Abstract
Given that few studies have examined the interaction between endocrine-inflammatory mediators and aerobic exercise training in hypertensive postmenopausal women, the aim of this study was to investigate whether aerobic exercise training (AET) for twenty-four sessions would alter cortisol, leptin and interleukin-1β (IL-1β) levels. To further analyze endothelium function in response to AET, we also examined redox state as well as NO/cGMP pathway in this population. Eighteen hypertensive postmenopausal women finished this study. AET program consisted of 24 sessions in treadmill, 3 times per week, duration of 30 up to 40 min for each session, for 8 weeks at intensity of 100% of the MLSS according to previous incremental test. Heart rate was monitored in all studied time (resting and during exercise sessions). After 48 h of the last exercise session, blood samples were collected for biochemical analyses (levels of cortisol, leptin, IL-1β, nitrite/nitrate (NOx−), cGMP, malondialdehyde (MDA) and asymmetric dimethylarginine (ADMA); superoxide and catalase activity). We also measured systolic and diastolic blood pressure. A significant reduction in body mass was observed. As expected, systolic and diastolic blood pressure values were significantly reduced after AET in hypertensive women. We also found a marked increase in NOx− levels as well as cGMP concentration in trained women, approximately 37.7 and 30.8%, respectively. No changes in cortisol, leptin, ADMA and IL-1β levels were observed after AET. Similarly, MDA levels and catalase activity were not affected by AET. In contrast, a marked increase in SOD activity was found (86.6%). In conclusion, our findings show that aerobic exercise training for twenty-four sessions promoted a significant reduction in blood pressure by activating NO/cGMP pathway as well as by promoting an up-regulation of SOD activity without changing in cortisol/leptin levels in postmenopausal hypertensive women.
Keywords: Cortisol, Leptin, Nitric oxide pathway, Blood pressure, Redox state
Introduction
Epidemiological studies have shown that the incidence of cardiovascular diseases (CVD) in women increases dramatically after menopause [1], [2]. However, the underlying mechanisms are not yet fully clarified. Several hypotheses have been proposed to explain this phenomenon in postmenopausal women. Estrogen deficiency has been pointed out to play a major role, but its deficiency partially explains the increased incidence of CVD since hormone replacement therapy did not prevent or mitigated cardiovascular events in this population [3], [4]. Oxidative stress is another explanation, where increased production of the inflammatory mediators would lead to a massive production of reactive oxygen species, which in turn, resulting in endothelium dysfunction with decrease in nitric oxide (NO) production or its bioavailability to the cells [5]. However, some studies found a positive association between CVD and inflammatory mediators [6], [7], [8] whereas others failed to detect any association [9] in climacteric phase. The hypothalamic-pituitary-adrenal axis has also been linked to the higher incidence of CVD in postmenopausal women [10], [11]. Nevertheless, the number of studies examining the interaction between menopause status and glucocorticoids is scarce. Therefore, the higher incidence of CVD in postmenopausal women still a complex issue and further studies should be carried out to look at the insight mechanisms as well as to get more information in an attempt to prevent cardiovascular events in this population. On the other hand, a plethora of studies has shown that physically active subjects have more longevity with reduction of morbidity and mortality [12], [13]. Given that few studies have examined the interaction between endocrine-inflammatory mediators and aerobic exercise training in hypertensive postmenopausal women, the aim of this study was to investigate whether aerobic exercise training for twenty-four sessions would alter cortisol, leptin and interleukin-1β levels. To further analyze endothelium function in response to exercise training, we also examined redox state as well as NO/cGMP pathway in this population.
Methodology
Study participants
This study was approved by the Ethical Committee of Institute of Bioscience at the University of São Paulo State (UNESP). All the volunteers were recruited through advertisements in the surrounding area of UNESP. A total of thirty-two volunteers were eligible to participate in the study. After all screening test, only eighteen women finished the study. Postmenopausal status was determined as the absence of menstruation for at least 1 year under natural or surgical causes were classified as hypertensive according to previous medical diagnosis (systolic blood pressure: 140–159 mm Hg, diastolic blood pressure: 90–99 mm Hg or using anti-hypertensive). The inclusion criteria of this study were: to be hypertensive; body mass index ≤30 kg/m2; sedentary (<150 min of moderate physical activity per week or <60 min of vigorous physical activity per week). The exclusion criteria were: smoking, taking hormone replacement therapy, diabetic, cardiovascular disease (stroke, heart failure); renal dysfunction; other condition that precludes the practice of physical exercise. Before starting the protocol, volunteers were informed about the procedures and risks of the study and signed a consent form in accordance with Ethical Committee of UNESP.
Study protocol
This clinical trial lasted 10 weeks and all parameters were evaluated at initial time and after 24 sessions of the aerobic exercise training (AET). Initially, the anthropometric and cardiovascular parameters were measured and volunteers were familiarized to the treadmill during 2–4 days, depending upon each participant. After familiarization, maximal lactate steady state (MLSS) was defined individually for prescription of AET intensity. Briefly, postmenopausal women performed two to five tests with fixed duration (30 min) and walking speed (5.5 km/h) on a treadmill (Movement RT 250 PRO) in accordance with previous study [14]. The inclination of the ergometer was used to control the intensity that ranged between 1 and 15%. The intensity was adjusted in each test according to the aerobic capacity of the participant. Measurement blood lactate concentration was performed at rest, 10th and 30th min during incremental test. MLSS was determined when the difference of blood lactate concentration between 10th and 30th min was not exceeded 1 mM [15].
AET program consisted of 24 sessions in treadmill, 3 times per week, duration of 30 up to 40 min for each session, for 8 weeks. The intensity of the AET was 100% of the MLSS according to previous incremental test. Heart rate was monitored and AET was supervised by exercise physiologists in an environmental with temperature (≈25 °C) and humidity (40–60%) controlled. Figure 1 illustrates the experimental design.
Figure 1.
Experimental design.
Anthropometric parameters
Body weight and height was determined using a scale and stadiometer (Toledo 2096 PP). Body mass index was calculated as the ratio body weight divided by the square of the height in meters. Waist circumference was measured at the midpoint between the last rib and iliac crest.
Cardiovascular parameters
Blood pressure (BP) – After 20 min of sitting position, three consecutives BP measurements using a semi-automatic equipment (Microlife MIB-P3BTOA). Resting BP was determined as the average of the measurements.
Heart rate (HR) – HR was measured using a heart rate monitor (Polar FT1 TRQ) after 20 min of seated position. At the final of the resting period the value of HR was obtained.
Blood samples
Blood samples were collected after 12 h of overnight fast (between 7:00 and 8:00 am). Blood samples were collected from the antecubital vein using standard venipuncture methods. Samples were centrifuged (3000 rpm, 12 min) and the supernatant (plasma and serum) were stored in aliquots at −80 °C for future analysis.
Biochemical analyses
Lipid profile and glycemia – Serum concentrations of total cholesterol, low-density lipoprotein-cholesterol, very low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides and glycemia were determined using automated standard method (Cobas Mira Plus).
Cortisol – Serum concentrations of cortisol were measured by the chemiluminescence (ADVIA Centaur®). This is a competitive immunoassay that uses direct chemiluminescent technology.
Leptin – Serum leptin concentrations were measured by ELISA using a commercial available kit (SPI Bio, Montigny-le-Bretonneux, France) according to the manufacturer's instructions.
Interleukin-1β (IL-1β) and guanosine cyclic monophosphate (cGMP) – Plasma concentrations of IL-1β and cGMP were measured by ELISA using a commercial available kit (R&D Systems, Mineapolis, MN, USA) according to the manufacturer's instructions.
Asymmetric dimethylarginine (ADMA) – Plasma concentrations of ADMA were measured by ELISA using a commercial available kit (Immunodiagnostik AG, Bensheim, Germany) according to the manufacturer's instructions.
Nitrite/Nitrate (NOx−) – Plasma concentrations of NOx− were measured to evaluate NO production by commercial available kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions. Before starting this assay samples were ultra-filtered through micro filter (Microcon Centrifugal Filter Units, 10 kDa, Millipore, Bedford, MA).
Superoxide dismutase (SOD), catalase and malondialdehyde (MDA) – All these markers were measured by ELISA using a commercial available kit (Cayman Chemical, Ann Arbor, MI, USA) according to the manufacturer's instructions. SOD's assay detects radicals superoxide generated by xanthine oxidase and hypoxanthine, revealing the plasmatic activity of this enzyme. Catalase's assay is based on the reaction of the enzyme with methanol in an optimal H2O2 concentration. Serum levels of MDA were determined to evaluate lipid peroxidation [16].
Statistical analysis
Data are presented as mean ± standard error mean. Normality of the data was verified by the Kolmogorov–Smirnov's test. A paired Student's t-test was used to analyze the effect of AET on the cardiometabolic and endocrine parameters. For all analyses, P < 0.05 was considered statistically significant.
Results
Body mass index values were significantly reduced in hypertensive postmenopausal women after 24 sessions of AET approximately 1.5% as compared to initial time. However, no changes were found in waist circumference, lipid profile and glycemia. On the other hand, the intensity of physical exercise employed in our study was effective to promote a significant increase in MLSS after AET, approximately 32.7%. Data are summarized in Table 1. Regarding antihypertensive therapy, 67% of the participants were on renin-angiotensin inhibitors or AT1 receptor blockers whereas six volunteers were on diuretics or beta-adrenergic receptor blocker therapy (33%). Regarding hypolipidemic compound, 50% of the volunteers were on therapy previously to the study.
Table 1.
Effects of aerobic exercise training on anthropometrical parameters, lipid profile, glycemia and MLSS in hypertensive postmenopausal women.
| Parameters (n = 18) | Initial | Final |
|---|---|---|
| Age (yrs) | 58.3 ± 1.2 | – |
| Time after menopause (yrs) | 10.3 ± 2.1 | – |
| BMI | 27.3 ± 0.5 | 26.9 ± 0.5* |
| WC | 94.4 ± 1.6 | 93.4 ± 1.5* |
| TC (mg/dL) | 195.4 ± 6.4 | 194.1 ± 5.5 |
| LDL-c (mg/dL) | 124.7 ± 6.4 | 124.2 ± 5.0 |
| HDL-c (mg/dL) | 46.9 ± 1.7 | 49.1 ± 1.5 |
| VLDL-c (mg/dL) | 23.8 ± 1.9 | 20.6 ± 1.5 |
| TG (mg/dL) | 113.8 ± 10 | 103.1 ± 7.8 |
| Glycemia (mg/dL) | 87.2 ± 2.2 | 90.3 ± 2.1 |
| Rest HR | 67.3 ± 2.7 | 64.7 ± 2.2* |
| MLSS exercise HR | 152 ± 4.7 | 151 ± 5.1 |
| MLSS threshold | 5.5 ± 0.4 | 7.3 ± 0.5* |
BMI = body mass index (kg/m2); WC = waist circumference (cm); TC = total cholesterol; LDL = low density lipoprotein; HDL = high-density lipoprotein; VLDL = very low-density lipoprotein; TG = triglycerides. HR = heart rate (bpm); MLSS = maximal lactate steady state; Data are mean ± SEM.
*P < 0.05 compared with initial time.
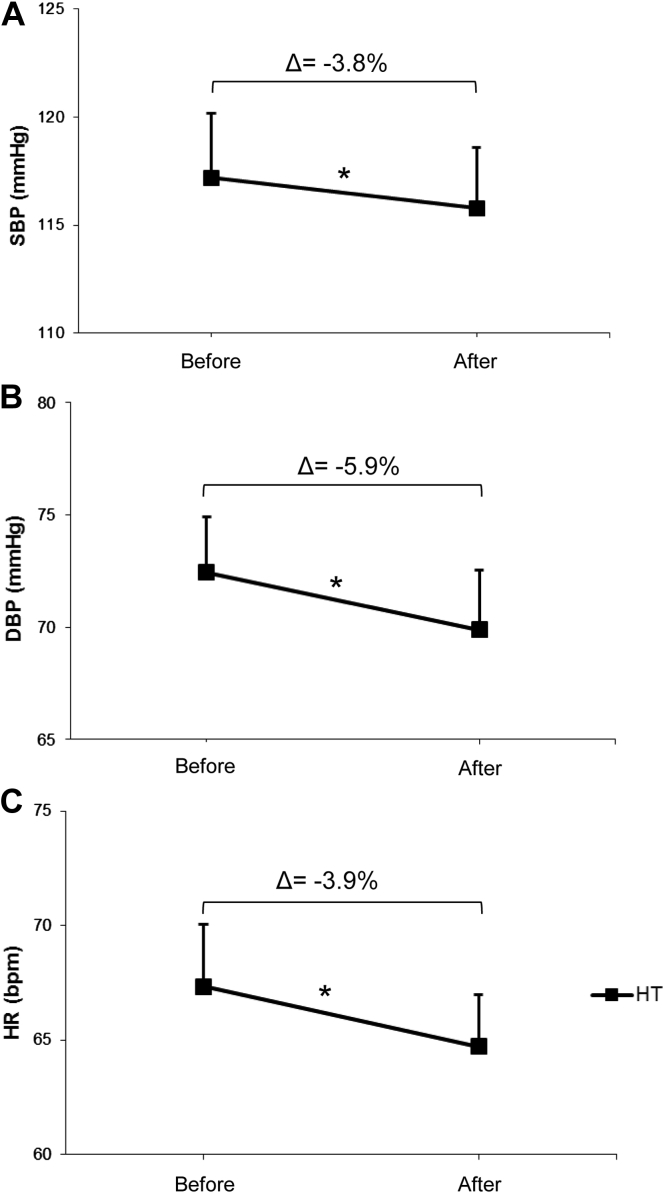
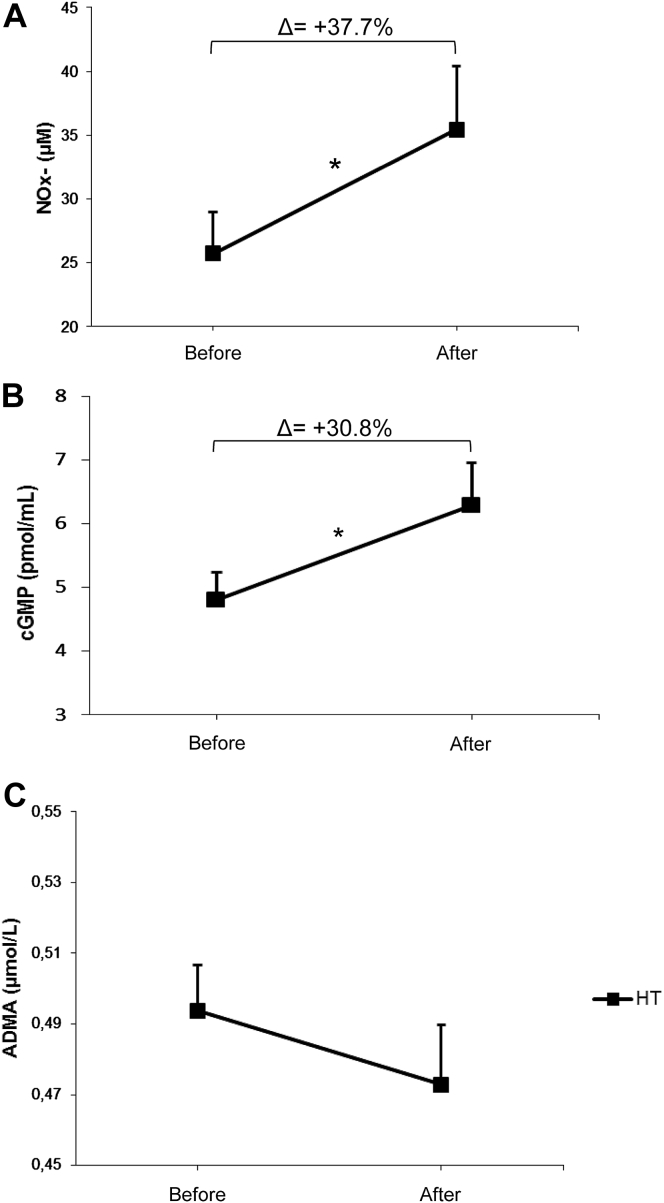
As expected, AET was effective in lowering arterial blood pressure in trained HT women, approximately −3.8% and −5.9% for systolic and diastolic blood pressure, respectively (Figure 2, panels A and B). Resting heart rate values were also decreased after 24 sessions of aerobic physical exercise (Table 1 and Figure 2C). Given that it is well established that the health beneficial effect of the exercise training is related to activation of NO/cGMP signaling pathway, we have evaluated these biomarkers before and after twenty-four sessions of aerobic physical exercise. We found a marked increase in NOx− levels, which reflect NO production, as well as cGMP concentration in trained women, approximately 37.7 and 30.8%, respectively (Figure 3, panels A and B). Interestingly, we found no changes in ADMA levels (Figure 3C).
Figure 2.
Effects of aerobic exercise training on systolic blood pressure (SBP, panel A), diastolic blood pressure (DBP, panel B) and resting heart rate (HR, panel C) in postmenopausal hypertensive women (n = 18). *P < 0.05 paired t test between initial and final time of the study.
Figure 3.
Effects of aerobic exercise training on NOx− (panel A), cGMP (panel B) and ADMA (panel C) levels in postmenopausal hypertensive women (n = 18). *P < 0.05 paired t test between initial and final time of the study.
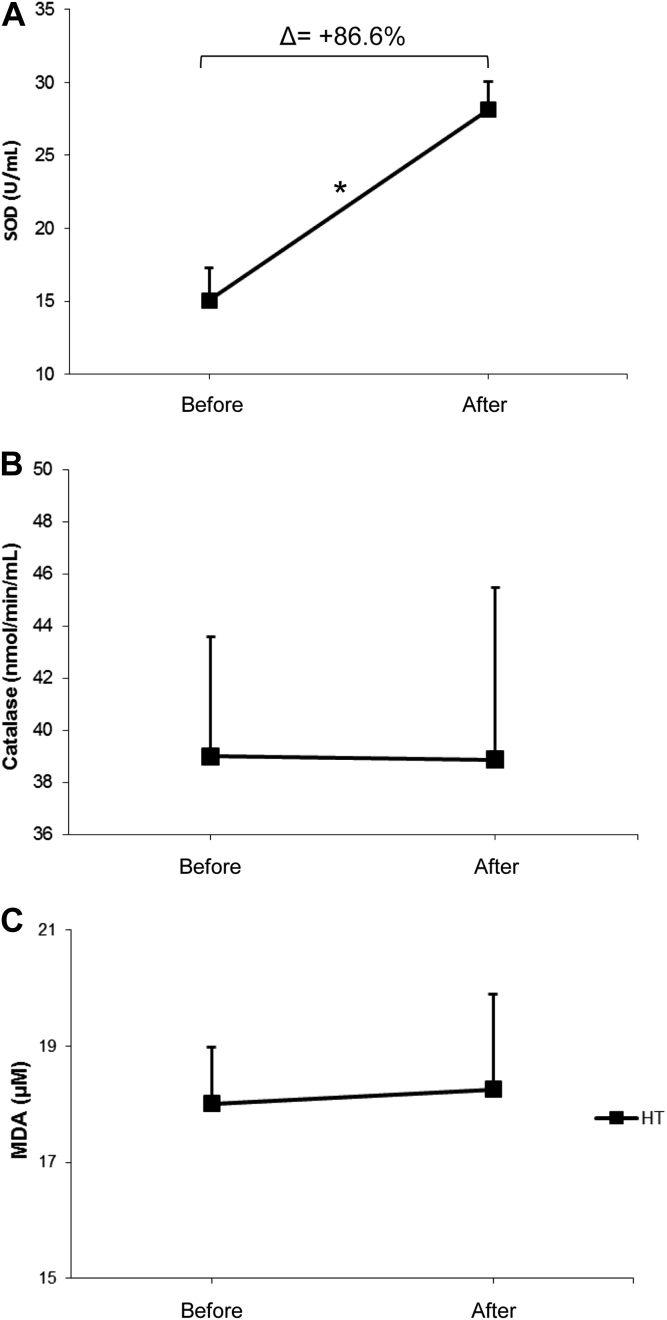
Furthermore, we also analyzed the redox state by measuring SOD and catalase activities and MDA concentration. Our findings show that AET promoted a profound increase in SOD activity, approximately 86.6% (Figure 4A). No changes were found catalase activity and MDA concentration (Figure 4, panels B and C).
Figure 4.
Effects of aerobic exercise training on SOD (panel A), catalase (panel B) activity and MDA concentration (panel C) in postmenopausal hypertensive women (n = 18). *P < 0.05 paired t test between initial and final time of the study.
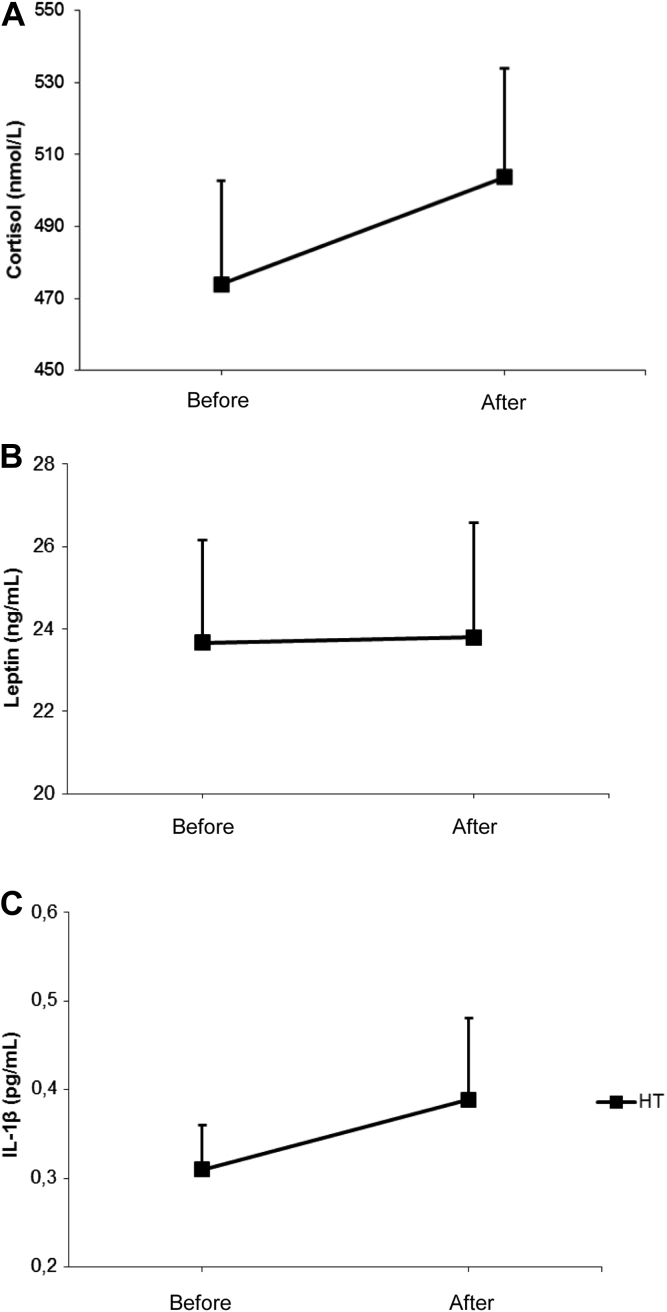
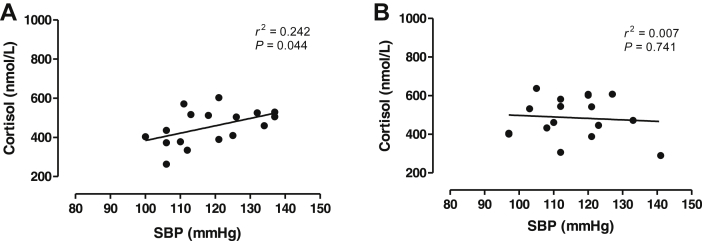
Regarding endocrine-inflammatory mediators, we found no effects of the AET on cortisol, leptin and IL-1β levels in postmenopausal hypertensive women (Figure 5, panels A, B and C, respectively). Interestingly, we found a positive relationship between systolic blood pressure values and cortisol levels at initial time of the study (r2 = 0.2422, P < 0.0448, n = 17, Figure 6A) whereas this correlation was abrogated after AET (P > 0.05, Figure 6B).
Figure 5.
Effects of aerobic exercise training on cortisol (panel A), leptin (panel B) and IL-1β levels (panel C) in postmenopausal hypertensive women (n = 18). *P < 0.05 paired t test between initial and final time of the study.
Figure 6.
Correlation between systolic blood pressure values and cortisol levels at initial time (panel A) and after twenty-four sessions (panel B) of physical exercise in postmenopausal hypertensive women.
Discussion
A number of studies have shown that cardiovascular diseases can be prevented by regular physical exercise either in human or in laboratory animals, which is strongly associated with an increase in NO bioavailability [17], [18], [19]. Regarding postmenopausal women, recent study found that aerobic exercise training even in a low intensity promoted reductions in blood pressure showing how changing in the lifestyle is favorable for this particular population in preventing cardiovascular events [20]. However, no mechanist events have been reported. Thus, one of the objectives of our study is to examine different pathways that regulate blood pressure in response to exercise training in hypertensive postmenopausal women. We found that twenty-four sessions of aerobic physical exercise was effective in reducing BMI as well as to improve the physical capacity of all volunteers. Additionally, the reductions of systolic and diastolic blood pressure were positively associated with an improvement of NO/cGMP pathway as well as an up-regulation of SOD activity. Taken together, these findings show that aerobic exercise training promoted an improvement of endothelium function in hypertensive postmenopausal women. Indeed, previous studies from our group have systematically demonstrated the beneficial effects of the exercise training on cardiovascular system by increased production of NO and/or its bioavailability in postmenopausal women [21], [22], [23], [24]. To further examine the association between NO inhibition and hypertension in postmenopausal women, we looked at ADMA levels since evidences have linked increased ADMA levels in patients with angina, arterial hypertension and immune dysfunction mainly in elderly and postmenopausal women [25], [26], [27]. We found no changes in ADMA levels in trained women excluding the contribution of this endothelial pathway on the beneficial effects of exercise training in blood pressure regulation. In contrast, two recent studies from the same group showed a decrease in plasma ADMA in healthy postmenopausal women in response to aerobic exercise training as well as an inverse relationship between aerobic fitness and levels of ADMA [28], [29]. However, these studies did not measure NO production, and blood pressure values were not modified by exercise training. Thus, more studies should be carried out to check the contribution of the endogenous inhibition of NO availability on arterial hypertension as well as the effects of exercise training on ADMA/NOS pathway.
Increased oxidative stress has been pointed out to play an important in the pathogenesis of arterial hypertension in men [30] as well as in postmenopausal women [5], [31]. Although evidences have demonstrated that trained [32] or physically active healthy postmenopausal women [33] showed a decreased in circulating concentrations of MDA as compared to sedentary one, no blood pressure measurements were performed in these studies. Thus, the data do not clarify the high incidence of arterial hypertension in postmenopausal women or explain the insight mechanisms in the phenomenon. In our study, we found no changes in MDA levels in response to exercise training in hypertensive postmenopausal women excluding the contribution of this biomarker in the beneficial effects of physical exercise on blood pressure regulation. We also found no changes in the pro-inflammatory mediators, IL-1β and leptin, in response to exercise training. It has been reported the influence of estrogen on pro- and anti-inflammatory pathway in different cells types [34]. Indeed, in whole human blood cultures, the addition of different concentration of estrogen decreases secretion of several pro-inflammatory mediators, mainly IL-1β [35]. Additionally, it has been demonstrated an increase in inflammatory mediators in experimental model of menopause [36]. Interestingly, a previous study showed that IL-1β levels increase in the early stage of the menopause (less than five years) and return to the normal levels in the late stage, with values similar to premenopause phase [8]. Thus, it is not clear whether estrogen deficiency could lead to an inflammatory state in postmenopausal women. Furthermore, the range of concentration of IL-1β levels in women is very wide varying between 0.30 and 0.081 pg/mL depending on the reproductive phase or not as well as the time after menopause [37]. Regarding leptin levels and postmenopausal women, the interaction is even more complex [38]. An increase [39], no variation [40] and a decrease [41], [42] in circulating leptin levels were detected after menopause. Previous studies reported that postmenopausal women under caloric restriction [43] or on tibolone therapy [42] showed a significant reduction in leptin levels. In our study, exercise training did not affect leptin levels even though there was a slight reduction in the BMI (1.5%). Indeed, previous studies have shown that changes in leptin levels are detected only when physical training is associated with caloric restriction in postmenopausal women [44], [45].
A number of studies have shown the importance of cortisol, the major glucocorticoid hormone produced in human, in elevating blood pressure [46], [47], [48]. Several signaling pathways seem to be involved in the mechanisms by which cortisol produces arterial hypertension including sodium/volume homeostasis, activation of the renin-angiotensin system, and increased sympathetic drive [49], [50], [51], [52]. Additionally, it has been reported that cortisol impairs NO production measured by NOx− levels attributing thus, the cortisol-induced hypertension to the NO deficiency in both human and experimental model [53], [54]. However, most of the studies have examined the interaction between cortisol and arterial hypertension in male subjects [55] and no one examined the effects of exercise training on endocrine-inflammatory mediators in hypertensive postmenopausal women. In our study, we found no effects of the exercise training in the cortisol concentration in hypertensive postmenopausal women. Thus, it is clear that the beneficial effects of exercise training on blood pressure were related to an improvement of NO/cGMP pathway without changing in serum cortisol levels. This is confirmed by the lack of positive correlation between cortisol and blood pressure after twenty-four sessions of exercise. Collectively, our findings show that the endocrine-inflammatory mediators: cortisol, leptin and IL-1β did not contribute to the beneficial effects of the exercise training on blood pressure in hypertensive postmenopausal women. Furthermore, our study confirms that aerobic exercise training for twenty-four sessions promoted a reduction in blood pressure by activating NO/cGMP pathway as well as by promoting an up-regulation of SOD in this particular population.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/3.0/).
Conflict of interest: The authors declare no conflict of interest.
Source of funding: Authors are grateful to Sao Paulo Research Foundation (FAPESP): 2001/17437-7 and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
References
- 1.Simkin-Silverman L.R., Wing R.R., Hansen D.H., Klem M.L., Pasagian-Macaulay A.P., Meilahn E.N. Prevention of cardiovascular risk factor elevations in healthy premenopausal women. Prev Med. 1995;24:509–517. doi: 10.1006/pmed.1995.1081. [DOI] [PubMed] [Google Scholar]
- 2.Simkin-Silverman L.R., Wing R.R., Boraz M.A., Kuller L.H. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med. 2003;26:212–220. doi: 10.1207/S15324796ABM2603_06. [DOI] [PubMed] [Google Scholar]
- 3.Shelley J.M., Green A., Smith A.M., Dudley E., Dennerstein L., Hopper J. Relationship of endogenous sex hormones to lipids and blood pressure in mid-aged women. Ann Epidemiol. 1998;8:39–45. doi: 10.1016/s1047-2797(97)00123-3. [DOI] [PubMed] [Google Scholar]
- 4.Maranon R., Reckelhoff J.F. Sex and gender differences in control of blood pressure. Clin Sci (Lond) 2013;125:311–318. doi: 10.1042/CS20130140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reckelhoff J.F. Gender differences in the regulation of blood pressure. Hypertension. 2001;37:1199–1208. doi: 10.1161/01.hyp.37.5.1199. [DOI] [PubMed] [Google Scholar]
- 6.Cioffi M., Esposito K., Vietri M.T., Gazzerro P., D'Auria A., Ardovino I. Cytokine pattern in postmenopause. Maturitas. 2002;4:187–192. doi: 10.1016/s0378-5122(01)00286-9. [DOI] [PubMed] [Google Scholar]
- 7.Yasui T., Uemura H., Tomita J., Miyatani Y., Yamada M., Kuwahara A. Association of interleukin-8 with hot flashes in premenopausal, perimenopausal, and postmenopausal women and bilateral oophorectomized women. J Clin Endocrinol Metab. 2006;91:4805–4808. doi: 10.1210/jc.2006-1100. [DOI] [PubMed] [Google Scholar]
- 8.Yasui T., Maegawa M., Tomita J., Miyatani Y., Yamada M., Uemura H. Changes in serum cytokine concentrations during the menopausal transition. Maturitas. 2007;56:396–403. doi: 10.1016/j.maturitas.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Rolland Y.M., Perry H.M., 3rd, Patrick P., Banks W.A., Morley J.E. Leptin and adiponectin levels in middle-aged postmenopausal women: associations with lifestyle habits, hormones, and inflammatory markers – a cross-sectional study. Metabolism. 2006;55:1630–1636. doi: 10.1016/j.metabol.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 10.Puterman E., O'Donovan A., Adler N.E., Tomiyama A.J., Kemeny M., Wolkowitz O.M. Physical activity moderates effects of stressor-induced rumination on cortisol reactivity. Psychosom Med. 2011;73:604–611. doi: 10.1097/PSY.0b013e318229e1e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S., Liu W., Wang L., Huang R., Chen Q., Wu Y. Effects of menopause on hepatic 11β-hydroxysteroid dehydrogenase type 1 activity and adrenal sensitivity to adrenocorticotropin in healthy non-obese women. Gynecol Endocrinol. 2011;27:794–799. doi: 10.3109/09513590.2010.507288. [DOI] [PubMed] [Google Scholar]
- 12.Artero E.G., España-Romero V., Lee D.C., Sui X., Church T.S., Lavie C.J. Ideal cardiovascular health and mortality: Aerobics Center Longitudinal Study. Mayo Clin Proc. 2012;87:944–952. doi: 10.1016/j.mayocp.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swift D.L., Lavie C.J., Johannsen N.M., Arena R., Earnest C.P., O'Keefe J.H. Physical activity, cardiorespiratory fitness, and exercise training in primary and secondary coronary prevention. Circ J. 2013;77:281–292. doi: 10.1253/circj.cj-13-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beneke R. Anaerobic threshold, individual anaerobic threshold, and maximal lactate steady state in rowing. Med Sci Sports Exerc. 1995;27:863–867. [PubMed] [Google Scholar]
- 15.Beneke R. Methodological aspects of maximal lactate steady state-implications for performance testing. Eur J Appl Physiol. 2003;89:95–99. doi: 10.1007/s00421-002-0783-1. [DOI] [PubMed] [Google Scholar]
- 16.Yagi K. Simple assay for the level of total lipid peroxides in serum or plasma. Methods Mol Biol. 1998;108:101–106. doi: 10.1385/0-89603-472-0:101. [DOI] [PubMed] [Google Scholar]
- 17.Zanesco A., Antunes E. Effects of exercise training on the cardiovascular system: pharmacological approaches. Pharmacol Ther. 2007;114:307–317. doi: 10.1016/j.pharmthera.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Delbin M.A., Davel A.P., Couto G.K., de Araújo G.G., Rossoni L.V., Antunes E. Interaction between advanced glycation end products formation and vascular responses in femoral and coronary arteries from exercised diabetic rats. PLoS One. 2012;7:e53318. doi: 10.1371/journal.pone.0053318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuler G., Adams V., Goto Y. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur Heart J. 2013;34:1790–1799. doi: 10.1093/eurheartj/eht111. [DOI] [PubMed] [Google Scholar]
- 20.Swift D.L., Earnest C.P., Blair S.N., Church T.S. The effect of different doses of aerobic exercise training on endothelial function in postmenopausal women with elevated blood pressure: results from the DREW study. Br J Sports Med. 2012;46:753–758. doi: 10.1136/bjsports-2011-090025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zanesco A., Zaros P.R. Physical exercise and menopause. Rev Bras Ginecol Obstet. 2009;31:254–261. doi: 10.1590/s0100-72032009000500009. [DOI] [PubMed] [Google Scholar]
- 22.Zaros P.R., Pires C.E., Bacci M., Jr., Moraes C., Zanesco A. Effect of 6-months of physical exercise on the nitrate/nitrite levels in hypertensive postmenopausal women. BMC Womens Health. 2009;19:9–17. doi: 10.1186/1472-6874-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sponton C.H., Rezende T.M., Mallagrino P.A., Franco-Penteado C.F., Bezerra M.A., Zanesco A. Women with TT genotype for eNOS gene are more responsive in lowering blood pressure in response to exercise. Eur J Cardiovasc Prev Rehabil. 2010;17:676–681. doi: 10.1097/HJR.0b013e32833a1301. [DOI] [PubMed] [Google Scholar]
- 24.Esposti R.D., Sponton C.H., Malagrino P.A., Carvalho F.C., Peres E., Puga G.M. Influence of eNOS gene polymorphism on cardiometabolic parameters in response to physical training in postmenopausal women. Braz J Med Biol Res. 2011;44:855–863. doi: 10.1590/s0100-879x2011007500106. [DOI] [PubMed] [Google Scholar]
- 25.Leiper J., Vallance P. Biological significance of endogenous methylarginines that inhibit nitric oxide synthases. Cardiovasc Res. 1999;43:542–548. doi: 10.1016/s0008-6363(99)00162-5. [DOI] [PubMed] [Google Scholar]
- 26.Hori T., Matsubara T., Ishibashi T., Ozaki K., Tsuchida K., Mezaki T. Significance of asymmetric dimethylarginine (ADMA) concentrations during coronary circulation in patients with vasospastic angina. Circ J. 2003;67:305–311. doi: 10.1253/circj.67.305. [DOI] [PubMed] [Google Scholar]
- 27.Schulze F., Maas R., Freese R., Schwedhelm E., Silberhorn E., Böger R.H. Determination of a reference value for N(G), N(G)-dimethyl-L-arginine in 500 subjects. Eur J Clin Invest. 2005;35:622–626. doi: 10.1111/j.1365-2362.2005.01561.x. [DOI] [PubMed] [Google Scholar]
- 28.Tanahashi K., Akazawa N., Miyaki A., Choi Y., Ra S.G., Matsubara T. Aerobic exercise training decreases plasma asymmetric dimethylarginine concentrations with increase in arterial compliance in postmenopausal women. Am J Hypertens. 2014;27:415–421. doi: 10.1093/ajh/hpt217. [DOI] [PubMed] [Google Scholar]
- 29.Tanahashi K., Akazawa N., Miyaki A., Choi Y., Ra S.G., Matsubara T. Plasma ADMA concentrations associate with aerobic fitness in postmenopausal women. Life Sci. 2014;108:30–33. doi: 10.1016/j.lfs.2014.05.003. pii: S0024-3205(14) 00474–3. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigo R., Prat H., Passalacqua W., Araya J., Guichard C., Bächler J.P. Relationship between oxidative stress and essential hypertension. Hypertens Res. 2007;30:1159–1167. doi: 10.1291/hypres.30.1159. [DOI] [PubMed] [Google Scholar]
- 31.Signorelli S.S., Neri S., Sciacchitano S., Pino L.D., Costa M.P., Marchese G. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas. 2006;53:77–82. doi: 10.1016/j.maturitas.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Karolkiewicz J., Michalak E., Pospieszna B., Deskur-Smielecka E., Nowak A., Pilaczyńska-Szcześniak Ł. Response of oxidative stress markers and antioxidant parameters to an 8-week aerobic physical activity program in healthy postmenopausal women. Arch Gerontol Geriatr. 2009;49:e67–71. doi: 10.1016/j.archger.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Bartfay W., Bartfay E. A case-control study examining the effects of active versus sedentary lifestyles on measures of body iron burden and oxidative stress in postmenopausal women. Biol Res Nurs. 2014;16:38–45. doi: 10.1177/1099800413501717. [DOI] [PubMed] [Google Scholar]
- 34.Straub R.H. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 35.Rogers A., Eastell R. The effect of 17beta-estradiol on production of cytokines in cultures of peripheral blood. Bone. 2001;29:30–34. doi: 10.1016/s8756-3282(01)00468-9. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton K.L., Lin L., Wang Y., Knowlton A.A. Effect of ovariectomy on cardiac gene expression: inflammation and changes in SOCS gene expression. Physiol Genomics. 2008;32:254–263. doi: 10.1152/physiolgenomics.00039.2007. [DOI] [PubMed] [Google Scholar]
- 37.Beavers K.M., Jonnalagadda S.S., Messina M.J. Soy consumption, adhesion molecules, and pro-inflammatory cytokines: a brief review of the literature. Nutr Rev. 2009;67:213–221. doi: 10.1111/j.1753-4887.2009.00191.x. [DOI] [PubMed] [Google Scholar]
- 38.Springer A.M., Foster-Schubert K., Morton G.J., Schur E.A. Is there evidence that estrogen therapy promotes weight maintenance via effects on leptin? Menopause. 2014;21:424–432. doi: 10.1097/GME.0000000000000117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baranowska B., Radzikowska M., Wasilewska-Dziubínska E., Roguski K., Pølonowski A. Relationship among leptin, neuropeptide Y, and galanin in young women and in postmenopausal women. Menopause. 2000;7:149–155. doi: 10.1097/00042192-200007030-00004. [DOI] [PubMed] [Google Scholar]
- 40.Kristensen K., Pedersen S.B., Richelsen B. Interactions between sex steroid hormones and leptin in women. Studies in vivo and in vitro. Int J Obes Relat Metab Disord. 2000;24:1438–1444. doi: 10.1038/sj.ijo.0801428. [DOI] [PubMed] [Google Scholar]
- 41.Rosenbaum M., Nicolson M., Hirsch J., Heymsfield S.B., Gallagher D., Chu F. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81:3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 42.Dedeoğlu E.N., Erenus M., Yörük P. Effects of hormone therapy and tibolone on body composition and serum leptin levels in postmenopausal women. Fertil Steril. 2009;91:425–431. doi: 10.1016/j.fertnstert.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 43.Deibert P., König D., Vitolins M.Z., Landmann U., Frey I., Zahradnik H.P. Effect of a weight loss intervention on anthropometric measures and metabolic risk factors in pre- versus postmenopausal women. Nutr J. 2007;6:31. doi: 10.1186/1475-2891-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryan A.S., Pratley R.E., Elahi D., Goldberg A.P. Changes in plasma leptin and insulin action with resistive training in postmenopausal women. Int J Obes Relat Metab Disord. 2000;24:27–32. doi: 10.1038/sj.ijo.0801080. [DOI] [PubMed] [Google Scholar]
- 45.Figueroa A., Vicil F., Sanchez-Gonzalez M.A., Wong A., Ormsbee M.J., Hooshmand S. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am J Hypertens. 2013;26:416–423. doi: 10.1093/ajh/hps050. [DOI] [PubMed] [Google Scholar]
- 46.Mantero F., Boscaro M. Glucocorticoid-dependent hypertension. J Steroid Biochem Mol Biol. 1992;43:409–413. doi: 10.1016/0960-0760(92)90077-v. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell B.M., Webb R.C. Impaired vasodilation and nitric oxide synthase activity in glucocorticoid-induced hypertension. Biol Res Nurs. 2002;4:16–21. doi: 10.1177/1099800402004001003. [DOI] [PubMed] [Google Scholar]
- 48.Whitworth J.A., Williamson P.M., Mangos G., Kelly J.J. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1:291–299. doi: 10.2147/vhrm.2005.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tam S.H., Kelly J.J., Williamson P.M., Whitworth J.A. Reflex sympathetic function in cortisol-induced hypertension in humans. Clin Exp Hypertens. 1997;19:479–493. doi: 10.3109/10641969709084509. [DOI] [PubMed] [Google Scholar]
- 50.Kelly J.J., Mangos G., Williamson P.M., Whitworth J.A. Cortisol and hypertension. Clin Exp Pharmacol Physiol Suppl. 1998;25:S51–S56. doi: 10.1111/j.1440-1681.1998.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 51.Wen C., Fraser T., Li M., Turner S.W., Whitworth J.A. Haemodynamic mechanisms of corticotropin (ACTH)-induced hypertension in the rat. J Hypertens. 1999;17(12 Pt 1):1715–1723. doi: 10.1097/00004872-199917120-00008. [DOI] [PubMed] [Google Scholar]
- 52.Whitworth J.A., Schyvens C.G., Zhang Y., Andrews M.C., Mangos G.J., Kelly J.J. The nitric oxide system in glucocorticoid-induced hypertension. J Hypertens. 2002;20:1035–1043. doi: 10.1097/00004872-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Kelly J.J., Tam S.H., Williamson P.M., Whitworth J.A. Decreased threshold for the nitric oxide donor glyceryl trinitrate in cortisol-induced hypertension in humans. Clin Exp Pharmacol Physiol. 2007;34:1317–1318. doi: 10.1111/j.1440-1681.2007.04700.x. [DOI] [PubMed] [Google Scholar]
- 54.Ong S.L., Whitworth J.A. How do glucocorticoids cause hypertension: role of nitric oxide deficiency, oxidative stress, and eicosanoids. Endocrinol Metab Clin North Am. 2011;40:393–407. doi: 10.1016/j.ecl.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 55.Williamson P.M., Kohlhagen J.L., Mangos G.J., Whitworth J.A., Kelly J.J. Acute effects of hydrocortisone on plasma nitrate/nitrite activity and forearm vasodilator responsiveness in normal human subjects. Clin Exp Pharmacol Physiol. 2005 Mar;32:162–166. doi: 10.1111/j.1440-1681.2005.04173.x. [DOI] [PubMed] [Google Scholar]