Abstract
Prevalence of hepatitis C infection, which is associated with mortality and morbidity, is higher in chronic kidney disease patients on hemodialysis and transplant recipients when compared to non HCV infected patients. In addition to the conventional risk factors, HCV infection maybe an additional risk factor in the development of chronic kidney disease. HCV causes adverse effects leading to the poor long term outcome in renal transplant recipients; hepatitis C infection can cause both hepatic as well as extra hepatic complications. Prior evaluation and management of HCV infection is recommended for better long term outcome as there are chances of higher rejection rates with HCV treatment. However transplantation is not contraindicated in those patients who cannot be treated prior to the transplantation as patient survival is better when compared to dialysis patients. Kidney Disease Outcome Quality Initiative Clinical Practice Guidelines recommend interferon based therapy only when there is a rapid worsening of HCV related hepatic injury in transplant recipients. HCV treatment has been improved by the addition of direct acting antiviral, protease inhibitors and polymerase inhibitors. Combination therapies are showing improved sustained virological response rates. NS3-4A protease inhibitors, nucleotidic/nucleosidic NS5A and NS5B polymerase inhibitors are promising treatments which are under trials with different combinations. The focus of this review is to evaluate and optimize the treatment options of co-existing HCV infection in renal transplant recipients and discuss more promising alternative treatment regimen.
Keywords: Transplantation, Hepatitis C virus, Treatment options, Diagnostics
Introduction
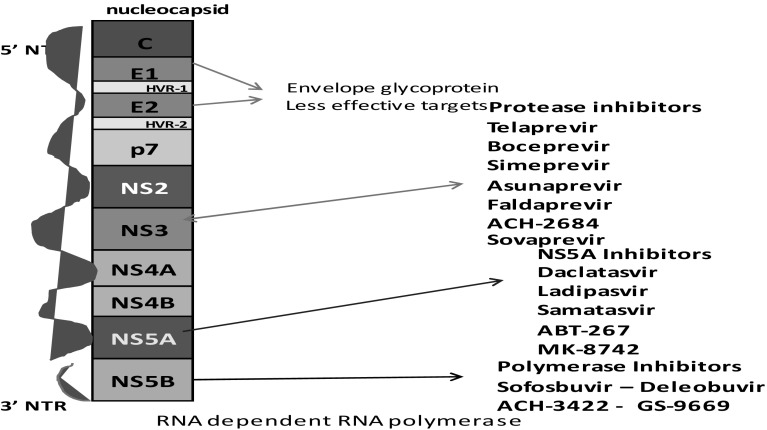
Hepatitis C virus is a small circular, enveloped, single stranded ribonucleic acid virus belonging to group IV (+) ssRNA of family Flaviviridae, genus Hepacivirus and species-Hepatitis C virus. The total genome is about 9.6 kb length with 5′ and 3′ un translated regions at both edges. It is comprised of 10 viral proteins including 3 structural proteins-C, E1 and E2, and 7 non-structural proteins which include P7, NS2, NS3, NS4A, NS4B, NS5A and NS5B. The structural proteins are important components of nucleocapsid, and core protein modulates gene transcription and cell proliferation.
There are nearly 500,000 deaths occurring globally in a calendar year due to hepatitis C infection [27]. The burden of HCV is immense in low and middle-income countries, and India contributes a significant share (1–1.5%) of global HCV infections. Chronic hepatitis C virus infection remains a major cause of morbidity and mortality in dialysis patients and kidney transplant recipients. Patients with chronic kidney disease are at an increased risk for acquiring HCV either because of their frequent exposure to blood from transfusions, or by exposure to HCV by nosocomial transmission, or during hemodialysis. The prevalence of HCV positivity varies from 5 to 60% in hemodialysis patients globally [56]. Around 12–18 million people are infected with HCV in India. It has been observed that anti HCV antibodies have an incidence ranging from 4.3 to 46% in dialysis patients [41].
Its prevalence in transplant recipients, when compared to that in the general population, is significantly higher, and is associated with lower long term graft survival as compared to HCV negative recipients. 8–28% deaths were reported due to liver failure in long term kidney transplant recipients [24]. In addition to hepatic complications, it is also associated with extra hepatic complications leading to chronic glomerulopathy [6], chronic allograft nephropathy [29], and new onset of diabetes, etc. [30]. As this population is immune compromised, the diagnosis of HCV requires reverse transcriptase polymerase chain reaction (RT-PCR). Despite recent advances in diagnosis and therapeutics, the management of HCV infection remains a great challenge. There are limited studies in renal transplant population and a few review articles discussed about the insights of HCV infection, liver disease and management [33, 35]. This review describes the disease mechanism, epidemiology, diagnosis, outcome of the allograft, emphasizing on the existing treatment and current advances. And also discusses the novel therapeutic approaches using direct acting antiviral (DAA) treatment protocols for HCV infected renal transplant patients.
“The World Health Organization (WHO) has recently recommended that the prevention of chronic kidney disease (CKD) within the non communicable disease (NCD) programs as CKD is a key determinant of poor health outcome of major NCDs”.
Disease mechanism
The role of HCV in morbidity and mortality of renal disease is often not considered in the care of patients with CKD; nonetheless, HCV infection has been proposed as a possible cause of CKD. It was reported that there is an association between HCV infection and glomerular disease in both native kidneys and in kidneys [6] post-transplant. The most common type of HCV-related glomerulonephritis is type 1 membranoproliferative glomerulonephritis, usually in the context of type II cryoglobulinemia. A meta-analysis of four clinical studies with 81,286 individuals stated that HCV sero-positivity was a significant independent factor for proteinuria in the general population [11]. It has also been reported that there is an association between proteinuria and components of metabolic syndrome, higher incidence of dyslipidemia, insulin resistance and arterial hypertension. Kidney disease improving global outcomes (KDIGO) guidelines recommended that patients infected with HCV be tested for proteinuria and hematuria at least once a year, as HCV associated glomerular disease manifests itself in the former. The disappearance of proteinuria after interferon therapy confirms its pathogenic role. Antigens of HCV were found in the kidney tissue of patients with HCV negative glomerulonephritis, occult HCV infection could be the underlying cause of glomerular disease.
Various causes, such as cytopathic activity of HCV on renal parenchyma, could be the underlying mechanisms of renal disease. CD8 and SR-B1 receptors of renal parenchyma allows the binding of HCV to the cell surface. Proteins of HCV were identified in the tubular epithelial cells, endothelial cell and mesangial cells of glomerular and tubular capillaries, which could reflect mesangial injury by HCV [42]. An over stimulation of B-lymphocytes and production of mixed cryoglobulins (Polyclonal immunoglobulins IgG or IgM) was caused by persistent HCV infection. Deposition of circulating immune complexes containing HCV proteins, anti-HCV antibody, is also associated with HCV related glomerular injury. The probable mechanism which aids in B-lymphocyte alteration need not require B cell infection, as this mechanism may occur between the surface CD81 protein and the extracellular E2 of the virion (Fig. 1). It has been proposed that anti-HCV-IgG and HCV lipoprotein complexes may act as B-cell antigens inducing the synthesis of non-HCV reactive IgM. Immune complexes are formed by these auto antibodies and are deposited in small to medium blood vessels, resulting in complement activation and extra hepatic injury.
Fig. 1.
Interaction between virions E2 and cell surface molecules CD81, SR-B1Occludin. Interaction of cell surface molecules induces expression of activation induced deaminases and mutations of immuniglobulin genes leading to the proliferation
An increase in the toll-like receptor 3 (TLR3) messenger RNA expressions has been observed in the glomeruli, mainly in the mesangial cells of the HCV induced glomerulo nephritis patients, when compared to HCV negative and healthy controls [55]. TLRs identify molecular patterns of microbial agents and are expressed on immune and non-immune cells, representing the innate immune response. An excess production of endothelin1, increased oxidative stress, insulin resistance, intra renal synthesis of IGF-1 and TGFβ promote proliferation of renal cells and lead to the progression of the kidney damage.
Membrano-proliferative glomerulonephritis (MPGN) and membranous glomerulonephritis (MGN), with or without cryoglobulinemia, are the most commonly occurring lesions associated with chronic HCV infection in renal transplant recipients. The probable reason for the formation of HCV-protein containing complexes in the glomeruliis rooted in immunosuppressive therapy, leading to the increase in the HCV viral load and reduction in immunoglobulin synthesis, thereby resulting in the imbalance of antigen antibody complex. This process may interfere with the clearance of the viral load, leading to the development of HCV protein deposits in the allograft [32].
Diagnosis and diagnostic methods
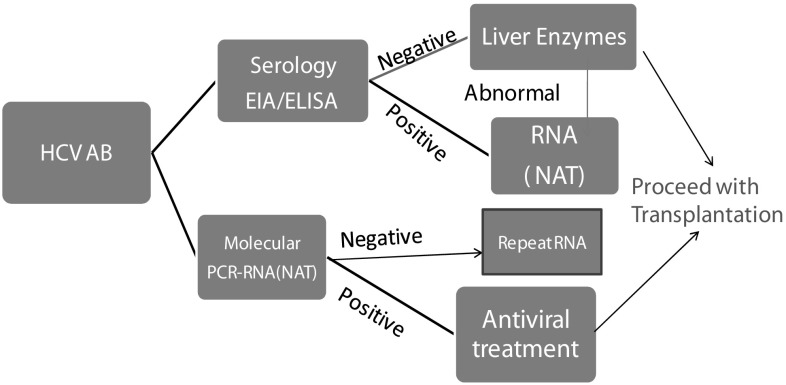
Hepatitis C virus is a small single stranded RNA virus, with six known genetic groups. Two types of assays are available in the detection of hepatitis infection. Serological assays which detect antibodies to HCV (antiHCV) are enzyme immunoassay (EIA) and enzyme linked immunosorbent assay (ELISA). Additionally, molecular diagnostic assays (HCV RNA) that detect viral nucleic acid have been frequently used to screen and diagnose HCV infection (Fig. 2).
Fig. 2.
Screening approach for HCV. EIA enzyme linked immunoassays, ELISA enzyme linked immune sorbent assay, NAT nucleic acid amplification test
Serologic tests
These are used to screen and diagnose infection. False negative results may occur with serological tests in immune compromised, immune suppressed patients (such as patients on long term hemodialysis and organ transplant patients), even though the specificity of these test is high. On the other hand, serological assays are less sensitive when the rates of anti-HCV antibodies diminish, especially in individuals undergoing dialysis [43]. However, third and fourth generation EIA anti HCV assays yield 100% sensitivity, which could be helpful in immune suppressed patients. There is an average 8-week serological window between the infection and the production of antibodies, and consequently, there is a delay/lack of production of antibodies in these patients. In order to confirm the results, molecular tests like HCV RNA-Nucleic acid amplification test (NAT) detection should be performed.
Molecular tests
Qualitative or quantitative tests with a detection level of 25 IU/ml or lower should be used to detect HCV RNA. All the recently available methods have high sensitivity and specificity. A quantitative HCV RNA testing is necessary to know the baseline viral load prior to the initiation of HCV therapy. Testing for specific genotypes may aid in selection of the appropriate therapeutic regimen. A positive anti HCV test and a negative HCV RNA may denote that there is no/little evidence of current active infection. False positive anti HCV tests are more common with low prevalence of HCV infection. To distinguish between false positivity and true positivity for HCV antibody, a method that is different from the assay used for initial testing should be employed (a biological false result ideally should not happen with two different methods).
Genotype tests
These assays are useful in the specific therapeutic approach. There have been seven major (1–7) genotypes which are divided into several subtypes. Genotype 1 and 3 are more common in India. HCV-1, HCV-2 and HCV-3 are distributed globally, whereas HCV-4, HCV-5 and HCV-6 are present in only certain parts of the world. The half life of virus is 45 min to 3 h.
HCV infection and renal transplant
The issue of graft function and patient survival has been discussed and debated in many clinical trials. The results are contradictory and complicated to interpret. Some studies reported lower patient survival rates in HCV infected recipients while the others reported similar rates of survival with HCV negative [2] recipients. Pereira et al. [38] observed an increased risk of deaths due to HCV infection in renal transplant recipients (RTx). It was also reported that HCV infected patients who underwent interferon therapy prior to the transplant (which cleared their viremia) have had similar transplant outcomes compared to HCV negative recipients [17]. There could be several factors resulting in the variations in these studies, chiefly false positivity, current active infection status, pre transplant liver pathology, HCV genotypes, serological response maybe delayed and presence of antibodies after the disappearance of viral RNA.
It is well known that transplant recipients with chronic HCV infection can develop progressive liver disease, a leading cause of morbidity and mortality. 7–24% of the transplant recipients demonstrated abnormal liver function tests. Deaths due to liver failure occurred 8–28% in this group [39]. It has been speculated that decreased survival rates could also be related to the increased risk of cardiovascular disease, new onset of diabetes after transplantation, and sepsis. There were contradicting reports about the progression of hepatic fibrosis: some studies reported accelerated progression while others observed slow progression after transplantation [45, 57]. Immunosuppressive therapy is a major cause for the development of rapid and aggressive HCV related infection and liver disease. However the impact of immunosuppression on the progression of liver injury remains unclear. Earlier studies reported that immunosuppression may prevent/delay the clearance of the virus, resulting in the worsening of the liver disease due to increase in HCV replication [57].
HCV infection in RTx recipients has been associated in the pathogenesis of acute glomerulopathy and de novo immune complex glomerulonephritis in the transplanted kidney. Decrease in allograft function/survival could be due to the development of HCV related de novo glomerulonephropathy. Moreover, chronic hepatitis C infection might also contribute in the development of chronic allograft dysfunction, which is the major cause of long term outcome failure [34]. Scott et al. [50] reported an increased rate of graft failure in HCV positive recipients with a hazard ratio of 1.87, owing to chronic allograft dysfunction. Spanish chronic allograft nephropathy group [36] analyzed 4304 RTx recipients and noticed the association of hepatitis infection with early proteinuria, CAD, and graft loss as compared to HCV negative recipients. They also observed lower rates of graft survival at 4 years after transplant when compared to HCV negative recipients. It was also mentioned in the same study that the presence of HCV associated GN and transplant glomerulopathy were higher in hepatitis C patients. 45.4 and 18.2% of de novo MPGN and MGN were reported by Cruzado et al. [7] in HCV positive RTx recipients compared to 5.7 and 7.7% in HCV negative recipients respectively. Despite all these adverse effects, renal transplantation improves the survival in HCV positive transplant patients compared to HCV positive dialysis patients [23].
Even higher rates of acute rejection (AR) due to HCV induced immune reactivity have been proposed [44]. Yet again there has been contradicting reports about the impact of HCV on incidence of acute rejection. Some groups noticed a decrease in the rate of AR [22], while the others have reported either similar or higher rates of AR in HCV positive RTx recipients [26].
Lopez et al. in their study of 105 HCV positive recipients stated that HCV infection was an independent risk factor for the recurrent rejections [26]; on the contrary, Forman et al. [14] stated that HCV is not at all an independent risk factor for AR when adjusted with covariates like panel reactive antibodies (PRA) more than 20%. In 2006 Moreso et al. [37] in their study with protocol biopsies found that both HCV infection and subclinical acute rejection were independent risk factors associated with late graft loss.
HCV infection has been associated with an increased incidence of new onset of diabetes after transplantation (NODAT). The probable mechanism involved could be insulin resistance with an altered glucose metabolism. The incidence may be higher when the traditional risk factors like metabolic syndrome, older age, BMI and family history of diabetes are involved [4]. Fabrizi et al. [9] performed a meta-analysis of HCV seropositivity and association of NODAT, and confirmed HCV as an independent risk factor (four fold increased risk) for the development of NODAT. There were no prospective studies that analyzed the impact of pre transplant HCV treatment and the development of NODAT, even though a relative risk of 1.3–1.4 is associated with HCV positivity [51]. However it has been reported that pre transplant IFN treated recipients with undetectable HCV RNA in their serum have a lower risk of incidence of NODAT [21]. Fenni et al. [13] reported a high frequency of NODAT in RTx recipients who were using tacrolimus as part of their immunosuppression.
There is an evidence of association of hepatitis C infection and non Hodgkin’s lymphoma in general population, while it is noted that there was a 5.7 fold increase in HCV infected transplant recipients [31]. While reports have suggested malignancy to be the third leading cause of the death [50], there is no substantial data on the HCV associated malignancies in RTx.
Impact of immunosuppression on HCV
Viral load tends to be higher after transplant, especially in the early post transplant period as compared to the pre-transplant period, which has been correlated with the use of immunosuppressive therapy. Currently, there is not much data available on the impact of immunosuppression on hepatitis C infected renal transplant recipients. It is very challenging to maintain drug–drug interaction between immunosuppression and HCV treatment in this patient population. Calcineurin inhibitors (CNI)—for instance, cyclosporine (CyA) and tacrolimus, which are the mainstay immunosuppressive drugs, inhibit calcineurin—a key enzyme in the production of IL-2 by T-cells, which in turn determines immune status. Immunosuppression has significant effect on HCV replication. HCV infection may influence CNI metabolism. Tacrolimus which is a preferred drug over cyclosporine has been shown to have a higher risk of NODAT in HCV positive patients. However, CyA has anti HCV activity leading to the inhibition of viral replication [54]. It is still unclear whether this is beneficial or not. In a large study of 75,000 RTx recipients of whom 3708 subjects were HCV infected patients, it was seen that both TAC and CyA are not associated with any benefit in hepatitis C infected patients and also that neither CNI has advantage over the other [28]. CNIs may reduce the clearance of ribavirin (RBV), leading to the ribavirin associated hemolytic anemia.
Mycophenolate mofetil also has an inhibitory effect on the viral replication in both non-transplant and transplant recipients, without adverse effect on patient and allograft outcome [1, 20]. Despite all the adversities, transplantation remains the best option for ESRD patients with HCV positivity.
Treatment of hepatitis C infection in renal impairment
It is highly challenging to treat transplant recipients as HCV infection can cause hepatic as well as extra hepatic consequences and the use of antiviral drugs, such as interferon/peginterferon (IFN/PEGIFN) can considerably increase the risk of graft rejection. Hence it was suggested that HCV diagnosis and management should be performed prior to the transplantation. Over the last two decades, HCV therapy has become successful with the introduction of IFN monotherapy. Interferons are a class of potent immune-modulators which activate interferon stimulated genes and release Th1 cytokines and decrease the activity of IL10, resulting in tissue damage and inflammation. Hepatitis C virus clearance during post-transplant IFN treatment would improve hepatic microsomal function, there by leading to decreased immunosuppressant levels resulting in the development of acute cellular rejection. New treatments involving addition of ribavirin and pegylated interferon have enhanced the cure rates with an increasing sustained viralogical response (SVR). There was an additional improvement in the treatment of HCV infection with the approval of direct acting anti-virals and protease inhibitors. Whatever be the advancements in the development of new generation antiviral therapies, it is very critical in treating renal transplant as well as renal insufficiency patients. The kidney plays a main role in the catabolism and filtration of these drugs, which could result in the reduction in the clearance of these drugs in the former groups of patients, leading to overexposure to these drugs. Especially in patients on hemodialysis the clearance is negligible as both interferon/peginterferon are very large molecular weight substances and dialysis cannot remove these substances resulting in drug induced toxicity. Moreover ribavirin can accentuate hemolytic anemia by accumulating its metabolites in erythrocytes and erythroblasts.
There are contradictory reports about the usage of conventional IFNα and PEG-IFN in these patients. A meta-analysis was conducted on 11 studies on hemodialysis patients [47]: 213 patients received IFN α and 153 patients received IFN α-2b for 6-12 months showed a overall SVR of 33%, while a sub analysis of 123 patients with genotype 1 showed a SVR of 26%. Although no treatment related deaths were reported, 30% of the patients were discontinued from the study due to drug related adverse effects. Further studies with IFN mono therapy treatment showed an SVR of 41% with a discontinuation of 26% due to drug related side effects [18]. Other meta-analysis with PEGIFN mono therapy in hemodialysis patients reported that there were no significant differences in the SVR [3, 10]. However KDIGO guidelines recommend IFN α monotherapy for CKD patients with hepatitis C infection. However, PEGIFN has replaced IFN due to its longer half life and is required once a week compared to the thrice a week IFN dosage.
Ribavirin, a nucleoside analogue, enhances the SVR rates and lowers relapse rate when taken in combination with IFN α [52]. It has been reported that a low dose of ribavirin in combination with PEGIFN has shown good efficacy and tolerability with higher SVR rates when compared to PEGIFN monotherapy [25]. In a meta-analysis conducted on 11 studies of hemodialysis patients with combined PEGIFNα2a/2b plus ribavirin, the SVR rate was 60% and there was no difference between PEGIFNα2a and α2b [12]. An individualized dosing of ribavirin (200 mg/day or/alternate days or/thrice weekly) post hemodialysis with supplements of erythropoietin and iron with strict monitoring of hemoglobin has been proposed in the international guidelines.
There is not much data available on the triple-drug regimen consisting of peginterferon plus ribavirin plus protease inhibitors (either boceprevir or telaprevir) in patients with CKD/transplant recipients (Tables 1, 2) [8].
Table 1.
HCV treatment—pre and post transplant approach
| Renal impairment | Treatment/dose | Comments |
|---|---|---|
| Mild CrCl-50-80 ml/mt | Peg IFN+ RBV/no dose adjustment required | |
| Moderate CrCl-30–50 ml/mt | Standard dose of DAA/PegIFNα2a daclatasvir, ledipasvir–sofosbuvir, ombitasvir–paritaprevir–ritonavir, ombitasvir–paritaprevir–ritonavir plus dasabuvir, simeprevir, sofosbuvir, orpeginterferon alfa-2a | PegIFNα-2b should be reduced by 25% (1.125 mcg/kg once weekly) RBV-200 mg alternating with 400 mg |
| Severe/ESRD CrCl-<30 ml/mt | IFN monotherapy Dual therapy-PegIFN+RBV (low dose) |
Based on the genotype RBV should be discontinued if Hb decreased v by 2 gm/dl despite using erythropoitin |
| Post transplant | Peg IFN—contraindicated Unless-worsening of liver damage IFN-associated with graft rejection |
Good recovery of renal function after Tx Use of new DAA agents may provide excellent interferon-free treatment options |
Guide lines of the American Association for the study of liver diseases and the infectious diseases society of America (AASLD/IDSA)
CrCl creatinine clearance, DAA direct acting antiviral, ESRD end stage renal disease, Hb hemoglobin, RBV ribavirin, IFN interferon
Table 2.
Génotypes and anti viral therapies
| Genotype | Treatment option | Comment |
|---|---|---|
| Genotype 1a | Ombitasvir–paritaprevir–ritonavir and dasabuvir + reduced-dose ribavirin (200 mg thrice weekly to 200 mg once daily) | Triple therapy- PegIFN + sofosbuvir/simeprevir/faldaprevir IFN free sofosbuvir + RBV |
| Genotype1b | Ombitasvir–ritonavir + dasabuvir | Limited data |
| Genotype 2 | IFN free sofosbuvir + RBV | |
| Genotype 3 | Triple therapy IFN free sofosbuvir + RBV |
|
| Genotype 4 | PegIFN + sofosbuvir + RBV | |
| Genotype 5/6 | PegIFN + sofosbuvir + RBV |
RBV ribavirin, IFN interferon
New approaches to the treatment
There is a vast development in the treatment of HCV such as direct acting antiviral agents (DAA)-namely-declatasvir, ombitasvir–paritaprevir–ribavirin, dasabuvir, Sofosbuvir and simeprevir etc. Boceprevir and telaprevir are inhibitors of NS3 protease. There are many clinical studies with DAA based therapy. In the study A1444-063, investigators studied the safety and the pharmacokinetics of a single dose of 60 mg of daclatasvir. They compared renal failure patients with healthy controls and found that there is good tolerability and that the increase in the drug level in CKD patients was within the normal range [15]. The pharmacokinetics studies of sofosbuvir with different combinations (sofosbuvir plus peginterferon plus ribavirin, sofosbuvir plus ribavirin,sofosbuvir plus simeprevir, and sofosbuvir plus simeprevir plus ribavirin) noted that the SVR rates were high, ranging from 81 to 89%. The metabolite (GS-331007) of sofosbuvir is eliminated via renal clearance. No dosage adjustment is required in patients with GFR ≥ 30 ml/mt. Pharmacokinetic studies of sofosbuvir in renal impaired HCV negative patients compared to normal renal function subjects showed a significant increase in the serum levels of sofosbuvir and the metabolite. However no data is available in patients with severe renal impairment. In a recent controlled prospective study of pharmacokinetics sofosbuvir plus declatasvir involving HCV positive kidney transplant patients with a GFR of 30–60 ml/mt reported that administration of these drugs is safe and efficient and does not lead to drug accumulation. No significant change in GFR was observed according to the baseline GFR level. Preliminary pharmacokinetic data of 400 mg/day in ESRD patients showed extreme high levels of drug and the metabolite, however half dose of sofosbuvir plus full dose simeprevir regimen showed safe and well tolerated in severe renal impairment [5].
Investigational agents like grazoprevir and elbasvir were studied in C-SURFER, a phase 2/3 trial using a 12 week course, enrolling HCV genotype 1 patients with ESRD who failed PEGIFN therapy and also untreated patients. 99% of the patients achieved SVR12 (Sustained viral response: undetectable viral load 12 weeks [46]).
Treatment approach in renal transplant recipients
There are limited options of antiviral treatment (Fig. 3) after transplantation especially early post transplant period, due to the risk of allograft rejection. The safety and efficacy of interferon therapy in renal transplant recipients is disappointing [49]. KDOQI clinical practice guidelines (2008 Kidney Disease Outcome Quality Initiative) recommended IFN therapy only when there is rapid worsening of HCV related hepatic injuries like cirrhosis, fibrosing cholestatic hepatitis or vasculitis in the allograft [16]. Although the SVR rates are disappointing, PEG IFN has proved better when compared to conservative IFN therapy in safety and efficacy. It has also been reported that the rejection rates were lower when treated with PEGIFN in combination with RBV compared to IFN mono therapy. This could be because of dual therapy, duration between the antiviral treatment and the transplantation, and lastly, the process of pegylation [40].
Fig. 3.
The HCV genome with 5′ and 3′ untranslated regions and ten viral proteins: structural proteins-C, E1 and E2 and non-structural proteins. P7, NS2, NS3, NS4A, NS4B, NS5A and NS5B C: core protein
IFN free therapy is a promising treatment option after transplantation because of its reduced toxicity and high efficacy. Sofosbuvir targets different steps of the Hepatitis C Virus (HCV) life cycle. It blocks the polymerase enzyme used in the replication of HCV virus. Sofosbuvir has no inhibitory effect, while simeprevir, a second wave protease inhibitor, shows mild inhibitory effect on hepatic Cyp3A4 enzyme [53]. Previous non-responders to the treatment and IFN contraindicated patients were recommended this regimen. Sofosbuvir holds several advantages over current therapy due of its safety, efficacy, tolerability and dosing simplicity in different patient populations and HCV genotypes. The combination of daclatasvir and sofosbuvir can be considered a good upcoming option in the post-transplantation setting.
Vitamin D levels may affect the HCV treatment response. It has been suggested that vitamin D supplementation may enhance the treatment response [19]. Inhibitors of non-structural protein5A and host targeted compounds like silibinin and cyclophilin inhibitors are under investigation [48]. Appropriate treatment strategies in the pre and post transplant stages will be helpful in achieving good long term outcome, preventing morbidity and mortality caused by HCV infection.
Recent Phase 3 trial TIGER was conducted in East Asian countries (China, South Korea) wherein the chronic hepatitis C genotype 1 was treated with OLYSIO (simeprevir) in combination with PEGIFNα and RBV. Reports indicated that the safety profile was similar to that of global trials; however they showed a higher incidence of hyper-bilirubinemia in patients receiving 150mgOLYSIO,PEGIFNα and RBV compared to those receiving placebo + PEGIFNα + RBV. The reports stated that the SVR12 rates were 91% in OLYSIO group compared to 76% in the placebo group.
Concluding remarks
Hepatitis C infection prevalence is higher in patients on hemodialysis and renal transplant recipients, causing morbidity and mortality in comparison with uninfected patients. Several factors influence the progression of HCV related CKD. In addition to the conventional risk factors, HCV infection contributes to the impairment of patient and graft survival. All patients on dialysis should be tested for HCV antibodies and depending on the results should be further investigated. Prior to the initiation of antiviral treatment HCV RNA test is necessary to know the genotypes which may aid in selection of appropriate therapeutic regimen. IFN free regimens are promising alternatives in renal transplant patients. Dual therapy of pegylated IFN in combination with ribavirin is a promising therapy in dialysis settings. Several combinations of two or three Direct Acting Antivirals can cure HCV in the majority of treatment-naive patients. Hepatitis C infection is not a contraindication for renal transplantation as it has been proved that the survival rates were better after transplantation than on dialysis. In conclusion there are several promising alternative treatment regimens with minimal side effects and high SVR for HCV infection in this unique group.
References
- 1.Abbott KC, Bucci JR, Matsumoto CS, Swanson SJ, Agoda LYC, Holtzmuller KC, et al. Hepatitis C and renal transplantation in the era of modern immunosuppression. J Am Soc Nephrol. 2003;14(11):2908–2918. doi: 10.1097/01.ASN.0000090743.43034.72. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal SK, Dash SC, Irshad M, Gupta S, Bhowmik D, Tiwari SC. Impact of hepatitis C virus infection on renal transplant outcomes in India—a single centre study. J Assoc Phys India. 2000;48:1155–1159. [PubMed] [Google Scholar]
- 3.Alavian SM, Tabatabaei SV. Meta-analysis of factors associated with sustained viral response in patients on hemodialysis treated with standard or pegylated interferon for hepatitis C infection. Iran J Kidney Dis. 2010;4:181–194. [PubMed] [Google Scholar]
- 4.Al-Ghareeb SM, El-Agroudy AE, Arrayed Al, Al SM, Arrayed A, Alhellow HA. Risk factors and outcomes of new-onset diabetes after transplant: single-centre experience. Exp Clin Transplant. 2012;10:458–465. doi: 10.6002/ect.2012.0063. [DOI] [PubMed] [Google Scholar]
- 5.Bhamidimarri KR, Czul F, Peyton A, Levy C, Hernandez M, Jeffers L, Roth D, Schiff E, O’Brien C, Martin P. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of hepatitis C in patients with end stage renal disease. J Hepatol. 2015;63(3):763–765. doi: 10.1016/j.jhep.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Cruzado JM, Carrera M, Torras J, Grinyó JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1(2):171–178. doi: 10.1034/j.1600-6143.2001.10212.x. [DOI] [PubMed] [Google Scholar]
- 7.Cruzado JM, Carrera M, Torras J, Grinyó JM. Hepatitis C virus infection and de novo glomerular lesions in renal allografts. Am J Transplant. 2001;1(2):171–178. doi: 10.1034/j.1600-6143.2001.10212.x. [DOI] [PubMed] [Google Scholar]
- 8.Dumortier J, Guillaud MC, Gagnieu MC, JanbonB JL, Morelon E, et al. Anti viral tripletherapy with telaprevir in hemodialysedpatients:is it feasible? J Clin Virol. 2013;56:146–149. doi: 10.1016/j.jcv.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Kanwal F, Dulai G. Post-transplant diabetes mellitus and HCV seropositive status after renal transplantation: meta-analysis of clinical studies. Am J Transplant. 2005;5:2433–2440. doi: 10.1111/j.1600-6143.2005.01040.x. [DOI] [PubMed] [Google Scholar]
- 10.Fabrizi F, Dixit V, Messa P, Martin P. Pegylated interferon monotherapy of chronic hepatitis C in dialysis patients: meta-analysis of clinical trials. J Med Virol. 2010;82:768–775. doi: 10.1002/jmv.21542. [DOI] [PubMed] [Google Scholar]
- 11.Fabrizi F, Martin P, Dixit V, Messa P. Hepatitis C virus infection and kidney disease: a meta-analysis. Clin J Am Soc Nephrol. 2012;7(4):549–557. doi: 10.2215/CJN.06920711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fabrizi F, Dixit V, Messa P, Martin P. Anti viral therapy (Pegylated interferon and ribavirin) of chronic hepatitis C in dialysis patients: meta-analysis of clinical trials. J Viral Hepat. 2014;21:681–689. doi: 10.1111/jvh.12276. [DOI] [PubMed] [Google Scholar]
- 13.Finni PF, Souza ER, Rioja S, Ventura S, Starling P, Almedia JR, et al. Is hepatitis C a risk factor for post transplant diabetes mellitus after renal transplantation in patients using tacrolimus. Transplant Proc. 2004;36:884–885. doi: 10.1016/j.transproceed.2004.03.091. [DOI] [PubMed] [Google Scholar]
- 14.Forman JP, Tolkoff-Rubin N, Pascual M, Lin J. Hepatitis C, acute humoral rejection, and renal allograft survival. J Am Soc Nephrol. 2004;15:3249–3255. doi: 10.1097/01.ASN.0000145896.16153.43. [DOI] [PubMed] [Google Scholar]
- 15.Garimella T, Wang R, Luo WL, Hwang C, Sherman D, Kandoussi H, Marbury TC, Alcorn H, Bertz R, Bifano M. Single-dose pharmacokinetics and safety of daclatasvir in subjects with renal function impairment. Antivir Ther. 2015;20:535–543. doi: 10.3851/IMP2941. [DOI] [PubMed] [Google Scholar]
- 16.Ghany MG, Strader DB, Thomas DL, Seeff LB, American Association for the Study of Liver Diseases Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Roncero F, Gentil MA, Valdivia MA, Algarra G, Pereira P, Toro J, et al. Outcome of kidney transplant in chronic hepatitis C virus patients: effect of pre transplantation interferon-alpha 2b monotherapy. Transplant Proc. 2003;35:1745–1747. doi: 10.1016/S0041-1345(03)00717-6. [DOI] [PubMed] [Google Scholar]
- 18.Gordon CE, Uhlig K, Lau J, Schmid Ch, Levey AS, Wong JB. Interferon treatment in hemodialysis patients with chronic hepatitis C virus infection. A systematic review of the literature and meta-analysis of treatment efficacy and harms. Am J Kidney Dis. 2008;51:263–277. doi: 10.1053/j.ajkd.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Halegoua-De Marzio D, Fenkel J. Alternative medications in Hepatitis C infection. World J Hepatol. 2014;6(1):9–16. doi: 10.4254/wjh.v6.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahraman A, Witzke O, Scherag A, Putter C, Miller M, Dechene A, et al. Impact of immunosuppressive therapy on hepatitis C infection after renal transplantation. Clin Nephrol. 2011;75(1):16–25. [PubMed] [Google Scholar]
- 21.Kamar N, Toupance O, Buchler M, Sandres-Saune K, Izopet J, Durand D, et al. Evidence that clearance of hepatitis C virus RNA after alpha-interferon therapy in dialysis patients is sustained after renal transplantation. J Am Soc Nephrol. 2003;14:2092–2098. doi: 10.1097/01.ASN.0000079613.81511.3C. [DOI] [PubMed] [Google Scholar]
- 22.Kliem V, Burg M, Haller H, Suwelack B, Abendroth D, Fritsche L, et al. Relationship of hepatitis B or C virus prevalences, risk factors, and outcomes in renal transplant recipinets: analysis of German data. Transplant Proc. 2008;40:909–914. doi: 10.1016/j.transproceed.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Knoll GA, Tankersley MR, Lee JY, Julian BA, Curtis JJ. The impact of renal transplantation on survival in hepatitis C positive end stage renal disease patients. Am J Kidney Dis. 1997;29:608–614. doi: 10.1016/S0272-6386(97)90345-0. [DOI] [PubMed] [Google Scholar]
- 24.Kokado Y, Takahara S, Ichimaru N, Toki K, Wang JD, Permpongkosol S, et al. Clinical outcome of HCV infection after renal transplantation. Transplant Proc. 2000;32(7):1940–1943. doi: 10.1016/S0041-1345(00)01503-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu CH, Huang CF, Liu CJ, Dai CY, Liang CC, Huang JF, Hung PH, Tsai HB, Tsai MK, Chen SI, et al. Pegylated interferon-α2a with or without low-dose ribavirin for treatment-naive patients with hepatitis C virus genotype 1 receiving hemodialysis: a randomized trial. Ann Intern Med. 2013;159:729–738. doi: 10.7326/0003-4819-159-11-201312030-00005. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Medrano F, Fernandez-Ruiz M, Morales JM, JuanSR CC, Carratala J, et al. Impact of hepatitis C virus infection on the risk of infectious complications after kidney transplantation: data from the RESITRA/REIPI cohort. Transplantation. 2011;92:543–549. doi: 10.1097/TP.0b013e318225dbae. [DOI] [PubMed] [Google Scholar]
- 27.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luan FL, Schaubel DE, Zhang H, Jia X, Pettetier SJ, Port FK, Magee JC, et al. Impact of immunosuppressive regimen on survival of kidney transplant recipients with hepatitis C. Transplantation. 2008;85:1601–1606. doi: 10.1097/TP.0b013e3181722f3a. [DOI] [PubMed] [Google Scholar]
- 29.Mahmoud IM, Elhabashi AF, Elsawy E, El-Husseini AA, Sheha GE, Sobh MA. The impact of hepatitis C virus viremia on renal graft and patient survival: a 9-year prospective study. Am J Kidney Dis. 2004;43(1):131–139. doi: 10.1053/j.ajkd.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 30.Martin P, Fabrizi F, Hepatitis C. Virus and kidney disease. J Hepatol. 2008;49(4):613–624. doi: 10.1016/j.jhep.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo K, Kusano A, Sugumar A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of epidemiological studies. Cancer Sci. 2004;95:745–752. doi: 10.1111/j.1349-7006.2004.tb03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morales JM. Hepatitis C virus infection and renal disease after renal transplantation. Transplant Proc. 2004;36(3):760–762. doi: 10.1016/j.transproceed.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 33.Morales JM, Aguado JM. Hepatitis C and renal transplantation. Curr Opin Organ Transplant. 2012;17(6):609–615. doi: 10.1097/MOT.0b013e32835a2bac. [DOI] [PubMed] [Google Scholar]
- 34.Morales JM, Campistol JM. Transplantation in the patient with hepatitis C. J Am Soc Nephrol. 2000;11:1343–1353. doi: 10.1681/ASN.V1171343. [DOI] [PubMed] [Google Scholar]
- 35.Morales JM, Fabrizi F. Hepatitis C and its impact on renal transplantation. Nat Rev Nephrol. 2015;11:172–182. doi: 10.1038/nrneph.2015.5. [DOI] [PubMed] [Google Scholar]
- 36.Morales JM, Marcén R, Andres A, Gil BD, Campistol JM, Gallego R, et al. Renal transplantation in patients with hepatitis C virus antibody. A long national experience. NDT Plus. 2010;3(suppl 2):ii41–ii46. doi: 10.1093/ndtplus/sfq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moreso F, Ibernon M, Goma M, Carrera M, Fulladosa X, Hueso M, et al. Subclinical rejection associated with chronic allograft nephropathy in protocol biopsies as a risk factor for late graft loss. Am J Transplant. 2006;6:747–752. doi: 10.1111/j.1600-6143.2005.01230.x. [DOI] [PubMed] [Google Scholar]
- 38.Pereira BJG, Natv SN, Bouthot BA, Murthy BV, Ruthazer R, Schmid CH, The New England Organ Bank Hepatitis C Study Group et al. Effect of hepatitis C infection and renal transplantation on survival in end stage renal disease. Kidney Int. 1998;53:1374–1381. doi: 10.1046/j.1523-1755.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 39.Perira BJ. Hepatitis C infection and post transplantation liver disease. Nephrol Dial Transplant. 1995;10(suppl. 1):58–67. doi: 10.1093/ndt/10.supp1.58. [DOI] [PubMed] [Google Scholar]
- 40.Reddy KR. Development and pharmacokinetics and pharmacodynamics of pegylated interferon alf-2a (40 kD) Seminliver Dis. 2004;24:33–38. doi: 10.1055/s-2004-832926. [DOI] [PubMed] [Google Scholar]
- 41.Reddy AK, Murthy KV, Lakshmi V. Prevalence of HCV infection in patients on haemodialysis: survey by antibody and core antigen detection. Indian J Med Microbiol. 2005;23:106–110. doi: 10.4103/0255-0857.16049. [DOI] [PubMed] [Google Scholar]
- 42.Rodríguez-Iñigo E, Casqueiro M, Bartolomé J, Barat A, Caramelo C, Ortiz A, et al. Hepatitis C virus RNA in kidney biopsies from infected patients with renal diseases. J Viral Hepat. 2000;7(1):23–29. doi: 10.1046/j.1365-2893.2000.00194.x. [DOI] [PubMed] [Google Scholar]
- 43.Rostami Z, Nourbala MH, Alavian SM, Bieraghdar F, Jahani Y, Einollahi B. The impact of hepatitis C virus infection on kidney transplantation outcomes: a systematic review of 18 observational studies: the impact of HCV on renal transplantation. Hepat Mon. 2011;11(4):247–254. [PMC free article] [PubMed] [Google Scholar]
- 44.Roth D, Zucker K, Cirocco R, Demattos A, Burke GW, Nery J, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238–244. doi: 10.1038/ki.1994.29. [DOI] [PubMed] [Google Scholar]
- 45.Roth D, GaynorJJ R, Ciancio C, Sageshima J, Kupin W, et al. Effect of kidney transplantation on outcomes among patients with hepatitis C. J Am Soc Nephrol. 2011;22:1152–1160. doi: 10.1681/ASN.2010060668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth D, Nelson DR, Bruchfeld A, Liapakis A, Silva M, Monsour H, Jr, et al. Grazoprevir plus elbasvir in treatment-naive and treatment-experienced patients with hepatitis C virus genotype 1 infection and stage 4–5 chronic kidney disease (the C-SURFER study): a combination phase 3 study. Lancet. 2015;386:1537–1545. doi: 10.1016/S0140-6736(15)00349-9. [DOI] [PubMed] [Google Scholar]
- 47.Russo MW, Goldsweig CD, Jacoson IM, Brown RS., Jr Interferon monotherapy for dialysis patients with chronic hepatitis C:an analysis of the literature on efficacy and safety. Am J Gastoenterol. 2003;98:1610–1615. doi: 10.1111/j.1572-0241.2003.07526.x. [DOI] [PubMed] [Google Scholar]
- 48.Sarrazin C, Hézode C, Zeuzem S, Pawlotsky JM. Antiviral strategies in hepatitis C virus infection. J Hepatol. 2012;56(Suppl 1):S88–S100. doi: 10.1016/S0168-8278(12)60010-5. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz V, Kiessling A, Bahra M, Puhl G, Kahl A, Berg T, Neuhaus R, et al. Peginterferon alfa-2b plus ribavirin for the treatment of hepatitis C recurrence following combined liver and kidney transplantation. Ann Transplant. 2007;12(3):22–27. [PubMed] [Google Scholar]
- 50.Scott DR, Wong JK, Spicer TS, Dent H, Mensah FK, McDonald S, et al. Adverse impact of hepatitis C virus infection on renal replacement therapy and renal transplant patients in Australia and New Zealand. Transplantation. 2010;90(11):1165–1171. doi: 10.1097/TP.0b013e3181f92548. [DOI] [PubMed] [Google Scholar]
- 51.Shah T, Kasravi A, Huang E, Hayashi R, Young B, Cho YW, et al. Risk factors for development of new onset of diabetes mellitus after kidney transplantation. Transplantation. 2006;82:1673–1676. doi: 10.1097/01.tp.0000250756.66348.9a. [DOI] [PubMed] [Google Scholar]
- 52.Thomas E, Feld JJ, Li Q, Hu Z, Fried MW, Liang TJ. Ribavirin potentiates interferon action by augumenting interferon-stimulated gene induction in hepatitis C virus cell culture models. Hepatology. 2011;53:32–41. doi: 10.1002/hep.23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tischer S, Fontana RJ. Drug-drug interactions with oral anti-HCV agents and idio syncratichepato toxicity in the liver transplant setting. J Hepatol. 2014;60:872–894. doi: 10.1016/j.jhep.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watashi K, Hijikata M, Hosaka M, Yamaji M, Shimotohno K. Cyclosporin A suppress replication of hepatitis C virus genome in cultured hepatocytes. Hepatology. 2003;38:1282–1288. doi: 10.1053/jhep.2003.50449. [DOI] [PubMed] [Google Scholar]
- 55.Wörnle M, Schmid H, Banas B, Merkle M, Henger A, Roeder M, et al. Novel role of toll-like receptor 3 in hepatitis C-associated glomerulonephritis. Am J Pathol. 2006;168(2):370–385. doi: 10.2353/ajpath.2006.050491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wreghitt TG. Blood-borne virus infections in dialysis units a review. Rev Med Virol. 1999;9:101–109. doi: 10.1002/(SICI)1099-1654(199904/06)9:2<101::AID-RMV234>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 57.Zylberberg H, Nalpas B, Carnot F, Skhiri H, Fontaine H, Legendre C, et al. Severe evolution of chronic hepatitis C in renal transplantation: a case control study. Nephrol Dial Transplant. 2002;17:129–133. doi: 10.1093/ndt/17.1.129. [DOI] [PubMed] [Google Scholar]