Abstract
Background
It is well documented that overt hypothyroidism is associated with adverse pregnancy outcomes, but studies of subclinical hypothyroidism have demonstrated conflicting results.
Objective
Thyroid hormones are known to regulate mitochondrial function, and the aim of this study was to examine the possible relationship of subclinical hypothyroidism and mitochondrial dysfunction to adverse pregnancy outcomes in pregnant women.
Methods
Women in their third trimester of pregnancy (n = 113) who did not receive thyroid medication were included in this cross-sectional study. All participants were interviewed, and their thyroid status was determined. All participants had concentrations of thyroid hormones (fT4 and tT3) within the reference range. In addition to thyroid status, mitochondrial membrane potential (MMP) and reactive oxygen species (ROS) were measured by flow cytometry. To establish a reference range of MMP and ROS, a group of euthyroid, nonpregnant women were used as euthyroid controls. Adverse pregnancy outcome was defined as preterm delivery, preeclampsia, placental abruption, Apgar score <7 points 1 minute after birth, or postpartum hemorrhage.
Results
The prevalence of subclinical hypothyroidism among pregnant women was 17% (n = 19), and the number of overall adverse pregnancy outcomes was increased (p = 0.02) compared with that in euthyroid pregnant women. Preeclampsia, poor Apgar score, and postpartum hemorrhage were more frequent in the subclinical hypothyroidism group than in the euthyroid group (p = 0.04, p = 0.001 and p = 0.03, respectively), and more women showed prolonged gestation and gave birth later than 41 weeks of gestation than in the euthyroid group (p = 0.04). Compared with euthyroid, nonpregnant controls, a physiological upregulation of mitochondrial function was observed in euthyroid pregnant women. This was impaired in pregnant women with subclinical hypothyroidism. Compared with euthyroid, nonpregnant controls, pregnant women had increased ROS regardless of their thyroid status.
Conclusion
We speculate that the unfavorable effects on mitochondrial function in women with subclinical hypothyroidism may be associated with higher prevalence of adverse pregnancy outcomes.
Keywords: Subclinical hypothyroidism, Pregnancy outcome, Mitochondrial dysfunction, Mitochondrial membrane potential, Reactive oxygen species
Abbreviations: TSH, thyroid-stimulating hormone; TPOAb, thyroid peroxidase antibody; fT4, free thyroxine; tT3, total triiodothyronine; TMRM, tetramethylrhodamine methyl ester; ROS, reactive oxygen species; PBMC, peripheral blood mononuclear cells; MMP, mitochondrial membrane potential; carboxy-H2DCFDA, 5(6)-carboxy-2'-7'-dichlorodihydrofluoresceindiacetate; GA, gestational age; BMI, body mass index
Introduction
Subclinical hypothyroidism is defined as increased level of thyroid-stimulating hormone (TSH) and levels of thyroid hormones within the reference range. Subclinical hypothyroidism is the most frequent thyroid disease during pregnancy, compared with overt hypothyroidism and overt and subclinical hyperthyroidism [1]. Depending on the cut-off values used for the definition of subclinical hypothyroidism, ethnicity, and study design, the reported prevalence varies between 1.5% and 4% [2], [3], [4], [5]. It is well documented that overt hypothyroidism is associated with increased risk of obstetric complications such as miscarriage, gestational hypertension, preterm delivery, stillbirths, and perinatal deaths [6], [7], [8]. However, although studies of maternal subclinical hypothyroidism have also suggested a relation to adverse effects [9], [10], [11], this relation is not as well established.
A number of cellular functions are regulated by thyroid hormones, and one major function is that of mitochondrial energy production and biogenesis [12]. Several studies have reported impaired thyroid hormone-regulated mitochondrial function in patients with subclinical hypothyroidism [13], [14], [15].
The aim of the present study was to examine pregnancy outcomes in women with subclinical hypothyroidism, and the relation to a possible mitochondrial dysfunction, as well as to examine whether mitochondrial function was influenced by pregnancy.
Material and methods
Women in their third trimester of pregnancy who consulted the Department of Obstetrics, Naestved Hospital, Region Zealand, Denmark, between June 2012 and January 2013 were included.
Women with known thyroid disease, diabetes, and those receiving any medical treatment for thyroid disease were excluded. In total, 115 pregnant women (all of Caucasian ethnicity) were included. Two women were subsequently excluded because they gave birth at home and their data were unavailable. The remaining 113 data sets were complete.
All participants were interviewed to ensure full investigation of their obstetric history. Body mass index (BMI) before pregnancy was obtained from general practitioner records.
All women were examined during the first trimester, and gestational age (GA) was determined by ultrasound. GA at delivery, placental abruption, preeclampsia, cesarean section, postpartum hemorrhage, Apgar score, birth weight, and any severe event occurring during the period from inclusion in the study until delivery were obtained from hospital records.
Adverse pregnancy outcome was defined as preterm delivery (GA <37 weeks), preeclampsia, placental abruption, Apgar score <7 points 1 minute after birth, postpartum hemorrhage (>500 mL), or delivery later than 41 weeks after gestation. Preeclampsia was defined as persistent blood pressure ≥140/90 mm Hg accompanied by proteinuria >300 mg/24 h or ++ proteinuria determined by urinary dipstick analysis (Multistix 7®, Siemens Healthcare, Tarrytown, NY, USA). The definition of adverse pregnancy outcome was based on former studies of hypothyroidism during pregnancy, and all criteria carry potential risks of increased mortality and morbidity.
To ensure that the control group of age-matched, euthyroid, nonpregnant women (n = 42) had not consulted the hospital regarding emergency or chronic disease, participants were recruited from a population study performed at Naestved Hospital during the same period [16]. The control group was included to establish the normal reference range of mitochondrial membrane potential (MMP) and production of reactive oxygen species (ROS). All laboratory tests were performed at the same laboratory using the same assays, and all nonpregnant euthyroid women had values of TSH, thyroid peroxidase antibody (TPOAb), and thyroid hormones within the reference ranges.
Biochemical variables
Measurements of TSH and thyroid hormones fT4 and tT3 were performed using an electrochemical luminescent immunoassay (Roche Cobas 6000, Basel, Switzerland). TPOAb was measured by KRYPTOR antiTPOn (BRAHMS, Hennigsdorf, Germany), detection limit 10 U/mL.
Normal values for thyroid hormones followed the standard references used at the Central Laboratory of Naestved Hospital: fT4 = 10.0–26.0 pmol/L and tT3 = 1.20–2.80 nmol/L. This reference range also covers the trimester-specific alterations (decrease of fT4 and rise of tT3 within the reference range).
Subclinical hypothyroidism was defined as raised serum concentration of TSH ≥3.40 mU/L (this cutoff value was chosen based on recommendations from the testing laboratory at the hospital) and thyroid hormones within the reference range. Thyroid peroxidase antibody positivity was defined by the cutoff value of TPOAb >60 U/mL.
Measurement of mitochondrial function
MMP and ROS production were measured using flow cytometry as previously described [15], [17], [18]. In brief, peripheral blood mononuclear cells (PBMC) were obtained from a freshly drawn blood sample and stained with 0.1 μmol/L tetramethylrhodamine methyl ester (TMRM) or 1 μM 5(6)-carboxy-2'-7'-dichlorodihydrofluoresceindiacetate (carboxy-H2DCFDA) (Invitrogen A/S, Taastrup, Denmark). The cells were incubated in the dark for 30 min at room temperature for TMRM and at 37 °C for carboxy-H2DCFDA. The cells were chilled on ice and immediately measured by flow cytometry (Accuri C6, BD Biosciences, Franklin Lakes, NJ, USA). A total of 20,000 leukocytes were acquired, and the lymphocytes were identified on the forward- and side-scatter parameters. The median TMRM and carboxy-H2DCFDA fluorescence intensities of PBMC were calculated using BD Accuri C6 software, and values were given as relative emission (arbitrary units, a.u.). The intra-assay variation of TMRM and carboxy-H2DCFDA measurements was <12%.
Because of the time-consuming procedure of performing flow cytometry on fresh blood specimens, the number of participants was limited.
Statistics
The Student's t-test or Mann–Whitney test was used to compare cases and controls. The Shapiro–Wilk test was used to test normality of the distribution of data, and the Wilcoxon signed-rank test was used to compare paired data. The discontinuous variables were compared using χ2-test. The Spearman correlation coefficient was used to evaluate the correlation of the variables. A value of p < 0.05 was considered statistically significant. All statistical analyses were performed using STATA version 11 for Windows (StataCorp, College Station, TX, USA).
Ethical considerations
The study was approved by the Regional Research Ethics Committee of Zealand, Denmark (Reg. no. RVK SJ-294). The clinical trial registration at ClinicalTrial.gov is NCT01335802.
Written consent was obtained from all participants before inclusion in the study, and the study conformed to the principles of the Declaration of Helsinki.
Results
Euthyroid pregnant women versus euthyroid nonpregnant women
The trimester-specific fT4 decreased and tT3 increased, although both hormones remained within the reference range. There was no difference in TSH between the pregnant and nonpregnant euthyroid groups.
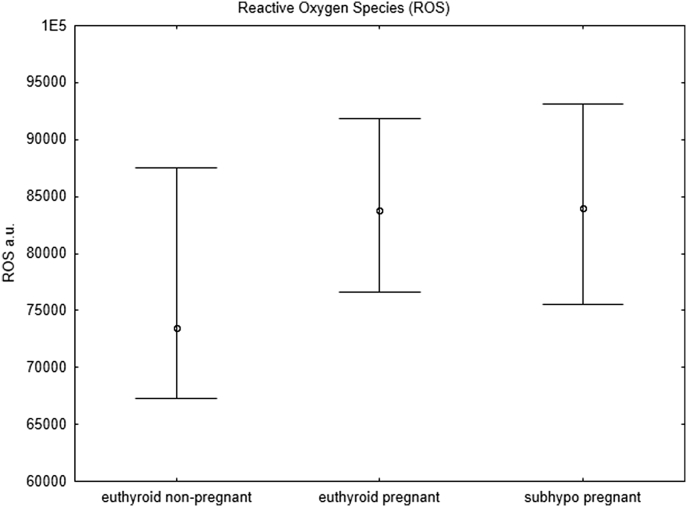
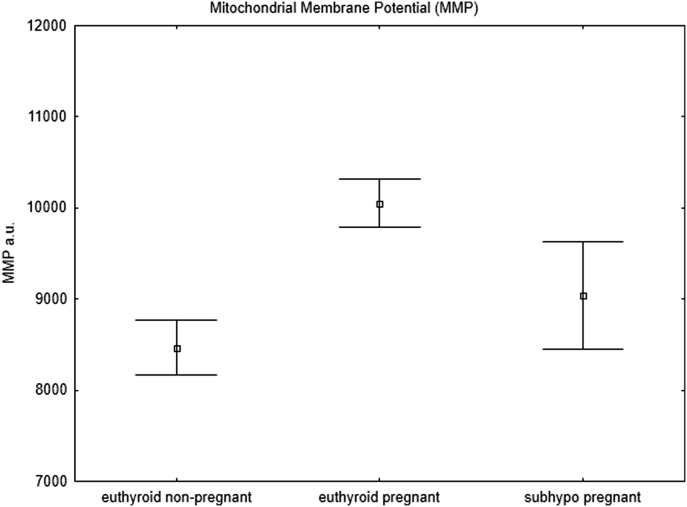
The mitochondrial parameters were also significantly different in the pregnant and nonpregnant euthyroid groups, which may represent a physiological adaptation to pregnancy. Both ROS (p = 0.002) (Figure 1) and MMP (p = 0.003) (Figure 2) were significantly increased in the euthyroid pregnant women, which in our opinion represents a physiological adaptation to pregnancy and to our knowledge has not been reported previously.
Figure 1.
Reactive oxygen species. Flow cytometry analysis of reactive oxygen species (ROS) in pregnant women with subclinical hypothyroidism (subhypo pregnant, n = 19), euthyroid pregnant women (n = 94), and euthyroid non-pregnant women (controls, n = 42). All points represent median values and 25th–75th percentiles (Mann–Whitney test).
Figure 2.
Mitochondrial membrane potential. Flow cytometry analysis of mitochondrial membrane potential (MMP) in pregnant women with subclinical hypothyroidism (subhypo pregnant, n = 19), euthyroid pregnant women (n = 94), and euthyroid non-pregnant women (controls, n = 42). All points represent mean values and standard errors (Student's t-test).
Pregnant women with subclinical hypothyroidism versus euthyroid pregnant women
Nineteen of the 113 pregnant women (17%) had subclinical hypothyroidism. The mean gestational age at inclusion was 257 days. As can be seen in Table 1, no differences were present between the subclinical hypothyroidism group and the euthyroid group regarding age, prepregnancy BMI, or parity. Significantly higher numbers of overall adverse pregnancy outcomes were observed among women with subclinical hypothyroidism than among euthyroid women (p = 0.02).
Table 1.
Clinical data of 113 pregnant women with or without subclinical hypothyroidism and pregnancy outcome
| Subclinical hypothyroidism |
Euthyroid |
p-value | |
|---|---|---|---|
| n = 19 (17%) | n = 94 (83%) | ||
| Characteristics | |||
| Age (years) | |||
| Mean (range) | 30 (21–41) | 29 (19–42) | 0.33a |
| BMI before pregnancy (kg/m2) | |||
| Mean (range) | 28 (22–46) | 26 (17–44) | 0.32b |
| Parity = 0 (n) | 10 (53%) | 43 (46%) | 0.58c |
| Complications | |||
| Preeclampsia (n) | 6 (32%) | 12 (13%) | 0.04c |
| Preterm delivery GA <37 (n) | 2 (5%) | 9 (10%) | 0.90c |
| Delivery GA >41 (n) | 7 (37%) | 15 (16%) | 0.04c |
| Placental abruption (n) | 0 (0%) | 1 (1%) | 0.65c |
| Caesarean section (n) | 8 (42%) | 37 (39%) | 0.82c |
| Postpartum hemorrhage >500 mL (n) | 10 (53%) | 26 (28%) | 0.03c |
| Apgar <7/1 min (n) | 5 (26%) | 4 (4%) | 0.001c |
| Birth weight (g) | |||
| Mean (range) | 3450 (1201–4500) | 3360 (1435–4700) | 0.39b |
| Neonatal intensive care unit (n) | 4 (21%) | 11(12%) | 0.27c |
| Adverse pregnancy outcome (n) | 15 (79%) | 47 (50%) | 0.02c |
| Biochemical | |||
| TSH (mU/L) | |||
| Median (IQR) | 4.2 (3.6–5.6) | 1.8 (1.1–2.3) | <0.001b |
| tT3 (nmol/L) | |||
| Median (IQR) | 2.67 (2.23–3.20) | 2.70 (2.34–2.92) | 0.80b |
| fT4 (pmol/L) | |||
| Median (IQR) | 11.4 (10.5–12.9) | 11.2 (10.2–12.8) | 0.71b |
| TPOAb positive (n) | 1 (5%) | 7 (7%) | 0.74c |
Subclinical hypothyroidism was defined by TSH (thyroid stimulating hormone) ≥3.4 mU/L and levels of tT3 (total triiodothyronine) and fT4 (free thyroxine) within the reference range. TPOAb positivity was defined by thyroid peroxidase antibody >60 U/mL. Values are mean (range) or median (IQR: interquartile range).
Student's t-test was used to compare age as these are considered normally distributed data.
Mann–Whitney test was used to compare biochemical values, BMI and birth weight.
Frequencies are noted as numbers (percent) and χ2-test was used to compare data. Boldface indicates p < 0.05.
Table 1 shows that the adverse outcomes were attributed to preeclampsia, postpartum hemorrhage, and higher numbers of newborns with Apgar score <7 points 1 minute after birth. In addition, women with subclinical hypothyroidism had higher frequencies of prolonged gestation (gestational age >41 weeks at delivery). In this study, no differences were observed in the number of preterm deliveries, caesarean sections, or birth weights related to gestational age.
Mitochondrial function
Pregnant women with subclinical hypothyroidism had significantly decreased MMP (Figure 2) compared with that of euthyroid pregnant women (p = 0.037), whereas no difference in ROS was observed between these two groups (p = 0.93) (Figure 1).
The relationship between tT4 and the mitochondrial parameters MMP and ROS was examined in euthyroid pregnant women and pregnant women with subclinical hypothyroidism. A significant correlation between MMP of TMRM-stained PBMCs and fT4 (r = −0.25, p = 0.03) and of ROS and fT4 (r = −0.28, p = 0.018) was observed in the euthyroid pregnant group, but no correlation was found in women with subclinical hypothyroidism (r = −0.29, p = 0.29 and r = 0.07, p = 0.78, respectively).
Discussion
In the present study we observed an association between subclinical hypothyroidism during the third trimester of pregnancy and adverse pregnancy outcomes. Mitochondrial function was altered during normal euthyroid pregnancy compared with that in nonpregnant women. However, this pregnancy-induced alteration was not seen in women with subclinical hypothyroidism.
The relation between subclinical hypothyroidism and adverse pregnancy outcomes has recently been a subject of discussion in the literature. Cleary-Goldman et al. concluded that there was no consistent pattern of adverse pregnancy outcomes in subclinical hypothyroid women [19]. In contrast, Wilson et al. reported that women with subclinical hypothyroidism identified during pregnancy had an increased risk of severe preeclampsia [20]. Our results also indicate an association between subclinical hypothyroidism and adverse pregnancy outcomes, although the number of participants in the present study necessarily was smaller than that used by Wilson et al. because of the comprehensive mitochondrial analyses.
Davis et al. reported a higher prevalence of postpartum hemorrhage in women with overt as well as subclinical hypothyroidism [21], but other studies found no association [22], [23]. We observed a significantly higher number of postpartum hemorrhages in women with subclinical hypothyroidism than in euthyroid women. In addition, the number of women with prolonged gestation (gestational age >41 weeks at delivery) was significantly higher in the group with subclinical hypothyroidism. The risk of maternal mortality and complications during birth is increased in post-term pregnancies independent of thyroid function [24]. Therefore, subclinical hypothyroidism carries an additional risk of birth complications due to prolonged gestation.
This study of an ethnically homogenous study population (Caucasian) found a higher prevalence of subclinical hypothyroidism than previously reported despite the fact that women with previous or present thyroid disease and those on antihypothyroid or antihyperthyroid medication were excluded from the study. In addition, we observed a higher frequency of adverse pregnancy outcomes such as preeclampsia, postpartum hemorrhage, poor Apgar score, and prolonged gestation in women with subclinical hypothyroidism. Finally, we present a possible link between the higher frequency of adverse pregnancy outcomes in women with subclinical hypothyroidism and changes in mitochondrial function.
The prevalence of subclinical hypothyroidism in pregnant women in their third trimester was 17%, which is higher than that found in other studies, which may be related to the mild iodine deficiency in Eastern Denmark that has been reported previously [25].
The cutoff value of TSH used in this study was 3.4 mU/L. However, because some recent studies have suggested use of the cutoff value 3.0 mU/L in third-trimester pregnant women, the data set was recalculated using this value. Although the prevalence of subclinical hypothyroidism was increased, adverse outcomes and mitochondrial function were not significantly changed. In consideration of this fact and because the department has previously conducted a number of studies of subclinical hypothyroidism using a cutoff value for TSH of 3.4 mU/L, it seems appropriate to maintain this cutoff value also in the present study. The higher prevalence of subclinical hypothyroidism found here may be caused by the fact that the inclusion took place at the department of obstetrics from patients who were visiting the department because they had symptoms of disease or other signs that needed evaluation by an obstetrician.
MMP reflects the functional state of mitochondria, as it is a driving force for ATP-synthesis. ROS are mainly generated by electron leaks from the electron transport chain and depend on MMP, as more ROS are produced when MMP is increased [26], [27].
In the present study we observed an increase in ROS in euthyroid pregnant women compared with euthyroid nonpregnant controls (p = 0.002). Since the increase is similar for euthyroid pregnant and subclinically hypothyroid pregnant women, we regard this result as a pregnancy-driven physiological regulation of mitochondrial function. Physiological adaptations in euthyroid pregnant women compensate for the increased ROS by increasing the MMP and thereby ensuring sufficient ATP production. Pregnant women with subclinical hypothyroidism did not present the expected MMP rise compared with that in euthyroid pregnant women and showed a significantly lower MMP. This observation suggests that the cells may not be able to compensate for the increased ROS and that ATP production may be insufficient. This conclusion is supported by the lack of correlation between fT4 and MMP in subclinical hypothyroid pregnant women and may provide a link to the adverse pregnancy outcomes observed.
Thyroid hormone regulates MMP [17]. In euthyroid pregnant women, we observed the expected inverse correlation between fT4 and the mitochondrial parameters MMP and ROS, which is likely caused by the fact that a product of deiodination of T4 (3',5'-diiodothyronine) stimulates MMP [17]. By contrast, no correlation was observed in the group with subclinical hypothyroidism, suggesting an impaired thyroid hormone regulation of the mitochondrial balance between ATP and ROS production.
Conclusion
In conclusion, we observed a physiological upregulation of mitochondrial function in euthyroid pregnant women, which was impaired in pregnant women with subclinical hypothyroidism. We hypothesize that unfavorable effects on mitochondrial function in women with subclinical hypothyroidism could be the link to the higher prevalence of adverse pregnancy outcomes due to insufficient ATP production.
Acknowledgments
The authors would like to thank technicians Carina Foldager and Jette Ellehauge at the Department of Clinical Biochemistry, Naestved Hospital, Region Zealand, Denmark for their skilled effort.
Funding statement: This study was supported by grants from Region Zealand’s Medical Research Foundation and the Medical Research Foundation of Hospital South, Region Zealand.
Footnotes
Conflicts of interest statement: The authors declare no conflicts of interest.
References
- 1.Cignini P., Cafa E.V., Giorlandino C., Capriglione S., Spata A., Dugo N. Thyroid physiology and common diseases in pregnancy: review of literature. J Prenat Med. 2012 October;6(4):64–71. [PMC free article] [PubMed] [Google Scholar]
- 2.Allan W.C., Haddow J.E., Palomaki G.E., Williams J.R., Mitchell M.L., Hermos R.J. Maternal thyroid deficiency and pregnancy complications: implications for population screening. J Med Screen. 2000;7(3):127–130. doi: 10.1136/jms.7.3.127. [DOI] [PubMed] [Google Scholar]
- 3.Vaidya B., Anthony S., Bilous M., Shields B., Drury J., Hutchison S. Detection of thyroid dysfunction in early pregnancy: Universal screening or targeted high-risk case finding? J Clin Endocrinol Metab. 2007 January;92(1):203–207. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 4.Casey B.M., Dashe J.S., Wells C.E., McIntire D.D., Byrd W., Leveno K.J. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005 February;105(2):239–245. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 5.Negro R., Mestman J.H. Thyroid disease in pregnancy. Best Pract Res Clin Endocrinol Metab. 2011 December;25(6):927–943. doi: 10.1016/j.beem.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Abalovich M., Gutierrez S., Alcaraz G., Maccallini G., Garcia A., Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12(1):63–68. doi: 10.1089/105072502753451986. [DOI] [PubMed] [Google Scholar]
- 7.Haddow J., Palomaki G., Allan W., Williams J., Knight G., Gagnon J. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999 August;341(8):549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 8.Leung A., Millar L.K., Koonings P.P., Montoro M., Mestman J.H. Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol. 1993 March;81(3):349–353. [PubMed] [Google Scholar]
- 9.Chang D.L., Pearce E.N. Screening for maternal thyroid dysfunction in pregnancy: a review of the clinical evidence and current guidelines. J Thyroid Res. 2013;2013:851326. doi: 10.1155/2013/851326. Epub 2013 May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey B.M., Leveno K.J. Thyroid disease in pregnancy. Obstet Gynecol. 2006 November;108(5):1283–1292. doi: 10.1097/01.AOG.0000244103.91597.c5. [DOI] [PubMed] [Google Scholar]
- 11.Behrooz H., Tohidi M., Mehrabi Y., Behrooz E., Tehranidoost M., Azizi F. Subclinical hypothyroidism in pregnancy: intellectual development of offspring. Thyroid. 2011 October;21(10):1143–1147. doi: 10.1089/thy.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weitzel J.M., Iwen K.A., Seitz H.J. Regulation of mitochondrial biogenesis by thyroid hormone. Exp Physiol. 2003 January;88(1):121–128. doi: 10.1113/eph8802506. [DOI] [PubMed] [Google Scholar]
- 13.Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008 February;18(2):141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- 14.Weitzel J.M., Iwen K.A. Coordination of mitochondrial biogenesis by thyroid hormone. Mol Cell Endocrinol. 2011 August 6;342(1–2):1–7. doi: 10.1016/j.mce.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 15.Kvetny J., Wilms L., Pedersen P.L., Larsen J. Subclinical hypothyroidism affects mitochondrial function. Horm Metab Res. 2010 May;42(5):324–327. doi: 10.1055/s-0030-1248261. [DOI] [PubMed] [Google Scholar]
- 16.Bergholdt H., Bathum L., Kvetny J., Rasmussen D., Bremmelgaard A., Moldow B. Study design, participation, and characteristics of The Danish General Suburban Population Study. Dan Med J. 2013 September;60(9):A4693. [PubMed] [Google Scholar]
- 17.Kvetny J., Bomholt T., Pedersen P., Wilms L., Anthonsen S., Larsen J. Thyroid hormone effect on human mitochondria measured by flow cytometry. Scand J Clin Lab Invest. 2009 August 12:1–5. doi: 10.3109/00365510903154752. [DOI] [PubMed] [Google Scholar]
- 18.Anthonsen S., Larsen J., Pedersen P.L., Dalgaard L.T., Kvetny J. Basal and T(3)-induced ROS production in lymphocyte mitochondria is increased in type 2 diabetic patients. Horm Metab Res. 2013 April;45(4):261–266. doi: 10.1055/s-0032-1327590. [DOI] [PubMed] [Google Scholar]
- 19.Cleary-Goldman J., Malone F.D., Lambert-Messerlian G., Sullivan L., Canick J., Porter T.F. Maternal thyroid hypofunction and pregnancy outcome. Obstet Gynecol. 2008 July;112(1):85–92. doi: 10.1097/AOG.0b013e3181788dd7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson K.L., Casey B.M., McIntire D.D., Halvorson L.M., Cunningham F.G. Subclinical thyroid disease and the incidence of hypertension in pregnancy. Obstet Gynecol. 2012 February;119(2 Pt 1):315–320. doi: 10.1097/AOG.0b013e318240de6a. [DOI] [PubMed] [Google Scholar]
- 21.Davis L.E., Leveno K.J., Cunningham F.G. Hypothyroidism complicating pregnancy. Obstet Gynecol. 1988 July;72(1):108–112. [PubMed] [Google Scholar]
- 22.Krassas G.E., Poppe K., Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010 October;31(5):702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 23.Matalon S., Sheiner E., Levy A., Mazor M., Wiznitzer A. Relationship of treated maternal hypothyroidism and perinatal outcome. J Reprod Med. 2006 January;51(1):59–63. [PubMed] [Google Scholar]
- 24.Caughey A.B., Stotland N.E., Washington A.E., Escobar G.J. Maternal complications of pregnancy increases beyond 40 weeks' gestation. Am J Obstet Gynecol. 2007 February;196(2):155.e1–155.e6. doi: 10.1016/j.ajog.2006.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurberg P., Jorgensen T., Perrild H., Ovesen L., Knudsen N., Pedersen I.B. The Danish investigation on iodine intake and thyroid disease, DanThyr: status and perspectives. Eur J Endocrinol. 2006 August;155(2):219–228. doi: 10.1530/eje.1.02210. [DOI] [PubMed] [Google Scholar]
- 26.Widlansky M.E., Wang J., Shenouda S.M., Hagen T.M., Smith A.R., Kizhakekuttu T.J. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res. 2010 July;156(1):15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korshunov S., Skulachev V., Starkov A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997 October;416(1):15–18. doi: 10.1016/s0014-5793(97)01159-9. [DOI] [PubMed] [Google Scholar]