Arrigo | |||
| CN | Cuneo | CAD A.S.O. Santa Croce e Carle | 39 | 32 |
| Fossano | CAD Osp. SS Trinita′ | |||
| Fossano | F.A.N.D. | |||
| TO | Torino | CAD A.S.O. C.T.O. (Città della Salute e della Scienza) | 116 | 91 |
| Torino | CAD Osp. Regina Margherita (Città della Salute e della Scienza) | |||
| Torino | CAD Via Monginevro ASL To1 | |||
| Torino | CAD Osp. Maria Vittoria | |||
| Torino | CAD S.G.A.S. (Città della Salute e della Scienza) | |||
| Torino | CAD A.S.O. Ordine Mauriziano Umberto I | |||
| BI VCO VL | Omegna | CAD Osp. Madonna del Popolo | 86 | 38 |
| Verbania | CAD Osp. Castelli | |||
| Domodossola | CAD Osp. San Biagio | |||
| Vercelli | CAD P.O. S. Andrea | |||
| Biella | CAD Osp. Degli Infermi | |||
| Total | 346 | 259 | ||
Table 2.
Key study questionsa
| How many injections do you give per day? |
| What injection sites do you use? |
| When do you give your injections? |
| What needle length do you use? |
| Do you use the pinch-up technique? |
| If so, when do you release the pinch? |
| How long do you leave the needle in the skin after the injection? |
| Does the injection cause bleeding or bruising? |
| How often do you use the same needle? |
| At what angle do you insert the needle? 45°, 90° or other? |
| How would you describe the experience of injection in terms of pain? |
| b How important was it for you to use a short needle? |
| b What did using a 4 mm × 32 g needle feel like? |
This is a selection of questions; the full questionnaire is available on request.
These questions were only posed at the end.
The nurses involved in this study were trained at the time of the Swansdown Survey [1] in 2011 on correct IT using the published New Injection Recommendations [10]. The questionnaire used by patients and nurses was reviewed point by point and each nurse was certified on proper methodology for administrating the questionnaire as well as for performing the physical exam. Shortly before our study began in 2012, the same nurses met for a 4-h roundtable in Turin, managed by the ANIED scientific board, to review and discuss the Swansdown results [1], and to create the ANIED Card. This card teaches patients correct IT. At the same roundtable the nurses reviewed the new trial protocol, with emphasis on inclusion and exclusion criteria. All participating nurses were then trained on the ‘ANIED Card’ which would then be used to train all patients on correct IT during the general education session. The entrance and exit questionnaires were reviewed point-by-point with explanation and discussion of every question. There were instructions on minimizing drop-out and ensuring follow-up. Each nurse had to demonstrate expertise in history taking, gathering of laboratory data in an appropriate manner as well as in the use of a uniform methodology for observing and palpating injection sites. Hands-on certification was required in the proper use of the Starter Kit® and Lipobox®. Structured education techniques were taught and certified including the correct explanation of injection rotation, the use of a 4 mm needle and detailed LH management.
Inclusion Criteria for patients included age >12 years, having type 1 diabetes (T1D) or type 2 (T2D) and being on injection therapy for at least four years. It was felt that subjects injecting for less than four years might have already been exposed to the most up-dated teaching in IT. By four years, however, subjects were felt to have already established habits which might need corrective education and training. Nevertheless, if subjects were still in their teens (13–19 years old) the four-year requirement was waived. Subjects of all body mass index (BMI) and using all marketed needle lengths were accepted into the study.
Because of the time commitments needed to administer the questionnaire and provide both general and individual training, it was often not possible to include all patients seen on a daily basis in any center. Hence nurses were instructed to admit to the study either the first one or two patients of the day who satisfied the inclusion criteria or the first and last patients of the day who satisfied the inclusion criteria. If time permitted, all patients who satisfied the inclusion criteria in a day were included. While not strict randomization, this approach helped prevent selection bias.
Nurses in all centers received standardized training on how to evaluate for LH and how to train patients on IT. Each patient was taught the correct way to inject using a 4 mm PN and were told they would be evaluated after the first 3 months in order to assess their IT, changes in clinical parameters, the state of their injection sites and their psychological reaction to and clinical impact of the 4 mm PN (see final questions in Table 2).
Every patient was informed that data from their questionnaire would be maintained in strict confidentiality, with only his/her doctor and nurse having access to their identity. They were told that it was not important to give the correct answer (‘this is not an examination!’) and that they should not try to give the answer they thought their nurse or doctor might want to know. It was reinforced that to help them optimize their care it was extremely important to give accurate, true answers. No patient was paid for participating or otherwise rewarded. They were informed that the reason for doing the study was to help them optimize their own therapy, to uncover issues which individually-tailored training could address and that the global information from the study would help them and other patients by providing an improved educational approach.
HbA1c values from the 18 centers were obtained from instruments which were calibrated every month and underwent an optical control every time they were moved. The instruments were qualified for use in the diagnosis of diabetes and were shown to give equivalent readings across centers.
Patients gave verbal consent to participate and the study was conducted in accordance with Good Clinical Practice, the clinical trials directives of the EU and the 1964 Declaration of Helsinki and its six revisions. The study started in January, 2012 when the nurses were trained. Patient recruitment began in February, 2012 and lasted to April, 2012 and 3-month follow up lasted from May, 2012 to July, 2012.
SPSS software (IBM Corporation, Armonk NY, USA, version 19) was used to analyze the data. Mean, median, standard deviation, minimum, maximum and standard error around the mean were measured for the entire population. Comparisons of parameters were performed using χ-square, ANOVA, log linear models and multivariate analysis. The threshold for statistical significance was α = 0.05.
Results
A total of 346 patients were included in the study. Demographic data are given in Table 3A, Table 3B and the key clinical parameter changes in Table 4A, Table 4B.
Table 3A.
Overall patient demographics
| N | Mean | Std. deviation | Minimum | Maximum | |
|---|---|---|---|---|---|
| Age (years) | 346 | 55.5 | 18.6 | 11.0 | 85.0 |
| Age at insulin inception (years) | 325 | 42.2 | 21.4 | 1.0 | 80.0 |
| Years on insulin | 332 | 13.0 | 9.8 | 0.5 | 50.0 |
| Injections/Day | 342 | 3.71 | 0.89 | 1.0 | 7.0 |
Table 3B.
Parameters at study entry
| N | % | |
|---|---|---|
| Females/Males | 166/176 | 48.1/51.9 |
| Visible lipohypertrophy | 124 | 35.7 |
| Visible lipoatrophy | 18 | 5.2 |
| Palpable lipohypertrophy | 159 | 45.8 |
| Total lipohypertrophya | 169 | 48.7 |
| 5 mm needle used | 111 | 33.3 |
| 6 mm needle used | 139 | 41.7 |
| 8 mm needle used | 79 | 23.7 |
| 12.7 mm needle used | 4 | 1.2 |
| Abdomen used primarily for injections | 163 | 47.0 |
| Thigh used primarily for injections | 83 | 23.9 |
| Buttocks used primarily for injections | 14 | 4.0 |
| Arm used primarily for injections | 79 | 21.8 |
Those with both visible and palpable lipohypertrophy are only counted once.
Table 4A.
Clinical parameters at study entry and at three months
| Clinical parameter | n | Mean | Δ | SD | Minimum | Maximum |
|---|---|---|---|---|---|---|
| HbA1c at entry | 346 | 8.49 | 2.86 | 5.2 | 14.0 | |
| HbA1c at 3 months | 259 | 7.91 | −0.58* | 1.30 | 5.1 | 14.1 |
| FBG (mg/dL)at entry | 249 | 186.7 | 49.9 | 90 | 410 | |
| FBG (mg/dL)at 3 months | 182 | 172.5 | −14.2* | 42.3 | 81 | 358 |
| TDD (IU) insulin at entry | 326 | 50.5 | 24.7 | 9 | 159 | |
| TDD (IU) insulin at 3 months | 256 | 48.5 | −2.0* | 24.8 | 9 | 150 |
| BMIa at entry | 304 | 28.2 | 7.77 | 17.0 | 103.0 | |
| BMI at 3 months | 235 | 27.7 | −0.5 | 8.20 | 16.5 | 102.0 |
*p < 0.05.
BMI = height (in meters)/(weight in kg)2.
Table 4B.
Injection parameters at study entry and at three months
| Practice parameter | N | % | Δ in % |
|---|---|---|---|
| Use of pinch up at entry | 121 | 34.9 | |
| Use of pinch up at 3 monthsa | 31 | 8.9 | −26.0* |
| <5 s dwell time after injection at entryb | 133 | 38.3 | |
| <5 s dwell time after injection at 3 months | 21 | 6.1 | −32.2* |
| 5–10 s dwell time after injection at entry | 193 | 55.6 | |
| 5–10 s dwell time after injection at 3 months | 125 | 36.0 | −19.6* |
| >10 s dwell time after injection at entry | 50 | 16.7 | |
| >10 s dwell time after injection at 3 months | 162 | 46.7 | +30.0* |
| Use needle only once at entry | 294 | 84.7 | |
| Use needle only once at 3 months | 301 | 86.7 | +2.0 |
| Consider injection technique VERY IMPORTANT at entry | 139 | 40.1 | |
| Consider injection technique VERY IMPORTANT at 3 months | 224 | 64.6 | +24.5* |
| Consider injection technique IMPORTANT at entry | 151 | 43.5 | |
| Consider injection technique IMPORTANT at 3 months | 68 | 19.6 | −23.9* |
| Consider injection technique SLIGHTLY IMPORTANT at entry | 39 | 11.2 | |
| Consider injection technique SLIGHTLY IMPORTANT at 3 months | 9 | 2.6 | −8.6 |
| Consider injection technique NOT IMPORTANT at entry | 13 | 3.7 | |
| Consider injection technique NOT IMPORTANT at 3 months | 6 | 1.7 | −2.0 |
| VERY HAPPY with current needle at entry | 255 | 73.5 | |
| VERY HAPPY with the 4 mm needle at 3 months | 314 | 88.9 | +15.4* |
| OK with current needle at entry | 82 | 23.6 | |
| OK with the 4 mm needle at 3 months | 31 | 8.9 | −14.7* |
| UNHAPPY with current needle at entry | 5 | 1.4 | |
| UNHAPPY with the 4 mm needle at 3 months | 3 | 0.9 | −0.5 |
*p < 0.05.
Pinching up the skin is not necessary with a 4 mm pen needle for patients older than 6 years (all subjects in this study).
Best practice is a dwell time of >10 s, next best is 5–10 s and worst is <5 s.
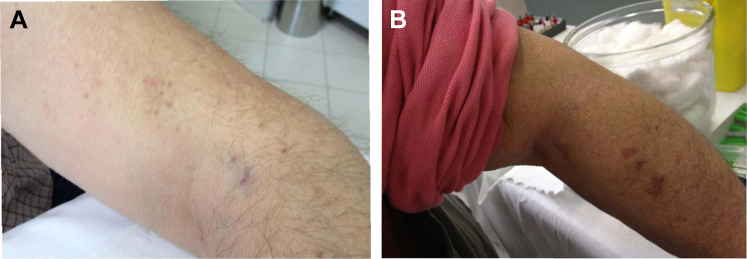
Age and gender parameters, years on insulin, total daily doses (TDD) of insulin and injections/day were all in keeping with previous studies in patients with diabetes 2, 4, 11, 12. At study entry patients were found to be using a variety of needle lengths, with a majority on PN ≤ 6 mm long; none used the 4 mm PN. Most patients used the four conventional injection sites (abdomen, thighs, upper arm and buttocks) although the buttock was used much less frequently than the others. A number of patients were found to be injecting in sites other than these four. Figure 1 illustrates some of these unusual practices which were addressed during individualized training.
Figure 1.
Injections into unconventional sites: Patient A, into the elbow region (see cluster of needle marks) and Patient B into the forearm (see bruises).
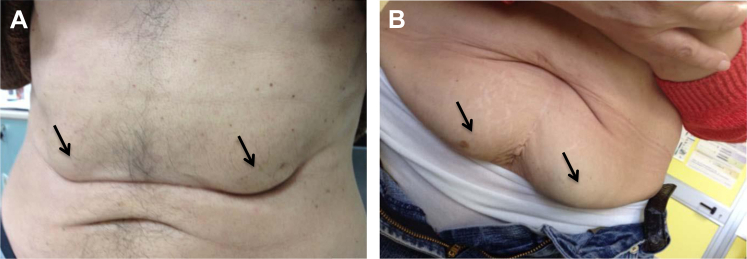
By visual inspection at study entry nurses found LH in 35.7% of patients and by palpation, in 45.8% (Figure 2). The overall LH percentage when visual and touch evaluations were combined was 48.7%. Those with both visible and palpable LH were only counted once in determining the latter percentage.
Figure 2.
Examples of visible lipohypertrophy (A. Bilateral upper abdomen; B. Bilateral lower abdomen); see arrows.
After three months, patients had mean reductions in HbA1c of 0.58% (0.50%–0.66%, 95% CI), in fasting blood glucose of 14 mg/dL (10.2–17.8 mg/dL, 95% CI) and in total daily insulin dose of 2.0 IU (1.4–2.5 IU, 95% CI) with a p < 0.05 for all differences (Table 4A, Table 4BA). Body mass index (BMI) decreased slightly over the three months of the study but this change was not statistically significant. LH rates were unchanged at three months. Follow-up HbA1c data were available on only 259 of the 346 subjects (74.9%) included at baseline (Table 1). However analysis of the demographic and clinical data on the 259 was compared to that of the 346 and similar results were obtained for HbA1c, fasting blood glucose and total insulin doses.
Patients were found to be using more optimal IT at the end of three months (Table 4A, Table 4BB). Specifically, most patients had abandoned the pinch-up technique when using the 4 mm PN. There was a significant shift in favor of longer ‘in-dwell’ times, with more patients leaving the needle under the skin for a full 10 s after depressing the plunger. Proper IT was rated as important or very important by significantly higher percentages of patients than at study initiation. And a majority of patients indicated high levels of satisfaction with the 4 mm PN. Needle reuse was not common at the start of the study (∼15% of subjects) and did not change significantly by the end of the three-month period.
Discussion
This study investigated the effects of an integrated IT educational approach which included general and targeted training, advice on rotation of sites and needle reuse as well as a switch to the shortest PN (4 mm). All patients underwent a multimodal intervention (training, needle length, questionnaire), thus retrospectively it is impossible to determine with certainty which was the causative component of the intervention. It is very possible that the combination of all three interventions was effective. We found that this approach can lead to improved glucose control, greater satisfaction with therapy and lower daily consumption of insulin over a relatively short period of time. These positive results could contribute to better therapy adherence and, if sustained, improved long-term clinical outcomes.
The glucose control improvements seen in our study differ from results in the Swansdown Survey [1] conducted in the same centers several months before. In the latter no improvements in control were seen although a majority of patients exhibited better understanding of injection technique. This difference may be explained by the nature of the intervention. In Swansdown there was a general education session regarding injection technique given once to patients, while in our study the same general education was given but a questionnaire was also used to identify specific patient needs. These were then addressed using a tailored individualized approach. All patients were assessed for LH and moved to healthy sites if they had LH and were injecting into it. And all patients were switched to a 4 mm needle in order to facilitate wider site rotation without increasing the risk of IM injections. It is this mosaic of interventions which, we feel, made the difference, and which we now recommend to others.
The percentage of our patients with LH (48.7%) was similar to that found in three other published studies 11, 13, 14. Other demographic results were also similar suggesting that our patient population is typical of others on a worldwide basis; hence we believe our findings can be widely applied.
A series of clinical trials evaluating different needle lengths and gauges 12, 15, 16 have shown advantages of shorter, finer-gauge needles over longer ones (principally in quality of life measures such as reduced pain and patient preference), but such studies have never shown an improvement in glucose control; all such studies have been of a non-inferiority design. Several studies have also shown a reduction in the frequency of IM injections when using shorter needles 7, 8, 17, 18 therefore it stood to reason that clinical parameters such as improved HbA1c might eventually be found to be associated with these needles. To our knowledge our study is the first to do so.
Traditionally the choice of needle length has been based on local habits or economic factors, or, at best, on an assessment of a patient's body habitus. However recent studies looking directly at the anatomy of the skin and SC tissue using ultrasound 8, 19, 20 have provided precise data on patients with diabetes and have made the choice of needle length more evidence-based. The skin thickness of adults [20] and children with diabetes [5] is approximately 1.6–2.5 mm, even in obese patients [19]. Therefore, a 4-mm needle is more than long enough to pass through the skin and enter the SC space.
However the distance from the skin surface to the muscle varies widely both from one person to another and within the usual injection sites in a single individual 8, 14, 19, 20. These results have given pause to those who once advocated routinely using longer needles (≥8 mm). In many normal to lean adults and in most children there is a substantial risk of IM injections with such needles. Even in obese patients who inject into the limbs (arms or legs) such risks are not trivial. Hence we have seen in recent years a shift to shorter needles. This was the rationale of ANIED in shifting all injecting patients in the Piedmont region of northern Italy over to the 4 mm PN, in the context of an educational IT intervention.
The 4 mm PN has been tested in a number of clinical trials to date 2, 3, 4, 6, 9 and has proven its safety and efficacy in both adults and children, as well as in persons spanning a range of BMIs, including in the obese. Hirsch et al. [2], for example, showed similar glycemic control (as measured by fructosamine values) during all stages of a 3-week two-period crossover study that compared the BD 32-gauge, 4-mm insulin PN with 31-gauge 5-mm and 31-gauge 8-mm needles [2]. In a follow-up randomized, controlled crossover study with 3-month treatment periods using HbA1c to measure control, the same results with the 4 mm PN were confirmed in an obese (BMI ≥ 30 kg/m2) diabetic population, many taking very large insulin doses 21, 22.
The latest consensus injection recommendations [10] state that 4 mm PN ‘may be used by any adult patient including obese ones and do not generally require the lifting of a skin fold’ and that children and adolescents ‘should use’ such needles. We found that educational interventions regarding IT coupled with a switch to the 4 mm PN are associated with improved glucose control in a relatively short period of time.
The weight of each of these interventions in the overall outcomes was not evaluated. Limitations of our study include the lack of a control group and the sequential nature (pre-post) of our analyses; furthermore, we do not yet have long-term data. We did not include a control group in this study because we felt obliged to give the same best-practice advice to all our patients. In fact IT review and a switch to a shorter needle is standard practice by the nurses and doctors in the participating centers. Lack of a control prevents absolute certainty that the 4 mm needle and IT training were the exclusive causes of the clinical improvements we observed. Lack of chronic follow-up data limits our knowledge as to how sustainable these improvements might be.
Another important limitation is that we have follow-up HbA1c data on only 259 of the 346 subjects (74.9%) included at baseline. Nevertheless, analysis of the data on the 259 was compared to that of the 346 and similar results were obtained, suggesting that results from the former can be extrapolated to the latter. Hypoglycemia was not assessed because of our inability to certify glucose values when hypoglycemic symptoms appeared; not all patients were using meters. Our findings however warrant further study in a prospective, randomized controlled clinical investigation. Follow-up for this trial was only planned for 3 months, but it has continued and will be the subject of an additional analysis and possible future paper.
The implications of our study are striking. Patients and professionals do not have to wait for months and years to see improvements in the most important clinical parameters when appropriate IT training and devices are provided. These improvements can be expected early enough in the course of insulin therapy to provide motivation for continuous improvement.
Acknowledgments
The authors wish to thank the ANIED Scientific Board—Dalmasso, Anna; Fiorito, Tiziana; Gaviglio, Donatella; Novo, Tommaso; Rosso, Germana; Scuntero, Paola; Trepiccioni, Rosalba. We would also like to thank each of the nurses from the participating centers—Alberti, Lidia; Albertini, Oriana; Araudo, Liliana; Baucero, M. Grazia; Bergonzini, Carla; Bertello, Stefania; Bono, Maddalena; Bosoni, Simona; Bragnuolo, Luisa; Bussone, Loredana; Campione, Gabriella; Cremonte, M. Rosa; Croce, Mariella; Curti, Fernanda; Cussotto, Giuseppina; De Murtas, Pietro; De Rossi, Cinzia; Farinetti, Marisa; Folchi, Luisa; Francioli, M. Grazia; Grillo, Nuccia; Guida, Danila; Incatasciato, Giovanna; Ingaramo, Anna; Lobina, Nadia; Lorenzini, Anna; Macrí, Katia; Maio, Paola; Maresca, Margherita; Massobrio, Luciana; Matteazzi, M. Teresa; Nistor, Marcella; Olivetti, Ombretta; Palenzona, Clara; Panero, Marina; Parrella, Ines; Pellanda, Sofia; Pellazza, Manuela; Peretto, Laura; Perlo, Barbara; Piana, Margherita; Piccirillo, Stefania; Rivabella, Ornella; Scaccabarozzi, Paola; Scognamiglio, Antonietta; Sordo, Mariella; Tagliente, Bruna; Torrisi, Loredana; Zizzari, Vittoria. Finally the authors would like to thank all the patients. We are also grateful to Dr. Laurence Hirsch for his expert review of the manuscript.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Conflict of interest: None declared.
Funding: No funding received.
References
- 1.Swansdown: la prima indagine piemontese sulla tecnica di iniezione. Una corretta tecnica di iniezione è importante quanto la terapia per ottenere un buon controllo glicemico. Poster presented at the 11th National Congress AME (Association of Endocrinologists). Update in Clinical Endocrinology, Nurse session, 14–16 October 2011, Udine.
- 2.Hirsch L.J., Gibney M.A., Albanese J., Qu S., Kassler-Taub K., Klaff L.J. Comparative glycemic control, safety and patient ratings for a new 4mm· 32 G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26:1531–1541. doi: 10.1185/03007995.2010.482499. [DOI] [PubMed] [Google Scholar]
- 3.Miwa T., Itoh R., Kobayashi T., Tanabe T., Shikuma J., Takahashi T. Comparison of the effects of a new 32 gauge – 4-mm pen needle and a 32-gauge – 6-mm pen needle on glycemic control, safety, and patient ratings in Japanese adults with diabetes. Diabetes Technol Ther. 2012;14:1084–1090. doi: 10.1089/dia.2012.0170. [DOI] [PubMed] [Google Scholar]
- 4.Nagai Y., Ohshige T., Arai K., Kobayashi H., Sada Y., Ohmori S. Comparison between shorter straight and thinner microtapered insulin injection needles. Diabetes Technol Ther. 2013;15:550–555. doi: 10.1089/dia.2012.0334. [DOI] [PubMed] [Google Scholar]
- 5.Lo Presti D., Ingegnosi C., Strauss K. Skin and subcutaneous thickness at injecting sites in children with diabetes: ultrasound findings and recommendations for giving injection. Pediatr Diabetes. 2012;13:525–533. doi: 10.1111/j.1399-5448.2012.00865.x. [DOI] [PubMed] [Google Scholar]
- 6.Hirose T., Ogihara T., Tozaka S., Kanderian S., Watada H. Identification and comparison of insulin pharmacokinetics injected with a new 4-mm needle vs 6- and 8-mm needles accounting for endogenous insulin and C-peptide secretion kinetics in non-diabetic adult males. J Diabetes Invest. 2013;10:1–10. doi: 10.1111/jdi.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birkebaek N., Solvig J., Hansen B., Jorgensen C., Smedegaard J., Christiansen J. A 4 mm needle reduces the risk of intramuscular injections without increasing backflow to skin surface in lean diabetic children and adults. Diabetes Care. 2008;22:e65. doi: 10.2337/dc08-0977. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch L., Gibney M. Reducing intramuscular (IM) injection risk: evidence-based recommendations. Diabetes. 2012;61(Suppl. 1):A224. Abstract presented at the American Diabetes Association annual meeting, Philadelphia, USA, June 2012. [Google Scholar]
- 9.Hirsch L.J., Gibney M.A., Li L., Bérubé J. Glycemic control, reported pain and leakage with a 4mm x 32G pen needle in obese and non-obese adults with diabetes: a post hoc analysis. Curr Med Res Opin. 2012;28:1305–1311. doi: 10.1185/03007995.2012.709181. [DOI] [PubMed] [Google Scholar]
- 10.Frid A., Hirsch L., Gaspar R., Hicks D., Kreugel G., Liersch J. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36:S3–S18. doi: 10.1016/S1262-3636(10)70002-1. [DOI] [PubMed] [Google Scholar]
- 11.Vardar B., Kizilci S. Incidence of lipohypertrophy in diabetic patients and a study of influencing factors. Diabetes Res Clin Pract. 2007;77:231–236. doi: 10.1016/j.diabres.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz S., Hassman D., Shelmet J., Sievers R., Weinstein R., Liang J. A multicenter, open-label, randomized, two-period crossover trial comparing glycemic control, satisfaction, and preference achieved with a 31 gauge – 6-mm needle versus a 29 gauge – 12.7-mm needle in obese patients with diabetes mellitus. Clin Ther. 2004;26:1663–1678. doi: 10.1016/j.clinthera.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 13.De Coninck C., Frid A., Gaspar R., Hicks D., Hirsch L., Kreugel G. Results and analysis of the 2008–2009 Insulin Injection Technique Questionnaire survey. J Diabetes. 2010;2:168–179. doi: 10.1111/j.1753-0407.2010.00077.x. [DOI] [PubMed] [Google Scholar]
- 14.Blanco M., Hernández M.T., Strauss K.W., Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445–453. doi: 10.1016/j.diabet.2013.05.006. (Epub 2013 Jul 22) [DOI] [PubMed] [Google Scholar]
- 15.Iwanaga M., Kamoi K. Patient perceptions of injection pain and anxiety: a comparison of NovoFine 32-gauge tip 6mm and Micro Fine Plus 31-gauge 5mm needles. Diabetes Technol Ther. 2009;11:81–86. doi: 10.1089/dia.2008.0027. [DOI] [PubMed] [Google Scholar]
- 16.Kreugel G., Keers J.C., Kerstens M.N., Wolffenbuttel B.H.R. Randomized trial on the influence of the length of two insulin pen needles on glycemic control and patient preference in obese patients with diabetes. Diabetes Technol Ther. 2011;13(7):737–741. doi: 10.1089/dia.2011.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tubiana-Rufi N., Belarbi N., Du Pasquier-Fediaevsky L., Polak M., Kakou B., Leridon L. Short needles (8mm) reduce the risk of intramuscular injections in children with type 1 diabetes. Diabetes Care. 1999;22:1621–1625. doi: 10.2337/diacare.22.10.1621. [DOI] [PubMed] [Google Scholar]
- 18.Hofman P.L., Derraik J.G., Pinto T.E., Tregurtha S., Faherty A., Peart J.M. Defining the ideal injection techniques when using 5-mm needles in children and adults. Diabetes Care. 2010;33:1940–1944. doi: 10.2337/dc10-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibney M.A., Arce C.H., Byron K.J., Hirsch L.J. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26:1519–1530. doi: 10.1185/03007995.2010.481203. [DOI] [PubMed] [Google Scholar]
- 20.Laurent A., Mistretta F., Bottigioli D., Dahel K., Goujon C., Nicolas J.F. Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine. 2007;25:6423–6430. doi: 10.1016/j.vaccine.2007.05.046. [DOI] [PubMed] [Google Scholar]
- 21.Bergenstal R., Strock E., Peremislov D., Parvu V., Gibney M., Hirsch L. Insulin therapy with a 4mm x 32G pen needle vs larger needles in obese subjects. Diabetes. 2013;62(Suppl. 1):A250. doi: 10.1016/j.mayocp.2014.12.014. Abstract presented at the American Diabetes Association annual meeting, Chicago, USA, June 21–24, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Strock E., Bergenstal R., Parvu V., Hirsch L., Gibney M. Glycemic control, pain and leakage with 4mm vs larger pen needles in obese patients treated with lantus® or high insulin doses: pre-specified subgroup analyses. Diabetes. 2013;62(Suppl. 1):A255. Abstract presented at the American Diabetes Association annual meeting, Chicago, USA, June 2013. [Google Scholar]