Abstract
Objective:
To evaluate pharmacists’ attitudes toward the Take Home Naloxone (THN) program and identify areas that could be improved to support pharmacists’ involvement.
Methods:
Pharmacists on the Alberta College of Pharmacists’ directory were invited to complete an online survey between July 10 and August 8, 2016. The survey consisted of 19 questions. Descriptive statistics were used to analyze the data.
Results:
Four hundred seventy pharmacists completed the survey (response rate = 11.2%). A total of 76.8% of respondents strongly agreed or agreed that pharmacists should be screening patients to identify those at risk of opioid overdose. Full-time pharmacists were more likely to agree (p = 0.02). A total of 79.8% of respondents strongly agreed or agreed that pharmacists should be recommending THN kits. Pharmacists working in large population centres (p = 0.008) and full-time pharmacists (p = 0.02) were more likely to agree with this statement. Furthermore, 60.6% of pharmacists were extremely willing or very willing to participate in the THN program. Pharmacists in practice for ≤15 years were more willing to participate in the THN program than pharmacists in practice >15 years (p = 0.03). The most common perceived barriers to implementation of the THN program were lack of time in pharmacists’ current work environment and education about the program.
Conclusions:
Overall, pharmacists had positive attitudes toward screening patients to identify those at risk of opioid overdose, recommending THN kits and willingness to participate in the program. Factors that may facilitate increased participation in the program include addressing time issues and improving education about the THN program.
Knowledge Into Practice.
There are currently no Canadian data looking at pharmacists’ perspectives on the Take Home Naloxone program. Engaging pharmacists in the opioid overdose epidemic is critical.
The article provides insight into barriers that pharmacists perceive regarding the Take Home Naloxone program.
Understanding how the Take Home Naloxone program is perceived and addressing potential barriers can help to engage more pharmacists and increase participation in harm reduction services.
These findings highlight the need for better education programs for pharmacists to develop their confidence and increase their comfort in order to maximize naloxone distribution.
Mise En Pratique Des Connaissances.
Pour l’instant, il n’existe aucune donnée concernant le point de vue des pharmaciens sur le programme de la naxolone à emporter à domicile. Il est crucial de mobiliser les pharmaciens dans cette crise épidémique de surdoses d’opioïdes.
L’article présente des exemples de ce qui, selon les pharmaciens, constitue des obstacles dans le cadre du programme de la naxolone à emporter à domicile.
Savoir comment le programme de la naxolone à emporter à domicile est perçu et surmonter les éventuels obstacles pourrait permettre de mobiliser davantage de pharmaciens et d’accroître l’utilisation des services de réduction des méfaits.
Les conclusions de l’article soulignent la nécessité de mettre en place de meilleurs programmes de formation pour permettre aux pharmaciens de faire plus confiance au programme et de l’apprivoiser pour maximiser la distribution de naxolone.
Background
Across Canada, opioid overdoses and related deaths are increasing.1-5 In response, there has been a broad range of implemented interventions. New federal prescription guidelines, removal of oxycodone slow release from provincial formularies, introduction of prescription-monitoring programs (PMP) and electronic and print mass media are some of the initiatives that have been attempted to control opioid-related morbidity and mortality.6 Despite these efforts, opioid overdoses continue to rise. In 2012, there were 1104 opioid-related emergency department visits in Alberta; by 2015, this number increased by 40% to 1547 visits.4 The number of calls to Health Link Alberta related to naloxone and/or an opiate also increased more than 60% in the first half of 2016 compared with the same period in 2015.4
Currently, there is no evidence that PMP and safe opioid prescribing guidelines are effective in reducing opioid-related deaths.7 However, harm reduction strategies such as syringe access programs,8-10 supervised injection facilities11 and, more recently, naloxone distribution programs12-16 have been shown to be successful and cost-effective.
Naloxone is an opioid antagonist given parenterally and intranasally to reverse the effects of opioids. In May 2016, Health Canada removed naloxone from the Prescription Drug List, thereby expanding access.17 Naloxone is now an unscheduled product in Alberta, no longer requiring a prescription through a licensed pharmacy.
Alberta Health implemented the Take Home Naloxone (THN) program in July 2015. The program provides THN kits (Figure 1) free of charge to any Albertan who is at risk of overdosing on opioids. To assist in the distribution of naloxone, Alberta Health Services (AHS) activated an Emergency Coordination Centre to manage the development of resources. The Alberta Health and AHS THN programs have now been consolidated into a single program with more than 700 naloxone distribution sites, including more than 65 walk-in clinics and more that 580 community pharmacies.5
Figure 1.
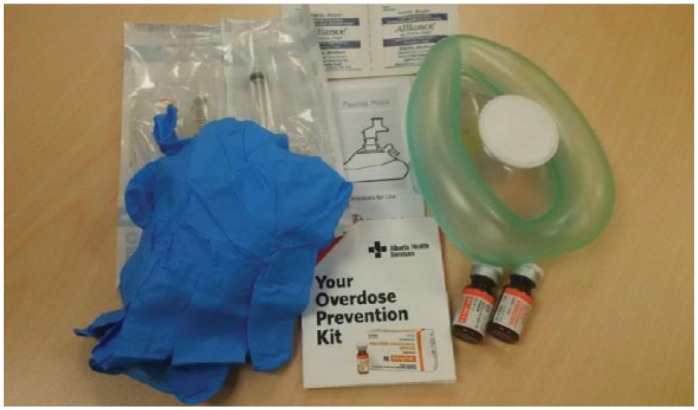
Contents of a Take Home Naloxone kit
Each kit contains 2 vials of naloxone 0.4 mg/mL, 2 retractable 3 mL 25 g 1 inch syringes, 2 alcohol swabs, 2 nitrile gloves, 1-way rescue breathing barrier mask, administration instructions and a kit label. For more information, including training requirements and training opportunities, visit www.drugsfool.ca.
Pharmacists are among the most accessible health care professionals and are uniquely positioned across the province, in the community and in hospitals, to prevent substance use disorder.18 To our knowledge, there are no data assessing pharmacists’ perceptions about the THN program in Alberta. Pharmacists’ engagement is critical in providing opportunities to increase naloxone distribution and prevent mortality.
The purpose of our study was to evaluate pharmacists’ perspectives on the Alberta THN program. A secondary purpose was to identify any areas that could be improved to support pharmacists’ involvement in the program.
Methods
We conducted a descriptive research study using an electronic survey. A directory of registered clinical pharmacists with the Alberta College of Pharmacists was used. This directory consisted of all registered clinical pharmacists who have consented to receive mailings for practice-based research. All pharmacists on this list were invited to complete the survey via e-mail. The e-mail provided a description of the study as well as a web link directing them to the online survey.
The survey was evaluated for face validity and pilot tested with a small group of licensed practising pharmacists to assess clarity and relevance prior to full-scale administration. The survey was developed using Survey Select (LCC Company, Kansas City, MO) in July 2016 and employed a modified Dillman Method.19 A response to each question was required to proceed to the next question. The link to the electronic survey was available for 4 weeks. A reminder e-mail about the study survey was sent out at the midpoint and 1 week prior to survey closure. Only surveys in which all questions were completed were included.
The survey consisted of 19 questions and included sections on baseline demographics, feedback on the THN training, pharmacist perceptions of the THN program and barriers to implementation into practice (Appendix 1, available at www.cpjournal.ca). We hypothesized that male pharmacists, community pharmacists, pharmacists practising in large urban populations, full-time pharmacists, younger aged pharmacists, pharmacists with additional education and pharmacists in practice for ≤15 years would be more supportive of the THN program.
Study approval was obtained from the Health Research Ethics Board of Alberta Community Health Committee. Consent to participate was implied by participant completion of the survey and was voluntary. All responses were anonymous and confidential.
Statistical analysis
Response rate was calculated with adjustment for surveys that could not be delivered because of inactive e-mail addresses and for e-mails with automatic replies specifying vacation times.
Descriptive statistics were presented for all study variables. Means and standard deviations were reported for continuous variables; frequency and proportions were reported for categorical variables. Pearson’s chi-square tests were conducted to compare 2 categorical variables. A p value <0.05 was used for statistical significance. SAS (SAS Institute Inc., Cary, NC) version 9.3 was used to conduct all statistical tests. Two-tailed tests were used for comparisons.
Results
During the study period, there were 5049 pharmacists registered with the Alberta College of Pharmacists. Of these, 4307 had consented to receive practice-based research mailings. Accounting for surveys that could not be delivered because of inactive e-mail accounts and for pharmacists who were unable to complete the survey because of vacation time yielded a total of 4195 delivered e-mails. From this, 470 surveys were completed with no missing data, resulting in a response rate of 11.2%.
Demographics and participation
Survey respondent demographics are shown in Table 1: 64.7% were female, 63.4% were community pharmacists, 27.2% were hospital pharmacists, 60.2% worked in a large urban population and 79.8% worked full time. Overall, 59% of survey respondents had completed the THN training requirements (Table 2). Of these, 83.5% felt the training was sufficient, but 15.5% were not even aware there was a THN training program.
Table 1.
Survey respondent demographics
| No. of survey respondents (N = 470) | |
|---|---|
| Gender | |
| Female | 304 (64.7) |
| Male | 166 (35.3) |
| Primary work setting | |
| Community pharmacy | 298 (63.4) |
| Hospital pharmacy | 128 (27.2) |
| Primary care network | 10 (2.1) |
| Academia/university affiliation | 5 (1.1) |
| Industry | 1 |
| Retired | 1 |
| Other | 27 (5.7) |
| Population of practice area | |
| Rural (<1000) | 11 (2.3) |
| Small population (1000 to 29,999) | 100 (21.3) |
| Medium population (30,000 to 99,999) | 76 (16.2) |
| Large urban population (≥100,000) | 283 (60.2) |
| Average hours worked per week | |
| Casual | 16 (3.4) |
| Part-time (<30 per week) | 79 (16.8) |
| Full-time (≥30 per week) | 375 (79.8) |
| Age group (years old) | |
| 20 to 29 | 111 (23.6) |
| 30 to 39 | 114 (24.3) |
| 40 to 49 | 113 (24.0) |
| 50 to 59 | 102 (21.7) |
| 60 to 69 | 27 (5.7) |
| ≥70 | 3 (0.6) |
| Current level of education | |
| Bachelor of science in pharmacy | 425 (90.4) |
| Pharmacy practice residency | 43 (9.1) |
| Master’s | 31 (6.6) |
| PharmD | 32 (6.8) |
| PhD | 4 (0.9) |
| Additional authorizations | |
| Additional Prescribing Authorization (APA) | 182 (38.7) |
| Administer drugs by injection | 342 (72.8) |
| Ability to order labs (PRAC ID) | 332 (70.6) |
| Years in practice | |
| 0 to 5 | 149 (31.7) |
| 6 to 10 | 62 (13.2) |
| 11 to 15 | 46 (9.8) |
| 16 to 20 | 49 (10.4) |
| 21 to 25 | 49 (10.4) |
| 26 to 30 | 38 (8.1) |
| 31 to 35 | 43 (9.4) |
| ≥36 | 33 (7.0) |
Table 2.
Pharmacist survey responses
| No. (%) of survey respondents (N = 470) | |
|---|---|
| Completed THN program training | |
| Yes | 278 (59.1) |
| No | 119 (25.3) |
| Was not aware there was a THN training program | 73 (15.5) |
| Did you feel the THN program training was sufficient? | |
| Yes | 234 (49.8) |
| No | 47 (10) |
| Have not completed the THN program training | 189 (40.2) |
| Pharmacists should be screening patients to identify those at risk of opioid overdose | |
| Strongly agree | 110 (23.4) |
| Agree | 251 (53.4) |
| Neutral | 76 (16.2) |
| Disagree | 23 (4.9) |
| Strongly disagree | 10 (2.1) |
| Scale (1 to 5) | 3.91 (SD = 0.88) |
| Pharmacists should be recommending and/or prescribing THN kits for patients at high risk for opioid overdose | |
| Strongly agree | 152 (32.3) |
| Agree | 223 (47.4) |
| Neutral | 56 (11.9) |
| Disagree | 26 (5.5) |
| Strongly disagree | 13 (2.8) |
| Scale (1 to 5) | 4.01 (SD = 0.96) |
| How confident are you in your ability to provide education/counsel on the THN kits? | |
| Very confident | 42 (8.9) |
| Confident | 154 (32.8) |
| Somewhat confident | 124 (26.4) |
| Not very confident | 93 (19.8) |
| Not at all confident | 57 (12.1) |
| Scale (1 to 5) | 3.07 (SD = 1.17) |
| How willing are you to participate in the THN program? | |
| Extremely willing | 91 (19.4) |
| Very willing | 194 (41.3) |
| Moderately willing | 107 (22.8) |
| A little willing | 51 (10.9) |
| Not at all willing | 27 (5.7) |
| Scale (1 to 5) | 3.58 (SD = 1.09) |
| How comfortable are you in engaging in conversation on the THN program with a patient? | |
| Very comfortable | 50 (10.6) |
| Comfortable | 151 (31.2) |
| Somewhat comfortable | 137 (29.1) |
| Slightly comfortable | 74 (15.7) |
| Not at all comfortable | 58 (12.3) |
| Scale (1 to 5) | 3.13 (SD = 1.18) |
| Are you already participating in the THN program? | |
| Yes | 218 (46.4) |
| No | 252 (53.6) |
| Do you or your pharmacy currently participate in other harm reduction initiatives? | |
| Yes | 341 (72.6) |
| No | 58 (12.3 ) |
| Not applicable | 71 (15.1) |
THN, Take Home Naloxone; SD, standard deviation.
Screening, recommending and willingness
A total of 76.8% of respondents strongly agreed or agreed that pharmacists should be screening patients to identify those at risk for opioid overdose, while 79.8% of respondents strongly agreed or agreed that pharmacists should be recommending and/or prescribing THN kits for patients at high risk for opioid overdose. Pharmacists who worked in large population centres (p = 0.008) and full-time pharmacists (p = 0.02) were significantly more likely to agree with this statement. Furthermore, 60.6% of pharmacists had been either extremely willing or very willing to participate in the THN program. Pharmacists who were in practice for ≤15 years were more willing to participate in the THN program than pharmacists in practice >15 years (p = 0.03)
Confidence and comfort
Forty-two percent of pharmacists were either very confident or confident in their ability to provide education on the THN kit. Male pharmacists (p < 0.0001) and community pharmacists (p < 0.0001) were significantly more confident than female pharmacists and hospital pharmacists. In addition, 42.8% of pharmacists were very comfortable or comfortable in engaging in conversation on the THN program with a patient. Pharmacists working in large population centres (p = 0.0081) and full-time pharmacists (p = 0.0423) were most comfortable.
Barriers to implementation
The most common perceived barriers to implementation of the THN program were the lack of time in pharmacists’ current work environment and lack of education about the program (Table 3). These 2 barriers remained the most common perceived barriers even when looking only at pharmacists who completed the THN program training. Of note, many respondents used the free-text option to comment on the perceived rushed implementation of the program (believed by some to be a political decision) as well as their resistance to harm reduction initiatives.
Table 3.
Perceived barriers to implementing the THN program by respondents
| No. (%) of survey respondents (N = 470) | |
|---|---|
| Lack of time in current work environment | 200 (42.6) |
| Lack of education about the program | 192 (40.9) |
| Inadequate financial compensation for service | 93 (19.8) |
| Do not see patients with opioid drug use in practice setting | 90 (19.1) |
| No perceived barriers to the THN program | 89 (18.9 ) |
| Fear of attracting drug-seeking clientele | 87 (18.5) |
| Belief that the THN program is enabling drug abuse | 84 (17.9) |
| Fear of legal liability | 73 (15.5) |
| Lack of support from management or organization | 68 (14.5) |
| Other identified barriers by respondents | |
| Lack of clientele buy-in | 12 (2.6) |
| THN kits are not user-friendly | 6 (1.3) |
| Poor advertisement of program | 6 (1.3) |
| Lack of treatment programs | 5 (1.1) |
| Personal opinion | 5 (1.1) |
| Lack of assessment tools | 3 (0.6) |
| Lack of patient education tools | 3 (0.6) |
| Poor implementation | 2 (0.4) |
| No access to THN kits | 2 (0.4) |
| Infrequent clientele | 2 (0.4) |
| Stigma from community | 1 (0.2) |
THN, Take Home Naloxone.
Discussion
This is the first study to look at pharmacists’ perceptions on the THN program in Canada. We found that, overall, pharmacists had positive attitudes toward screening patients to identify those at risk of opioid overdose and recommending and/or prescribing THN kits for patients at high risk of opioid overdose. Most pharmacists were willing to participate in the THN program. However, pharmacists were not very confident in their ability to provide education on the THN kits and were generally only somewhat comfortable in doing this.
A low degree of confidence and comfort from pharmacists could be associated with lack of education about the program itself. This was felt to be one of the biggest barriers to implementing the THN program. Despite low confidence and comfort, the fact that pharmacists are willing and have positive attitudes toward screening and recommending THN kits is extremely encouraging. Of note, lack of education for pharmacists is a modifiable barrier and can be addressed to improve pharmacists’ attitudes.
Lack of time in the current work environment was a noteworthy barrier to implementing the THN program. The estimated time for teaching, completion of documentation and dispensing of a THN kit is approximately 10 to 15 minutes. Recognizably, this estimated time can increase depending on numerous different uncontrollable factors, but it emphasizes the importance and the need for time efficient educational resources for pharmacists.
Another perceived barrier to the THN program noted by respondents was that the kit was not user friendly. Many respondents felt that in the event of an overdose, patients or bystanders would have difficulties administering the injection. As many respondents noted, naloxone is also available as an autoinjector and in intranasal formulation; however, the costs for these are considerably greater. A nasal formulation would also remove the risk of needle-stick injuries and would be easier for bystanders to administer.
Our findings are similar to a recent study looking at pharmacists’ knowledge, attitudes and confidence regarding naloxone for overdose reversal in Australia.20 Nielsen et al.20 distributed an online survey to community pharmacists (n = 595) to assess their attitudes toward naloxone supply and support for overdose prevention and found that pharmacists were willing to supply naloxone but few were confident they could identify appropriate patients and educate them. The survey also tested pharmacists’ level of knowledge about naloxone pharmacokinetics and administration. Participants scored a mean of 1.8 out of 5, with a score of 5 meaning highly knowledgeable.20 This knowledge score could be a reflection of the education programs targeted at pharmacists. The authors found that lack of time, training, knowledge and reimbursement were the most common barriers to supplying naloxone. This is consistent with our findings and reiterates a similar perspective of pharmacists toward naloxone programs across the globe.
There are limited data available looking at pharmacists’ perspectives on naloxone distribution programs. A few studies have completed small interview-based discussions with pharmacists on their experience with naloxone supply and looked at prescribing patterns; however, only 1 other study has widely surveyed community pharmacists.20-24 In our study, 15.5% of pharmacists did not even know there was a THN training program available. This emphasizes the need to revisit the implementation of the program and further echoes the need for more support for pharmacists to help them gain confidence and comfort with supplying naloxone.
In 2011, Alberta witnessed 6 fentanyl-related overdose deaths.4 This number increased to 120 deaths in 2014 and more than doubled to 274 deaths in 2015.4,5 In response to this rapid increase, Alberta Health implemented the THN program expeditiously. Given their accessibility, pharmacists are critical to the success of such an extensive and critical program. Our results suggest that pharmacists recognize the importance of such a program and they aspire to be involved; however, there remain substantial barriers to comprehensive endorsement by all pharmacists. We found that pharmacists were not very confident or comfortable and found time and education to be the biggest barriers to implementation of the program. This reiterates the need for a more strategic approach to increase support for pharmacists’ involvement in the THN program. Addiction is a primary, chronic brain disease,25 and like other chronic diseases, clinical interventions by pharmacists can have an important impact on the health of these patients.
Limitations
A potential limitation of our study was the response rate of 11.2%; however, this response rate is in keeping with rates from other studies using online surveys of pharmacists.26,27 The low response rate may have introduced sampling bias as well as missing different opinions from those who did not respond. Other methods such as using the telephone, mailing hard copies and/or using incentives to motivate survey participation were not used because of methodological and financial constraints, but these may have yielded different response rates.27 Our choice of the summer months may have also affected the response rate; however, the response rate was no different for studies completed in the nonsummer months.26
Conclusion
We found that pharmacists had positive attitudes toward screening patients for risk of opioid overdose and recommending naloxone kits for patients at high risk of opioid overdose, and they were willing to participate in the THN program. However, pharmacists were not as confident or as comfortable in actual participation in the program. Addressing factors such as time and education may help increase pharmacists’ attitudes and involvement toward the program. In light of the current opioid crisis, pharmacists’ perspectives are key, as their role in harm reduction is essential to provide an opportunity to expand naloxone supply and reduce morbidity and mortality. ■
Supplementary Material
Footnotes
Author Contributions:J. Edwards initiated the project, was responsible for design and methodology, completed data collection, revised work for intellectual content and wrote the final draft. D. Bates initiated the project, contributed to the design of the work and revised the work for intellectual content. M. Yarema contributed to the design of the work and revised the work for intellectual content. B. Edwards completed data collection and revised the work for intellectual content. S. Ghosh completed statistics for the project. All authors approved the final version of the article.
Declaration of Conflicting Interests:The authors declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding:The authors received no financial support for the research, authorship and/or publication of this article.
References
- 1. Carter CI, Graham B. Opioid Overdose Prevention and Response in Canada. 2013;1-17. Available: www.drugpolicy.ca/publications/ (accessed May 17, 2016).
- 2. Canadian Center on Substance Abuse. Canadian drug summary: prescription opioids. July 2015. Available: www.ccsa.ca/ResourceLibrary/CCSA-Canadian-Drug-Summary-Prescription-Opioids-2015-en.pdf (accessed May 17, 2016).
- 3. Coroners Service of British Columbia. Fentanyl-detected illicit drug overdose deaths. 2016;1-6. Available: www2.gov.bc.ca/gov/content/safety/public-safety/death-investigation/statistical-reports (accessed May 17, 2016).
- 4. Etches N. Fentanyl and Take Home Naloxone: The Alberta experience. Apr. 28, 2016. Available: www.albertahealthservices.ca/assets/healthinfo/mh/hi-amh-thn-presentation-accpa.pdf (accessed May 13, 2017).
- 5.Fentanyl and the Take-Home Naloxone program Alberta Health. Available: www.health.alberta.ca/health-info/AMH-Naloxone-Take-home.html (accessed May 17, 2016).
- 6. Murphy Y, Goldner EM, Fischer B. Prescription opioid use, harms and interventions in Canada: a review update of new developments and findings since 2010. Pain Physician 2015;18:605-614. [PubMed] [Google Scholar]
- 7. Samuels EA, Dwyer K, Mello MJ, Baird J, Kellogg AR, Bernstein E. Emergency department-based opioid harm reduction: moving physicians from willing to doing. Acad Emerg Med 2016;23(4):455-465. Available: http://doi.wiley.com/10.1111/acem.12910 (accessed May 17, 2016). [DOI] [PubMed] [Google Scholar]
- 8. Jacobs P, Calder P, Taylor M, Houston S, Duncan Saunders L, Albert T. Cost effectiveness of Streetworks’ needle exchange program of Edmonton. Can J Public Health 1999;90(3):168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wodak A, Cooney A. Effectiveness of sterile needle and syringe programmes. Int J Drug Policy 2005;16(Suppl. 1). [Google Scholar]
- 10. Centres for Disease Control and Prevention (CDC). Syringe exchange programs—United States. Morb Mortal Wkly Rep 2007;56(44):1164-1167. Available: www.cdc.gov.login.ezproxy.library.ualberta.ca/mmwr/preview/mmwrhtml/mm5644a4.htm (accessed May 17, 2016). [PubMed] [Google Scholar]
- 11. Bayoumi AM, Zaric GS. The cost-effectiveness of Vancouver’s supervised injection facility. CMAJ 2008;179(11):1143-1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coffin PO, Sullivan SD. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann Intern Med 2013;158(1):1–9. [DOI] [PubMed] [Google Scholar]
- 13.Preventing fatal overdoses: a systematic review of the effectiveness of take-home naloxone. EMCDDA Pap 2015:1-37. [Google Scholar]
- 14. Clark AK, Wilder CM, Winstanley EL. A systematic review of community opioid overdose prevention and naloxone distribution programs. J Addict Med 2014;8(3):153-163. Available: www.ncbi.nlm.nih.gov/pubmed/24874759 (accessed Aug. 17, 2016). [DOI] [PubMed] [Google Scholar]
- 15. Giglio RE, Li G, DiMaggio CJ. Effectiveness of bystander naloxone administration and overdose education programs: a meta-analysis. Inj Epidemiol 2015;2:10. Available: www.injepijournal.com/content/2/1/10 (accessed Aug. 18, 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piper TM, Stancliff S, Rudenstine S, et al. Evaluation of a naloxone distribution and administration program in New York City. Subst Use Misuse 2008;43(7):858-870. [DOI] [PubMed] [Google Scholar]
- 17. Health Canada, Government of Canada. Notice: prescription drug list (PDL): naloxone. Mar. 22, 2016. Available: www.hc-sc.gc.ca/dhp-mps/prodpharma/pdl-ord/pdl-ldo-noa-ad-naloxone-eng.php (accessed May 17, 2016).
- 18. Watson T, Hughes C. Pharmacists and harm reduction: a review of current practices and attitudes. Can Pharm J (Ott) 2012;145(3):124-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillman DA, Smyth JD, Christian LM. Internet, phone, mail and mixed-mode surveys. 4th ed. New York: Wiley; 2014. Available: https://ebookcentral-proquest-com.login.ezproxy.library.ualberta.ca/lib/ualberta/detail.action?docID=1762797 (accessed Jul. 5, 2016). [Google Scholar]
- 20. Nielsen S, Menon N, Larney S, Farrell M, Degenhardt L. Community pharmacist knowledge, attitudes and confidence regarding naloxone for overdose reversal. Addiction 2016;111(12):2177-2186. Available: http://doi.wiley.com/10.1111/add.13517 (accessed Aug. 18, 2016). [DOI] [PubMed] [Google Scholar]
- 21. Green TC, Dauria EF, Bratberg J, Davis CS, Walley AY. Orienting patients to greater opioid safety: models of community pharmacy-based naloxone. Harm Reduct J 2015;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bachyrycz A, Shrestha S, Bleske BE, Tinker D, Bakhireva LN. Opioid overdose prevention through pharmacy-based naloxone prescription program: innovations in healthcare delivery. Subst Abus 2017;38(1):55-60. Available: www.ncbi.nlm.nih.gov/pubmed/27164192\nhttp://www.tandfonline.com/doi/full/10.1080/08897077.2016.1184739 (accessed Sep. 28, 2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bailey AM, Wermeling DP. Naloxone for opioid overdose prevention: pharmacists’ role in community-based practice settings. Ann Pharmacother 2014;48(5):601-606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nielsen S, Van Hout MC. What is known about community pharmacy supply of naloxone? A scoping review. Int J Drug Policy 2016;32:24-33. Available: http://dx.doi.org/10.1016/j.drugpo.2016.02.006 (accessed Aug. 18, 2016). [DOI] [PubMed] [Google Scholar]
- 25.American Society of Addiction Medicine (ASAM). Definition of addiction: Available: www.asam.org/quality-practice/definition-of-addiction (accessed Oct. 6, 2016). [Google Scholar]
- 26. Canadian Pharmacists Association. Pharmacy practice research abstracts: special supplement to the Canadian Pharmacists Journal. Can Pharm J (Ott) 2015;148(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hardigan PC, Popovici I, Carvajal MJ. Response rate, response time and economic costs of survey research: a randomized trial of practicing pharmacists. Res Soc Adm Pharm 2016;12:141-148. Available: www.sciencedirect.com/science/article/pii/S1551741115001278 (accessed Aug. 17, 2016). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


