Abstract
Patients with Turner syndrome (TS) require close medical follow-up and management for cardiac abnormalities, growth and reproductive issues. This review summarizes current controversies in this condition, including: 1) the optimal genetic testing for Turner syndrome patients, particularly with respect to identification of Y chromosome material that may increase the patient's risk of gonadoblastoma and dysgerminoma, 2) which patients should be referred for bilateral gonadectomy and the recommended timing of such referral, 3) options for assisted reproduction in these patients and associated risks, 4) the increased risk of mortality associated with pregnancy in this population, and 5) how best to assess and monitor cardiovascular risks.
Keywords: Turner syndrome, Genetic testing, Gonadoblastoma, Cardiac MRI, Infertility, Aortic dissection
Abbreviations: FISH, fluorescent in situ hybridization; SRY, sex-determining region of Y; PCR, polymerase chain reaction; TSPY, testes-specific protein Y-linked; MRI, magnetic resonance imaging; CAIS, complete androgen insensitivity syndrome; FSH, follicle stimulating hormone; AMH, anti-Mullerian hormone; ART, assisted reproductive technology; IVF, in vitro fertilization; PAPVR, partial anomalous pulmonary venous return; BSA, body surface area; ASI, aortic size index; EKG, electrocardiogram
Highlights
-
•
Patients with non-mosaic TS must be tested for cryptic Y chromosome material.
-
•
Y chromosome material increases gonadoblastoma risk, so gonadectomy is recommended.
-
•
Cardiac MRI is preferred to echocardiogram for detecting cardiac anomalies in TS.
-
•
TS increases risk of cardiovascular anomalies and death, particularly in pregnancy.
-
•
TS is a relative contraindication to reproduction; cardiac anomalies are absolute.
Introduction
Turner syndrome is a heterogenous genetic disorder caused by loss of the short arm of the X chromosome, and it affects approximately 1 out of 2500 newborn females. Classic Turner syndrome associated with 45,X karyotype occurs in approximately 45% of cases and is characterized by short stature, ovarian insufficiency, nuchal folds, low hairline, low set ears, high-arched palate, wide-spaced nipples (shield chest), left-sided cardiac anomalies, cubitus valgus (wide carrying angle), shortened fourth metacarpal, and nail abnormalities. Mosaic Turner syndrome accounts for the remaining 55% of cases and has a highly variable phenotype depending on the region(s) of missing X chromosome and/or the proportion and location of affected cells. Due to the variable and often more subtle phenotypic characteristics caused by mosaic X chromosome loss, diagnosis of these patients is often delayed or missed. Conversely, as genetic techniques become more sensitive, lower levels of mosaicism are being identified. It is unclear whether patients with low-level mosaicism share similar risks and require similar monitoring as patients with classic Turner syndrome.
Endocrinologists are charged with evaluation and management of growth failure, ovarian insufficiency and estrogen replacement, and infertility. In addition, endocrinologists guide families toward subspecialty management of additional issues, such as cardiac abnormalities. For general reviews of these topics, readers are referred to Refs. [1], [2], [3]. This review focuses on the controversial issues of genetic testing for Y chromosome material as it relates to risk of gonadoblastoma and need for prophylactic gonadectomy, as well as assisted reproduction in patients with significant peri-partum cardiovascular risks. Although firm guidelines have not yet been established regarding these issues, we aim to provide the reader with recommendations for their clinical practices.
Genetic testing for Y chromosome material
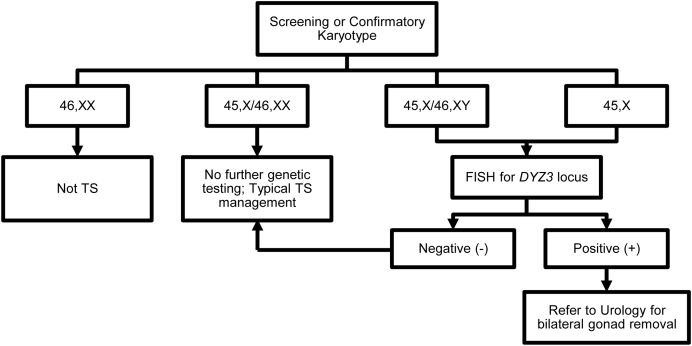
The presence of Y chromosome material is associated with increased risk of gonadoblastoma and germ cell tumors in patients with Turner syndrome (reviewed in Ref. [4]). Unfortunately, cryptic mosaicism for Y chromosome material may not be detected by standard cytogenetic techniques, which typically analyze 20–30 peripheral lymphocytes in metaphase [5]. Therefore, specific molecular testing for Y chromosome material must be performed in patients for whom the diagnosis is not clear (i.e. patients with a 45,X karyotype, see Figure 1). The preferred method for such testing is fluorescent in situ hybridization (FISH) for the Y-centromere using a probe to the DYZ3 locus because this region is linked to gonadoblastoma risk [6], [7], [8]. Importantly, FISH for the SRY gene is not specific to this region, and polymerase chain reaction (PCR) is susceptible to contamination [9].
Figure 1.
Recommended genetic testing for Turner syndrome.
All patients with 45,X karyotype should be evaluated specifically for Y chromosome material. Patients with mosaic Turner syndrome identified cytogenetically whose second cell line contains an additional chromosome that is either an X or derived from an X do not require further molecular testing because, by definition, the origin of their mosaicism has been determined. True sex chromosome monosomy (45,X or 45,Y) is incompatible with life; the presence of at least a partial second sex chromosome in a mosaic fashion is necessary [10]. The mosaicism is hypothesized to arise from the presence of a second (“rescue”) embryonic cell line that comprises only a small percentage of adult cells and consequently is not detected by conventional cytogenetic analysis. Thus, patients with 45,X karyotype may have a second cell line containing Y chromosome material that increases their risk of gonadoblastoma.
There is wide variability in the reported prevalence of Y chromosome material, gonadoblastoma, and dysgerminomas in Turner syndrome, likely due to differences in clinical practice regarding methods of genetic testing, recommendation for gonadectomy, and pathology analysis. A review of three studies in 2005 revealed that Y chromosome material is present in 8–12% of patients with Turner syndrome. Approximately 27% of this subset of patients have histologically-confirmed gonadoblastoma and 4% of these patients have evidence of malignant transformation (14% of patients with gonadoblastoma) [11]. The etiology of gonadoblastoma and mechanism of transformation to germ cell tumors are unclear [12]. Although a specific gene mutation has not been associated with this neoplasm, several groups have provided evidence that TSPY is the gonadoblastoma-susceptibility gene located within the centromeric region of the Y chromosome (reviewed in Ref. [8]). Interestingly, there is some evidence that gonadoblastoma is a congenital, rather than progressive, disorder due to fetal germ cell dysgenesis [13]. In fact, gonadoblastoma has been identified in infants with Turner syndrome. There is a theoretical concern that growth hormone therapy may increase the risk of gonadoblastoma and/or malignant transformation, but no evidence supporting this has been presented to date.
Because of the uncertain pathophysiology and natural history of gonadoblastoma, it is recommended that all patients with either 45,X/46,XY mosaic karyotype or 45,X with positive DYZ3 FISH analysis be referred for bilateral gonadectomy [14]. However, it is important to keep in mind that identification of Y chromosome material in peripheral blood samples does not necessarily reflect presence of Y chromosome material in gonadal tissue [15], which is most likely directly related to gonadoblastoma formation. An age threshold for gonadectomy has not been established in this patient population, particularly because evidence is lacking that screening with ultrasound, MRI, or serum tumor markers are sufficiently sensitive to identify gonadoblastoma prior to transformation to germ cell tumor [14]. Unfortunately, gonadal biopsy is likely also insufficiently sensitive to identify all cases of gonadal Y chromosome material, gonadoblastoma, and/or germ cell tumor, as only a small tissue region is analyzed.
It is unclear if patients with previously established 45,X nonmosaic Turner syndrome who had never been assessed for Y material in the past should now undergo retrospective targeted search, and if there should be an age cutoff for this. In the absence of evidence, it is reasonable to retroactively perform FISH for DYZ3 locus in patients with previously-identified 45,X karyotypes. Those patients with positive DYZ3 FISH should be referred to Urology and Reproductive Endocrinology for discussion of the risks and benefits of gonadectomy. Further studies are needed to address these important questions.
Because patients with classic and 45,X/46,XY mosaic Turner syndrome have low fertility potential, gonadectomy may be considered of little consequence. However, many patients and their families may have difficulty consenting to a procedure that reduces or eliminates that potential, particularly when the risks associated with delaying or refusing gonadectomy are somewhat unclear. Patients with complete androgen insensitivity syndrome (CAIS) face similar considerations because they are also at increased risk for gonadoblastoma and germ cell tumors, although they are unable to carry a pregnancy [16]. Bilateral gonadectomy is recommended in all patients with CAIS but is often delayed until after puberty because most patients with CAIS develop secondary female sex characteristics at the appropriate pubertal age due to conversion of elevated testosterone levels to estrogen. The decision to delay gonadectomy also may be more appropriate in the CAIS population because of the lower rate of gonadal dysgenesis compared to patients with Turner syndrome, although there certainly are proponents of early gonadectomy in all cases given the malignant potential of intra-abdominal dysgenic gonadal tissue.
Reproductive potential
Between 15% and 40% of adolescents with Turner syndrome undergo spontaneous puberty, although only 2–10% have spontaneous menarche [17], [18]. The prevalence of these events in patients with mosaic X chromosome disomy tends to be higher [18]. Therefore, current guidelines recommend waiting until 12–13 years of age before initiating estrogen replacement therapy in the absence of appropriate pubertal progression [1], [19]. Elevated follicle stimulating hormone (FSH) and anti-Mullerian hormone (AMH) levels also indicate ovarian insufficiency/failure and may represent need for exogenous estrogen. Only 2–5% of patients with Turner syndrome become pregnant spontaneously [17], [20]. Thus, many patients seek assisted reproductive technology (ART) in their young adult and adult years.
Notably, in 2012, the American Society of Reproductive Medicine identified Turner syndrome as a relative contraindication to pregnancy, and an absolute contraindication in those with documented cardiac anomalies [48]. This is due to high risk of cardiac-associated death, even in those with normal cardiac evaluations. Thus, the topics of preserving or assisting reproductive capabilities in patients with Turner syndrome are controversial, and safer alternatives such as surrogacy or adoption should be considered.
For those who wish to pursue assisted reproduction, one must proceed cautiously. The most commonly utilized method is via vitro fertilization (IVF) of the patient's harvested oocytes and embryo transfer to the patient's uterus. The oocytes may be either fresh or derived from previously cryo-preserved ovarian tissue. Importantly, ovarian follicle cryopreservation may be a consideration for patients undergoing gonadectomy due to presence of Y centromeric material, particularly because there may be more viable follicles at younger ages (reviewed in Refs. [17], [21], [22]). Although this procedure has resulted in successful pregnancies in females without Turner syndrome (i.e. cancer survivors whose ovarian tissue was cryopreserved prior to gonadotoxic chemotherapy), it has not yet been reported to lead to successful pregnancy in the Turner syndrome population. Overall, the success rate of IVF producing a live birth in patients with Turner syndrome is approximately 50%, although at least half of these pregnancies have significant complications including pregnancy-induced hypertension, preterm delivery, low birth weight, and need for Caesarean section [20], [23], [24]. Furthermore, in addition to the typical risks associated with IVF, patients with Turner syndrome have significantly increased mortality during and after pregnancy related to cardiovascular abnormalities.
Increased risk of cardiovascular death
Patients with Turner syndrome are at increased risk of having congenital left-sided cardiac anomalies and/or developing cardiovascular abnormalities during their lifetime, associated with significantly increased morbidity and mortality (reviewed in Ref. [26]). Kim et al. [27] found that 16% of patients with Turner syndrome have aortic coarctation, 39% have bicuspid aortic valve, and 16% had partial anomalous pulmonary venous return (PAPVR). Olivieri et al. [28] similarly identified that 36% of patients with Turner syndrome have some type of aortic valve anomaly (23% bicuspid, 12% partially fused, 1% unicuspid), and that these anomalies are associated with increased diameter of the ascending aorta. These left-sided cardiac anomalies, in addition to hypertension, increase the risk of aortic dissection and death. Aortic dissection is six times more likely to occur in patients with Turner syndrome compared to the general female population [29] and at a significantly younger age (average 30 years versus 68 years in the general population). Importantly, aortic dissection occurs in patients with Turner syndrome with aortic root and ascending aorta diameters that are well below the standard threshold of concern for the general population [30]. Thus, it is important to identify early and small increases in aortic root and ascending aorta diameter.
Cardiac MRI has been proposed to have higher sensitivity than echocardiography for identification of cardiac abnormalities in patients with Turner syndrome (reviewed in Ref. [31]). This seems to be related both to technological differences, as well as increased thoracic antero-posterior diameter and increased lymphatic tissue in patients with Turner syndrome. Importantly, aorta measurements should take into account the smaller size of patients with Turner syndrome; the most widely accepted method is normalization to patient body surface area (BSA), which is termed aortic size index (ASI = diameter/BSA). However, normal ASI values for different ages, as well as thresholds of concern, in patients with Turner syndrome are not firmly established.
Notably, some patients with Turner syndrome without any evidence of cardiac anomaly or cardiovascular disease develop aortic dissection, and the associated mortality rate is quite high (63%) [30]. There is also evidence that aortic dilation is not necessarily progressive in this population [32], [33], [34], in contrast to other patient populations at increased risk for aortic dilation and dissection such as Marfan, Ehlers-Danlos, and Loeys-Dietz syndromes [35]. These other syndromes are all classified as connective tissue disorders, of which Turner syndrome shares some characteristics. In fact, patients with Turner syndrome exhibit decreased aortic distensibility, particularly in the setting of aortic dilation [36], as well as increased carotid intima-media thickness and pulse wave velocity [37], [38]. Furthermore, at least 50% of patients with Turner syndrome develop hypertension, half of these by adolescence [39]. It is important to note that growth hormone and estrogen replacement therapies have not been associated with significant changes in aortic diameter when normalized to body size [40], [41]. Although it is clear that all patients with Turner syndrome require careful assessment of cardiovascular risk, it has been difficult to establish definitive guidelines for cardiac monitoring due to the unclear pathophysiology and prognosis associated with identifiable anomalies.
Pregnancy seems to be an independent risk factor for aortic dissection in Turner syndrome, as women with a prior pregnancy were over-represented (15%) in a registry of patients who suffered from aortic dissection [30]. Partly driven by this evidence, abnormalities on cardiac imaging is also considered a contraindication to pregnancy. Overall, pregnancy is associated with a 2% mortality risk in patients with Turner syndrome, primarily due to aortic dissection [24], [42], whereas the risk for maternal death in the general population is 1/10,000 [48]. The precise mechanisms responsible for this increased risk are not clear, although it is theorized to be related to the normal increases in stroke volume and cardiac output during pregnancy [43], [44], [45], which place stress on the heart and vasculature. This also may explain why multiple gestation pregnancies, which result in further increases in cardiac output [46], are associated with even higher cardiovascular mortality risk [25]. It is important to remember that the cardiovascular changes that occur during pregnancy are chronic and increase mortality risk for the remainder of the patient's lifetime.
Currently, cardiovascular screening recommendations for patients with Turner syndrome include EKG and imaging at the time of diagnosis (echocardiography in young patients who would otherwise require sedation for MRI, and cardiac MRI in older children and adults), follow-up imaging every 5–10 years if normal, and annual blood pressure measurements [1]. Patients should be referred to and followed by a cardiologist. Prior to pregnancy, women should be screened with blood pressure measurement and cardiac imaging, and they should also be counseled regarding the crucial risks of pregnancy. Blood pressure and cardiac imaging assessments should be repeated frequently throughout pregnancy and the early post-partum period [47]. In general, abnormal cardiac imaging or measurements, such as an ASI of >2 cm/m2, is considered an absolute contraindication to pregnancy in patients with Turner syndrome, due to risk of aortic dissection and death [48].
Discussion
Here we reviewed several current controversial topics in the comprehensive care of patients with Turner syndrome, and we identify several areas that need further study. As more has been learned regarding the etiology of Turner syndrome, it has become clear that nearly all patients have a mosaic cell line with a second sex chromosome, which results in a broad spectrum of clinical phenotypes and associated risks. An important implication of having Y chromosome material is the risk of malignant transformation of gonadoblastoma, necessitating molecular genetic testing. Specifically for patients in whom a second X chromosome or a marker chromosome was not detected by karyotype, FISH for the DYZ3 locus should be performed followed by immediate bilateral gonadectomy if such genetic testing is positive. However, further studies are needed to evaluate the pathophysiology and prevalence of gonadoblastoma and germ cell tumors in these patients in order to provide stronger evidence for optimal genetic testing and for recommendations regarding timing of gonadectomy. Furthermore, in light of recognized increase in mortality and morbidity, it is imperative to recognize and counsel patients regarding the significant cardiovascular and mortality risks associated with pregnancy. Additional studies to elucidate the underlying pathophysiology and prognosis of cardiovascular abnormalities in these patients are critical so that evidence-based recommendations can be made regarding method(s) and timing of cardiovascular evaluation.
In the absence of strong evidence-based guidelines, current practice with respect to these issues relies on the principle of “first do no harm.” As a result, some patients may be undergoing unnecessary (and expensive) testing, whereas other patients may not be receiving sufficient testing or intervention and likely are suffering from increased morbidity and mortality. It is important to educate patients and their families regarding these controversies and discuss them openly in order to provide a context for the recommendations.
Acknowledgments
We thank Holly Dubbs, MS at The Children's Hospital of Philadelphia for helpful discussions and critical review of this manuscript.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
References
- 1.Bondy C.A. Care of girls and women with Turner syndrome: a guideline of the Turner Syndrome Study Group. J Clin Endocrinol Metab. 2007;92:10–25. doi: 10.1210/jc.2006-1374. [DOI] [PubMed] [Google Scholar]
- 2.Davenport M.L. Approach to the patient with Turner syndrome. J Clin Endocrinol Metab. 2010;95:1487–1495. doi: 10.1210/jc.2009-0926. [DOI] [PubMed] [Google Scholar]
- 3.Pinsker J.E. Clinical review: Turner syndrome: updating the paradigm of clinical care. J Clin Endocrinol Metab. 2012;97:E994–E1003. doi: 10.1210/jc.2012-1245. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira R.M., Verreschi I.T., Lipay M.V., Eça L.P., Guedes A.D., Bianco B. Y chromosome in Turner syndrome: review of the literature. Sao Paulo Med J. 2009;127:373–378. doi: 10.1590/S1516-31802009000600010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff D.J., Van Dyke D.L., Powell C.M. Laboratory guideline for Turner syndrome. Genet Med. 2010;12:52–55. doi: 10.1097/GIM.0b013e3181c684b2. [DOI] [PubMed] [Google Scholar]
- 6.Tsuchiya K., Reijo R., Page D.C., Disteche C.M. Gonadoblastoma: molecular definition of the susceptibility region on the Y chromosome. Am J Hum Genet. 1995;57:1400–1407. [PMC free article] [PubMed] [Google Scholar]
- 7.Hanson L., Bryman I., Janson P.O., Jakobsen A.M., Hanson C. Fluorescence in situ hybridisation analysis and ovarian histology of women with Turner syndrome presenting with Y-chromosomal material: a correlation between oral epithelial cells, lymphocytes and ovarian tissue. Hereditas. 2002;137:1–6. doi: 10.1034/j.1601-5223.2002.1370101.x. [DOI] [PubMed] [Google Scholar]
- 8.Lau Y.F., Li Y., Kido T. Gonadoblastoma locus and the TSPY gene on the human Y chromosome. Birth Defects Res C Embryo Today. 2009;87:114–122. doi: 10.1002/bdrc.20144. [DOI] [PubMed] [Google Scholar]
- 9.Nishi M.Y., Domenice S., Medeiros M.A., Mendonca B.B., Billerbeck A.E. Detection of Y-specific sequences in 122 patients with Turner syndrome: nested PCR is not a reliable method. Am J Med Genet. 2002;107:299–305. doi: 10.1002/ajmg.10168. [DOI] [PubMed] [Google Scholar]
- 10.Hook E.B., Warburton D. Turner syndrome revisited: review of new data supports the hypothesis that all viable 45,X cases are cryptic mosaics with a rescue cell line, implying an origin by mitotic loss. Hum Genet. 2014;133:417–424. doi: 10.1007/s00439-014-1420-x. [DOI] [PubMed] [Google Scholar]
- 11.Mazzanti L., Cicognani A., Baldazzi L., Scarano E., Strocchi S., Nicoletti A. Gonadoblastoma in Turner syndrome and Y-chromosome-derived material. Am J Med Genet A. 2005;135:150–154. doi: 10.1002/ajmg.a.30569. [DOI] [PubMed] [Google Scholar]
- 12.Pauls K., Franke F.E., Buttner R., Zhou H. Gonadoblastoma: evidence for a stepwise progression to dysgerminoma in a dysgenetic ovary. Virchows Arch. 2005;447:603–609. doi: 10.1007/s00428-005-1272-9. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen N., Muller J., Jaubert F., Skakkebaek N.E. Heterogeneity of gonadoblastoma germ cells: similarities with immature germ cells, spermatogonia and testicular carcinoma in situ cells. Histopathology. 1997;30:177–186. doi: 10.1046/j.1365-2559.1997.d01-580.x. [DOI] [PubMed] [Google Scholar]
- 14.McCann-Crosby B., Mansouri R., Dietrich J.E., Sutton V.R., Austin E.G., Schlomer B. State of the art review in gonadal dysgenesis: challenges in diagnosis and management. Int J Pediatr Endocrinol. 2014;2014:4. doi: 10.1186/1687-9856-2014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guedes A.D., Bianco B., Lipay M.V., de Lourdes Chauffaille M, Verreschi IT. Determination of the sexual phenotype in a child with 45,X/46,X,Idic(Yp) mosaicism: importance of the relative proportion of the 45,X line in gonadal tissue. Am J Med Genet A. 2006;140A:1871–1875. doi: 10.1002/ajmg.a.31363. [DOI] [PubMed] [Google Scholar]
- 16.Hughes I.A., Davies J.D., Bunch T.I., Pasterski V., Mastroyannopoulou K., MacDougall J. Androgen insensitivity syndrome. Lancet. 2012;380:1419–1428. doi: 10.1016/S0140-6736(12)60071-3. [DOI] [PubMed] [Google Scholar]
- 17.Karnis M.F. Fertility, pregnancy, and medical management of Turner syndrome in the reproductive years. Fertil Steril. 2012;98:787–791. doi: 10.1016/j.fertnstert.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Zhong Q., Layman L.C. Genetic considerations in the patient with Turner syndrome–45,X with or without mosaicism. Fertil Steril. 2012;98:775–779. doi: 10.1016/j.fertnstert.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez L., Witchel S.F. The patient with Turner syndrome: puberty and medical management concerns. Fertil Steril. 2012;98:780–786. doi: 10.1016/j.fertnstert.2012.07.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hadnott T.N., Gould H.N., Gharib A.M., Bondy C.A. Outcomes of spontaneous and assisted pregnancies in Turner syndrome: the U.S. National Institutes of Health experience. Fertil Steril. 2011;95:2251–2256. doi: 10.1016/j.fertnstert.2011.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasserman D., Asch A. Reproductive medicine and Turner syndrome: ethical issues. Fertil Steril. 2012;98:792–796. doi: 10.1016/j.fertnstert.2012.08.036. [DOI] [PubMed] [Google Scholar]
- 22.Hewitt J.K., Jayasinghe Y., Amor D.J., Gillam L.H., Warne G.L., Grover S. Fertility in Turner syndrome. Clin Endocrinol (Oxf) 2013;79:606–614. doi: 10.1111/cen.12288. [DOI] [PubMed] [Google Scholar]
- 23.Alvaro Mercadal B., Imbert R., Demeestere I., Englert Y., Delbaere A. Pregnancy outcome after oocyte donation in patients with Turner's syndrome and partial X monosomy. Hum Reprod. 2011;26:2061–2068. doi: 10.1093/humrep/der166. [DOI] [PubMed] [Google Scholar]
- 24.Chevalier N., Letur H., Lelannou D., Ohl J., Cornet D., Chalas-Boissnonas C. Materno-fetal cardiovascular complications in Turner syndrome after oocyte donation: insufficient prepregnancy screening and pregnancy follow-up are associated with poor outcome. J Clin Endocrinol Metab. 2011;96:E260–E267. doi: 10.1210/jc.2010-0925. [DOI] [PubMed] [Google Scholar]
- 25.Hovatta O. Pregnancies in women with Turner's syndrome. Ann Med. 1999;31:106–110. [PubMed] [Google Scholar]
- 26.Bondy C.A. Congenital cardiovascular disease in Turner syndrome. Congenit Heart Dis. 2008;3:2–15. doi: 10.1111/j.1747-0803.2007.00163.x. [DOI] [PubMed] [Google Scholar]
- 27.Kim H.K., Gottliebson W., Hor K., Gutmark-Little I., Salisbury S.R., Racadio J.M. Cardiovascular anomalies in Turner syndrome: spectrum, prevalence, and cardiac MRI findings in a pediatric and young adult population. AJR Am J Roentgenol. 2011;196:454–460. doi: 10.2214/AJR.10.4973. [DOI] [PubMed] [Google Scholar]
- 28.Olivieri L.J., Baba R.Y., Arai A.E., Bandettini W.P., Rosing D.R., Bakalov V. Spectrum of aortic valve abnormalities associated with aortic dilation across age groups in Turner syndrome. Circ Cardiovasc Imaging. 2013;6:1018–1023. doi: 10.1161/CIRCIMAGING.113.000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gravholt C.H., Landin-Wilhelmsen K., Stochholm K., Jorgensen J.O., Laurberg P., Andersen M. Clinical and epidemiological description of aortic dissection in Turner's syndrome. Cardiol Young. 2006;16:430–436. doi: 10.1017/S1047951106000928. [DOI] [PubMed] [Google Scholar]
- 30.Carlson M., Airhart N., Lopez L., Silberbach M. Moderate aortic enlargement and bicuspid aortic valve are associated with aortic dissection in Turner syndrome: report of the international turner syndrome aortic dissection registry. Circulation. 2012;126:2220–2226. doi: 10.1161/CIRCULATIONAHA.111.088633. [DOI] [PubMed] [Google Scholar]
- 31.Gutmark-Little I., Backeljauw P.F. Cardiac magnetic resonance imaging in Turner syndrome. Clin Endocrinol (Oxf) 2013;78:646–658. doi: 10.1111/cen.12157. [DOI] [PubMed] [Google Scholar]
- 32.Ilyas M., Chu C., Ettles D., Mathew V., Atkin S. Evaluation by magnetic resonance imaging of aortic dilatation and coarctation in adult Turner syndrome patients. Clin Endocrinol (Oxf) 2006;65:154–157. doi: 10.1111/j.1365-2265.2006.02557.x. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen K.H., Hjerrild B.E., Stochholm K., Andersen N.H., Sørensen K.E., Lundorf E. Dilation of the ascending aorta in Turner syndrome – a prospective cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. 2011;13:24. doi: 10.1186/1532-429X-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lanzarini L., Larizza D., Prete G., Calcaterra V., Klersy C. Prospective evaluation of aortic dimensions in Turner syndrome: a 2-dimensional echocardiographic study. J Am Soc Echocardiogr. 2007;20:307–313. doi: 10.1016/j.echo.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 35.Bondy C.A. Aortic dissection in Turner syndrome. Curr Opin Cardiol. 2008;23:519–526. doi: 10.1097/hco.0b013e3283129b89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharma J., Friedman D., Dave-Sharma S., Harbison M. Aortic distensibility and dilation in Turner's syndrome. Cardiol Young. 2009;19:568–572. doi: 10.1017/S1047951109990874. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen K.H., Andersen N.H., Hjerrild B.E., Hørlyck A, Stochholm K, Højbjerg Gravholt C. Carotid intima-media thickness is increased in Turner syndrome: multifactorial pathogenesis depending on age, blood pressure, cholesterol and oestrogen treatment. Clin Endocrinol (Oxf) 2012;77:844–851. doi: 10.1111/j.1365-2265.2012.04337.x. [DOI] [PubMed] [Google Scholar]
- 38.Baguet J.P., Douchin S., Pierre H., Rossignol A.M., Bost M., Mallion J.M. Structural and functional abnormalities of large arteries in the Turner syndrome. Heart. 2005;91:1442–1446. doi: 10.1136/hrt.2004.048371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turtle E.J., Sule A.A., Bath L.E., Denvir M., Gebbie A., Mirsadraee S. Assessing and addressing cardiovascular risk in adults with Turner syndrome. Clin Endocrinol (Oxf) 2013;78:639–645. doi: 10.1111/cen.12104. [DOI] [PubMed] [Google Scholar]
- 40.Bondy C.A., Van P.L., Bakalov V.K., Ho V.B. Growth hormone treatment and aortic dimensions in Turner syndrome. J Clin Endocrinol Metab. 2006;91:1785–1788. doi: 10.1210/jc.2005-2625. [DOI] [PubMed] [Google Scholar]
- 41.Cleemann L., Mortensen K.H., Holm K., Smedegaard H., Skouby S.O., Wieslander S.B. Aortic dimensions in girls and young women with turner syndrome: a magnetic resonance imaging study. Pediatr Cardiol. 2010;31:497–504. doi: 10.1007/s00246-009-9626-8. [DOI] [PubMed] [Google Scholar]
- 42.Karnis M.F., Zimon A.E., Lalwani S.I., Timmreck L.D., Klipstein S., Reindollar R.H. Risk of death in pregnancy achieved through oocyte donation in patients with Turner syndrome: a national survey. Fertil Steril. 2003;80:498–501. doi: 10.1016/s0015-0282(03)00974-9. [DOI] [PubMed] [Google Scholar]
- 43.van Oppen A.C., van der Tweel I., Alsbach G.P., Heethaar R.M., Bruinse H.W. A longitudinal study of maternal hemodynamics during normal pregnancy. Obstet Gynecol. 1996;88:40–46. doi: 10.1016/0029-7844(96)00069-5. [DOI] [PubMed] [Google Scholar]
- 44.Desai D.K., Moodley J., Naidoo D.P. Echocardiographic assessment of cardiovascular hemodynamics in normal pregnancy. Obstet Gynecol. 2004;104:20–29. doi: 10.1097/01.AOG.0000128170.15161.1d. [DOI] [PubMed] [Google Scholar]
- 45.D'Silva L.A., Davies R.E., Emery S.J., Lewis M.J. Influence of somatic state on cardiovascular measurements in pregnancy. Physiol Meas. 2014;35:15–29. doi: 10.1088/0967-3334/35/1/15. [DOI] [PubMed] [Google Scholar]
- 46.Kuleva M., Youssef A., Maroni E., Contro E., Pilu G., Rizzo N. Maternal cardiac function in normal twin pregnancy: a longitudinal study. Ultrasound Obstet Gynecol. 2011;38:575–580. doi: 10.1002/uog.8936. [DOI] [PubMed] [Google Scholar]
- 47.Cabanes L., Chalas C., Christin-Maitre S., Donadille B., Felten M.L., Gaxotte V. Turner syndrome and pregnancy: clinical practice. Recommendations for the management of patients with Turner syndrome before and during pregnancy. Eur J Obstet Gynecol Reprod Biol. 2010;152:18–24. doi: 10.1016/j.ejogrb.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Practice Committee of American Society for Reproductive Medicine Increased maternal cardiovascular mortality associated with pregnancy in women with Turner syndrome. Fertil Steril. 2012;97:282–284. doi: 10.1016/j.fertnstert.2011.11.049. [DOI] [PubMed] [Google Scholar]