Abstract
Aim
To compare the performance of HbA1c with established glucose criteria during an oral glucose tolerance test (OGTT) and to assess HbA1c as a screening test for undiagnosed diabetes and pre-diabetes after gestational diabetes mellitus (GDM).
Methods
Glucose homeostasis was re-evaluated 1–5 years after delivery in 140 women with previous GDM, by means of OGTT and simultaneous HbA1c measurement. Glucose tolerance was defined according to World Health Organisation criteria. HbA1c ≥6.5% (≥48 mmol/mol) was used for diabetes diagnosis and HbA1c ≥5.7% (≥39 mmol/mol) to define abnormal glucose homeostasis.
Results
HbA1c had low sensitivity (14.3%) and high specificity (99.1%) in diabetes diagnosis. Sensitivity and specificity of HbA1c to detect abnormal glucose tolerance were 29.5% and 95.2%, respectively. The consistency in classifying abnormal glucose tolerance between HbA1c and OGTT criteria was 59% (κ = 0.227) and the area under the receiver operating characteristic curve was 0.708. The combined use of HbA1c and fasting glucose criteria showed similar performance to that of fasting glucose criteria alone. The latter identified 63% of the women with pre-diabetes or diabetes in the study cohort. However, by lowering the cut-point of HbA1c to ≥5.0% (≥31 mmol/mol), an additional proportion (27%) with isolated post-glucose load hyperglycaemia was identified.
Conclusion
Proposed thresholds of HbA1c had low diagnostic sensitivity. Combined with a fasting glucose test, the performance was no better than with using a fasting glucose test alone. Combining a fasting glucose test with a lower HbA1c cut-point may be an alternative approach for selection of women for an OGTT.
Keywords: Diabetes, Gestational diabetes, HbA1c, Oral glucose tolerance test, Post-partum screening
Highlights
-
•
We compare the performance of HbA1c with established glucose criteria during an oral glucose tolerance test (OGTT).
-
•
We also assess HbA1c as a screening test for undiagnosed diabetes and pre-diabetes after gestational diabetes mellitus.
-
•
Proposed thresholds of HbA1c had low diagnostic sensitivity relative to OGTT.
-
•
Combining HbA1c with a fasting glucose test the performance was no better than using a fasting glucose test alone.
-
•
Combining a fasting glucose test with a lower HbA1c cut-point may be an option for selection of women for an OGTT.
Introduction
HbA1c has recently been approved by the World Health Organisation (WHO) as an alternative to the oral glucose tolerance test (OGTT) for the diagnosis of diabetes mellitus outside pregnancy [1]. A diagnostic cut-point of ≥6.5% (≥48 mmol/mol) was recommended based on the risk of developing microvascular complications such as retinopathy. No formal recommendations on the interpretation of HbA1c levels below this cut-point were made. However, the International Expert Committee (IEC) recommended that high-risk individuals with HbA1c levels between 6.0% (42 mmol/mol) and 6.4% (47 mmol/mol) should be considered for diabetes prevention and interventions [2], and the American Diabetes Association (ADA) suggested that HbA1c levels between 5.7% (39 mmol/mol) and 6.4% (47 mmol/mol) indicate intermediate hyperglycaemia [3].
Women with gestational diabetes mellitus (GDM) are a high-risk group for development of type-2 diabetes [4]. According to Swedish national guidelines, lifestyle intervention and follow-up of these women after pregnancy should have high priority, but it is not clear which measures should be followed. In the primary care setting, the use of HbA1c for screening and diagnostic purposes would be practical, possibly in combination with a fasting glucose test. Both are quick and easy to perform, are more convenient for and acceptable to patients, and are less expensive than the OGTT [5].
Using the slightly modified European Association for the Study of Diabetes criteria, defining GDM as a 2-h capillary blood glucose concentration of ≥9.0 mmol/l during a universal 75-g OGTT [6], the estimated prevalence of GDM in southern Sweden over the past decade has increased from 1.9 to 2.6% [7]. In a previous study from our area, it was reported that 30% of the women with GDM in the study cohort had developed diabetes 5 years after delivery [8]. Furthermore, fasting blood glucose levels of ≥5.2 mmol/l and HbA1c levels of ≥5.7% (≥38 mmol/mol) during pregnancy were found to be associated with a four-to six-fold increased risk.
The aim of the present study was to compare the performance of HbA1c testing with that of established glucose criteria during the OGTT at 1- to 5-year follow-up post-partum in this historical cohort of women with GDM, and to assess HbA1c as a screening test (alone or combined with a fasting glucose test) for undiagnosed diabetes and abnormal glucose tolerance.
Material and methods
All women who are diagnosed with GDM in the region of Malmö and Trelleborg in southern Sweden are referred to the Department of Endocrinology in Malmö for follow-up during pregnancy. Women referred between 1996 and 1999 were invited to take part in a 5-year follow-up program, including measurement of HbA1c and a 75-g OGTT at 1, 2, and 5 years after delivery. The study design has been described previously in detail [8]. Of 182 eligible women, a total of 174 were finally included. Only women with complete glucose data at follow-up, i.e. simultaneous measurements of fasting and 2-h glucose values during the OGTT, in addition to an HbA1c test, were selected for the present evaluation. Altogether, 122 women with complete glucose data attended the 1-year follow-up, 84 attended the 2-year follow-up, and 55 attended the 5-year follow-up. Since the incidence of type-2 diabetes is known to increase cumulatively within the first 1–5 years after GDM in pregnancy, we used the latest available set of complete glucose data from each woman for the present evaluation to ensure the longest possible follow-up time [9]. We also wanted to minimize the risk of selection bias by using data taken from the same woman on several occasions. Accordingly, the final evaluation was based on data from 55 women at 5-year follow-up, 48 women at 2-year follow-up, and 37 women at 1-year follow-up.
Of the 140 women who were included, 72 (51%) were of Nordic origin (all but two of them Swedish). Women of non-Nordic origin were immigrants from different countries in Southern and Eastern Europe, Asia, South America, and Africa, with Arab women form the Middle East (17%) and women from former Yugoslavia (10%) comprising the largest groups.
A standard 75-g OGTT was performed after overnight fasting. A Venflon catheter (Becton Dickinson, Helsingborg, Sweden) was inserted into an antecubital vein. Blood samples were drawn in duplicate at 0 and 120 min for determination of glucose concentrations, and the mean value was calculated. A blood sample for determination of HbA1c was collected in an EDTA-containing tube. Weight and height were recorded and body mass index (BMI) was calculated.
Based on the results of the OGTTs, four subgroups were defined according to the WHO (1999) criteria: (1) normal glucose tolerance, fasting blood glucose (FBG) <5.6 mmol/l, and 2-h blood glucose (2-h BG) <6.7 mmol/l; (2) impaired fasting glucose (IFG), FBG 5.6–6.0 mmol/l, and 2-h BG <6.7 mmol/l; (3) impaired glucose tolerance (IGT), FBG <6.1 mmol/l, and 2-h BG 6.7–9.9 mmol/l; and (4) diabetes mellitus, FBG ≥6.1 mmol/l, and/or 2-h BG ≥10 mmol/l [10]. Glucose homeostasis was also determined based on HbA1c levels according to the WHO and ADA recommendations; ≥ 6.5% (≥48 mmol/mol) suggesting diabetes; 5.7–6.4% (39–47 mmol/mol) suggesting high risk (pre-diabetes); and <5.7% (<39 mmol/mol) suggesting normal glucose homeostasis 1, 3. For comparison, the combined category “IFG and IGT” was used to represent pre-diabetes and the combined category “IFG, IGT, and diabetes” was used to represent abnormal glucose tolerance. Similarly, HbA1c levels of ≥5.7% (≥39 mmol/mol) were used to define abnormal glucose homeostasis.
Informed consent was obtained from all participants, and the study protocol was approved by the Ethics Committee of Lund University.
Assays
The HemoCue glucose system (HemoCue AB, Ängelholm, Sweden) was used for immediate measurement of whole blood glucose concentrations (in mmol/l). The mean coefficient of variation (CV) of the duplicate analyses performed in this study was 3.1% for fasting samples and 1.9% for 2-h samples. HbA1c was analyzed by ion-exchange chromatography, Mono S-HPLC [11]. The within-assay CV (on the Mono S scale) of this method is 0.47–0.94% and the between-assay CV is 1.68%. The Mono S method, together with the reference method from NGSP, is a designated comparison method in the IFCC (International Federation of Clinical Chemistry) Reference System [12]. Numbers given in % (Mono S) were converted to NGSP units (%) and IFCC units (mmol/mol) using the regression equations developed by the IFCC Working Group [12].
Statistical analysis
The agreement between diagnoses resulting from HbA1c and OGTT criteria was estimated by constructing cross tables. The κ coefficient (κ) was calculated, where the closer the value is to 1, the better the agreement [13]. Spearman's correlation was used to analyze the relationship between glucose and HbA1c values. A receiver operating characteristic (ROC) curve was constructed for HbA1c using OGTT as the gold standard for the diagnosis of abnormal glucose tolerance, and the area under the curve (AUC) was calculated. Diagnostic accuracy was assessed using sensitivity, specificity, positive predicted value (PPV), and negative predictive value (NPV).
Statistical analyses were performed with IBM SPSS Statistics 22 for Windows (IBM Corporation, New York, NY). Any p-value of less than 0.05 was considered statistically significant.
Results
Mean (±SD) values for age and BMI of the women included were 35.4 ± 5.6 years and 26.6 ± 2.3 kg/m2, respectively. A median (interquartile range) of 26 (21–60) months had elapsed since their GDM pregnancy. Based on the OGTT, 62 women (44.3%) had normal glucose tolerance, 50 (35.7%) had pre-diabetes (13 IFG, 37 IGT), and 28 (20.0%) had diabetes. Among the 37 women with IGT, 12 had FBG values within the IFG range. In 8 women, the diagnosis of diabetes was based on the 2-h glucose value alone and in 6 women it was based on the fasting glucose value alone. In contrast, using the HbA1c criteria for definition, the corresponding figures for normal glucose homeostasis, pre-diabetes, and diabetes were 114 (81.4%), 21 (15.0%), and 5 (3.6%), respectively. In four of the five HbA1c tests that were consistent with a diagnosis of diabetes, the OGTT revealed diabetes, and in the remaining test it revealed IGT. The sensitivity of HbA1c for diabetes diagnosis was 14.3% and the specificity was 99.1%. The agreement between HbA1c and OGTT in classifying diabetes or non-diabetes was poor, as indicated by a κ coefficient of 0.194.
Altogether 23 of 140 women (16.4%) met the combined criteria for abnormal glucose tolerance (both OGTT criteria and HbA1c criteria) (Table 1). The consistency in classifying abnormal glucose tolerance between HbA1c and OGTT criteria was 59% (82/140) and κ was 0.227, indicating poor agreement. Similar results were obtained when evaluating Nordic and non-Nordic women as separate groups (κ 0.278 and κ 0.166, respectively), or when evaluating the 1-, 2- and 5-year results separately (κ 0.260, κ 0.072 and κ 0.337, respectively). Combining HbA1c criteria with fasting glucose criteria improved the agreement for the total group to fair (79%, κ = 0.596), although it was no better than between FBG criterion alone and OGTT criteria (79%, κ = 0.599).
Table 1.
Cross-tabulation between HbA1c, fasting blood glucose, and oral glucose tolerance test criteria in categorization of abnormal glucose metabolism
| Test criteria | Normal OGTT | Abnormal OGTT |
|---|---|---|
| HbA1c ≥5.7% (≥39 mmol/mol) | 3 | 23 |
| HbA1c <5.7% (<39 mmol/mol) | 59 | 55 |
| FBG ≥5.6 mmol/l | 0 | 49 |
| FBG <5.6 mmol/l | 62 | 29 |
| HbA1c ≥5.7% (≥39 mmol/mol) or FBG ≥5.6 mmol/l | 3 | 52 |
| HbA1c <5.7% (<39 mmol/mol) and FBG <5.6 mmol/l | 59 | 26 |
FBG, fasting blood glucose; OGTT, oral glucose tolerance test.
Correlations of HbA1c with FBG were 0.353 (p < 0.001) at 1- to 2-year follow-up and 0.613 (p < 0.001) at 5-year follow-up. The corresponding figures for HbA1c versus 2-h glucose were 0.380 (p < 0.001) and 0.430 (p < 0.001), respectively.
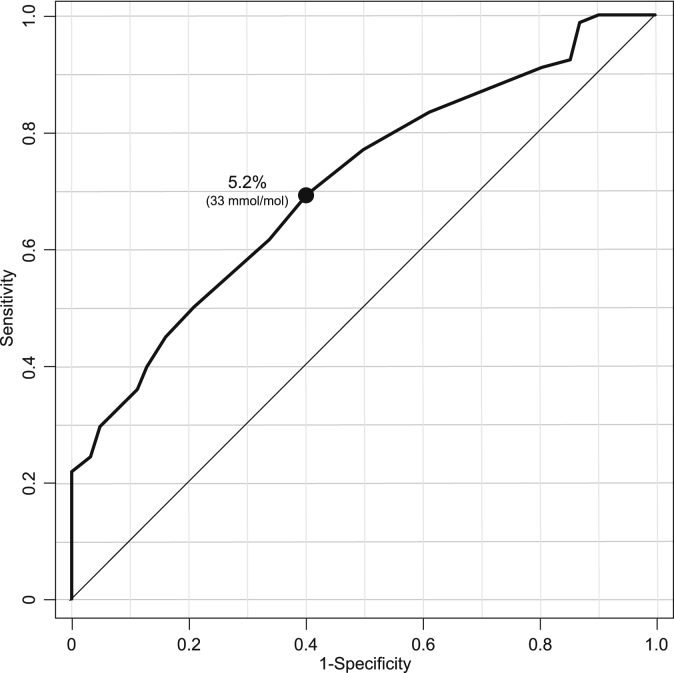
An ROC curve was constructed to evaluate the sensitivity and specificity of HbA1c in detection of abnormal glucose tolerance, as defined by the OGTT (Fig. 1). The optimal cut-off point of HbA1c for predicting abnormal glucose tolerance was 5.2% (33 mmol/mol) (AUC = 0.708, 95% CI 0.624–0.793), sensitivity was 69.2%, and specificity was 59.7%.
Figure. 1.
Receiver operating characteristic curve for HbA1c for detection of abnormal glucose tolerance by the oral glucose tolerance test. The optimal cut-off point of HbA1c is indicated.
Table 2 shows the sensitivity, specificity, PPV, and NPV of HbA1c and FBG, or a combination of both diagnostic tests, relative to the OGTT (the gold standard) for various cut-offs. Overall, the FBG test alone showed better performance than the HbA1c test alone in detecting abnormal glucose tolerance. Of those who screened positive using the FBG test alone, all had (by definition) abnormal glucose tolerance (13 IFG, 12 IGT, 24 diabetes), as compared to 32% of those who screened negative (25 IGT, 4 diabetes). The combined use of HbA1c and FBG criteria showed performance similar to that with use of the FBG test alone.
Table 2.
Diagnostic indices of various criteria using HbA1c or fasting blood glucose to detect abnormal glucose tolerance
| Diagnostic test | na | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|
| HbA1c ≥5.7% (≥39 mmol/mol) | 26 | 29.5 | 95.2 | 88.5 | 51.8 |
| FBG ≥5.6 mmol/l | 49 | 62.8 | 100.0 | 100.0 | 68.1 |
| HbA1c ≥5.7% (≥39 mmol/mol) or FBG ≥5.6 mmol/l | 55 | 66.7 | 95.2 | 94.5 | 69.4 |
| HbA1c ≥6.0% (≥42 mmol/mol) | 17 | 21.8 | 100.0 | 100.0 | 50.4 |
| HbA1c ≥6.0% (≥42 mmol/mol) or FBG ≥5.6 mmol/l | 51 | 65.4 | 100.0 | 100.0 | 69.7 |
| HbA1c ≥5.0% (≥31 mmol/mol) | 103 | 83.3 | 38.7 | 63.1 | 64.9 |
| HbA1c ≥5.0% (≥31 mmol/mol) or FBG ≥5.6 mmol/l | 108 | 89.7 | 38.7 | 64.8 | 75.0 |
FBG, fasting blood glucose; PPV, positive predictive value; NPV, negative predictive value.
Number of women who met cut-off values.
We then tested a combination of FBG (≥5.6 mmol/l) with various cut-points of HbA1c to increase the sensitivity and NPV of the combined test. From this, HbA1c ≥5.0% (≥31 mmol/mol) was judged as an optimal cut-point, according to which, in addition to the 49 women who screened positive by FBG criterion alone, another 59 women were identified (38 with normal glucose tolerance, 17 with IGT, and four with diabetes by OGTT). Of the remaining 32 women who screened negative using this combination, 8 had abnormal glucose tolerance (all IGT) by OGTT.
Discussion
In this historical cohort of women who had had GDM and who were prospectively followed for up to 5 years after delivery, we found suboptimal performance of proposed cut-points of HbA1c relative to OGTT in diagnosis of diabetes and abnormal glucose tolerance post-partum. Combined with a fasting glucose test, the diagnostic accuracy improved—although to an extent similar to that obtained using the fasting glucose test alone.
Women with a history of GDM have a 7.7-fold increased risk of future development of type-2 diabetes [4]. It has been shown previously that lifestyle intervention can prevent or delay the onset of type-2 diabetes in women with IGT and a history of GDM [14]. Thus, re-evaluation after pregnancy is essential. However, studies have repeatedly shown poor compliance with recommended guidelines in clinical practice, and the women fail to attend the post-partum visit, even in a research setting 15, 16, 17, 18. Easy, cost-effective, and less time-consuming screening strategies are required to capture as many women as possible who are at risk of type-2 diabetes. In this context, the HbA1c test appears to be attractive and its validity as a screening tool for abnormal glucose metabolism after GDM has only been examined in a few studies, with somewhat conflicting results 17, 19, 20, 21, 22.
Using the HbA1c test alone, we found that less than 5% of the women classified as having normal glucose tolerance by OGTT criteria would be misclassified as having abnormal glucose homeostasis, and more importantly, that 71% of the women classified as having abnormal glucose tolerance by OGTT criteria would be misclassified as having normal glucose homeostasis. Proposed cut-points of HbA1c had low sensitivity and modest NPV in detection of any degree of abnormal glucose tolerance, and therefore do not appear to be suitable for screening in these women. However, because of high PPV and high specificity, it may be used as a confirmatory test of the actual glucose tolerance status. The FBG test criterion had moderate sensitivity and NPV in detection of abnormal glucose tolerance. Megia et al. reported almost identical results to ours regarding HbA1c for diabetes diagnosis, with a sensitivity of 16.7% and a specificity of 100% [19]. However, using HbA1c of 5.7 (39 mmol/mol) as cut-off for any kind of impaired glucose tolerance, the sensitivity was comparatively low (13.5%). In contrast, Katreddy et al. reported a sensitivity of 71% and a specificity of 99% (AUC 0.98) in the diagnosis of diabetes, although the sensitivity of HbA1c ≥6.0% (≥42 mmol/mol) for detecting abnormal glucose tolerance was low (28%) [20]. Another study by Kim et al., based on a small group of women who had had GDM, found a sensitivity 65% and a specificity 68% for HbA1c ≥ 5.7% (≥39 mmol/mol) in detection of abnormal glucose tolerance [21]. ROC curves gave results similar to ours, with an AUC for any degree of impaired glucose tolerance of 0.76.
In line with previous studies, we found poor agreement in the consistency between HbA1c and OGTT criteria in classifying diabetes and abnormal glucose tolerance post-partum, although correlations between HbA1c and glucose values obtained during the OGTT indicated fairly good agreement 17, 19, 21.
Based on the present findings, the combination of HbA1c and FBG criteria classified 33% of the women who were classified as having abnormal glucose tolerance by OGTT criteria, as having normal glucose homeostasis. The specificity and PPV were high, but this combination did not improve the sensitivity and specificity obtained by FBG criterion alone. Similar observations for the combined test relative to the fasting glucose test alone were made by Picon et al. and Megia et al., albeit with higher sensitivities (83% and 82%, respectively), which might in turn be partly explained by their use of somewhat lower cut-offs 17, 19. Predictive values were only reported in the study by Picon et al., who found an NPV of 85%. Noctor et al. used a similar approach with cut-offs identical to those used by Picon et al., and reported sensitivity of 90% and NPV of 97% in detecting abnormal glucose tolerance, thereby reducing the proportion of women requiring confirmatory testing to 31%, as compared to 29% in the study by Megia et al. and 47% in the study by Picon et al. [22].
There are several plausible explanations for the discrepant results between studies. Firstly, differences in diagnostic criteria for the diagnosis of GDM imply that more or less high-risk women will be identified, rendering comparisons less reliable. If high glucose cut-points are used more severely affected women will be selected. We have recently evaluated how the introduction of the new International Association of the Diabetes and Pregnancy Study Groups (IADPSG) criteria and the 1999 WHO criteria would affect the prevalence of GDM in our population [23]. The results indicate that 26% more women would be identified by the IADPSG criteria and 20% more women by the WHO criteria, compared with the modified EASD criteria presently in use. The prevalence of abnormal glucose tolerance post-partum observed in the present study was relatively high (55.7%) in comparison with other studies, ranging from 18.4% in the study by Noctor et al. to 68.5% in the study by Kim et al. This could partly be attributed to the different glucose cut-points used for the diagnosis of GDM. Differences in the interval to post-partum retesting between studies would also affect the results. Median interval to follow-up in the present study was 2.1 years as compared with 3 months (Megia et al.), 1 year (Picon et al.), 1.5 years (Kim et al.) and 2.6 years (Noctor et al.). Other important differences include patient characteristics, such as BMI and age, and the ethnic composition of the cohorts, which in turn may have an impact on the interpretation of HbA1c data per se [24]. Also, the HbA1c assays used may differ and may not be fully comparable.
The rationale for recommending OGTT post-partum in women with GDM is not only to detect women with apparent diabetes but also to identify women with pre-diabetes and IGT in whom diabetes can be delayed or prevented 14, 25. We therefore hypothesized that a reasonable screening model would be to accept all women with IFG for intensive follow-up and prevention without retesting—in our sample, corresponding to 35% of the study population (49/140). If we then accept HbA1c 5.0% (31 mmol/mol) as a cut-off for further identification, that would leave 59 women for confirmatory testing by OGTT, among whom 36% (21/59) would be diagnosed with diabetes or IGT based on the 2-h glucose value alone. Of the remaining 32 women, 25% would be misclassified as having normal glucose metabolism, i.e. 10% (8/78) of the women with any kind of abnormal glucose tolerance in the study cohort.
Several limitations of this study must be considered. Since it was based on historical data, the results may not be completely representative of the contemporary population. Furthermore, glucose concentrations were determined in whole blood, which was the routine in Sweden at the time of the study. Converted glucose thresholds provided by the WHO (1999) for whole blood were used for classification. Due to the higher water concentration in plasma than erythrocytes, glucose concentration in plasma is higher than glucose in whole blood. A constant factor of 1.11 for the conversion between concentration of glucose in blood and the equivalent concentration in plasma is recommended, assuming a normal hematocrit [26]. Since the concentration of glucose in whole blood depends on the hematocrit the WHO conversion tables may be inaccurate in some situations. We have no information on the hematocrit in these women, but they were all apparently healthy and we do not believe this should have any major impact on the results. One further limitation of the study is that each test was only performed once and the diagnosis of diabetes was not confirmed by a repeat test. Moreover, hemoglobinopathies were not systematically assessed but are generally more common in the Mediterranean and not-white populations. However, the agreement between HBA1c and OGTT results did not markedly improve by excluding non-Nordic women from the evaluation (κ 0.278 as compared with κ 0.227).
This is the first study in Sweden comparing the performance of HbA1c with that of established glucose criteria during the OGTT in women with previous GDM. The strengths of the study include the prospective design with long-term follow-up of a relatively large number of women after GDM pregnancy. The uniform diagnostic procedure for GDM used in southern Sweden since 1995 is also noteworthy: it is based on a universal 75-g OGTT and there have been no major changes. Furthermore, the HbA1c assay used has a known and constant relation to the IFCC standard [12].
In summary, proposed thresholds of HbA1c (≥6.5% [48 mmol/mol] and ≥5.7% [≥39 mmol/mol]) had low sensitivity in diagnosis of diabetes and abnormal glucose tolerance in the present study cohort. Combined with a fasting glucose test, the performance was no better than using a fasting glucose test alone. Considering that early detection of pre-diabetes is of utmost importance in these women to prevent the development of diabetes, combining a fasting glucose test with a lower cut-point of HbA1c may be an alternative approach to select women for an OGTT—to identify those who have isolated post-glucose load hyperglycaemia. With an HbA1c cut-off of ≥5.0% (≥31 mmol/mol), the number of women who would need a confirmatory OGTT decreased by almost 60%, thus overlooking 10% of those with abnormal glucose tolerance in the study cohort.
Acknowledgments
We thank Ylva Wessman and Vera Gunnarsson for skilful technical assistance and Helene Jacobsson, biostatistician of the Department of Medical Statistics and Epidemiology, County of Skåne, Sweden, for statistical support.
Footnotes
Funding: This study was supported by grants from the Research Funds of Malmö University Hospital and Skåne County Council Research and Development Foundation (Grant number: REGSKANE-271001, REGSKANE-351271 and REGSKANE-360381).
Conflict of interest: The authors declare that there are no conflicts of interests associated with this manuscript.
References
- 1.World Health Organization . World health Organization; Geneva: 2011. Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Abbreviated Report of a WHO Consultation. [PubMed] [Google Scholar]
- 2.International Expert C International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;3:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl. 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellamy L., Casas J.P., Hingorani A.D., Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–1779. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 5.Sacks D.B. A1C versus glucose testing: a comparison. Diabetes Care. 2011;34:518–523. doi: 10.2337/dc10-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lind T., Phillips P.R. Influence of pregnancy on the 75-g OGTT. A prospective multicenter study. The Diabetic Pregnancy Study Group of the European Association for the Study of Diabetes. Diabetes. 1991;40(Suppl. 2):8–13. doi: 10.2337/diab.40.2.s8. [DOI] [PubMed] [Google Scholar]
- 7.Ignell C., Claesson R., Anderberg E., Berntorp K. Trends in the prevalence of gestational diabetes mellitus in southern Sweden, 2003–2012. Acta Obstet Gynecol Scand. 2014;9:420–424. doi: 10.1111/aogs.12340. [DOI] [PubMed] [Google Scholar]
- 8.Ekelund M., Shaat N., Almgren P., Groop L., Berntorp K. Prediction of postpartum diabetes in women with gestational diabetes mellitus. Diabetologia. 2010;53:452–457. doi: 10.1007/s00125-009-1621-3. [DOI] [PubMed] [Google Scholar]
- 9.Kim C., Newton K.M., Knopp R.H. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;2:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization . World Health Organization; Geneva: 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. [Google Scholar]
- 11.Jeppsson J.O., Jerntorp P., Sundkvist G., Englund H., Nylund V. Measurement of hemoglobin A1c by a new liquid-chromatographic assay: methodology, clinical utility, and relation to glucose tolerance evaluated. Clin Chem. 1986;32:1867–1872. [PubMed] [Google Scholar]
- 12.Hoelzel W., Weykamp C., Jeppsson J.O., Miedema K., Barr J.R., Goodall I. IFCC reference system for measurement of hemoglobin A1c in human blood and the national standardization schemes in the United States, Japan, and Sweden: a method-comparison study. Clin Chem. 2004;50:166–174. doi: 10.1373/clinchem.2003.024802. [DOI] [PubMed] [Google Scholar]
- 13.Altman D.G. Charpman and Hall; London: 1991. Practical statistics for medical research. [Google Scholar]
- 14.Ratner R.E., Christophi C.A., Metzger B.E., Dabelea D., Bennett P.H., Pi-Sunyer X. Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab. 2008;93:4774–4779. doi: 10.1210/jc.2008-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tovar A., Chasan-Taber L., Eggleston E., Oken E. Postpartum screening for diabetes among women with a history of gestational diabetes mellitus. Prev Chronic Dis. 2011;8:A124. [PMC free article] [PubMed] [Google Scholar]
- 16.Shah B.R., Lipscombe L.L., Feig D.S., Lowe J.M. Missed opportunities for type 2 diabetes testing following gestational diabetes: a population-based cohort study. BJOG. 2011;118:1484–1490. doi: 10.1111/j.1471-0528.2011.03083.x. [DOI] [PubMed] [Google Scholar]
- 17.Picon M.J., Murri M., Munoz A., Fernandez-Garcia J.C., Gomez-Huelgas R., Tinahones F.J. Hemoglobin A1c versus oral glucose tolerance test in postpartum diabetes screening. Diabetes Care. 2012;35:1648–1653. doi: 10.2337/dc11-2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderberg E., Landin-Olsson M., Kalen J., Frid A., Ursing D., Berntorp K. Prevalence of impaired glucose tolerance and diabetes after gestational diabetes mellitus comparing different cut-off criteria for abnormal glucose tolerance during pregnancy. Acta Obstet Gynecol Scand. 2011;90:1252–1258. doi: 10.1111/j.1600-0412.2011.01214.x. [DOI] [PubMed] [Google Scholar]
- 19.Megia A., Naf S., Herranz L., Serrat N., Yanez R.E., Simon I. The usefulness of HbA1c in postpartum reclassification of gestational diabetes. BJOG. 2012;119:891–894. doi: 10.1111/j.1471-0528.2012.03325.x. [DOI] [PubMed] [Google Scholar]
- 20.Katreddy M.V., Pappachan J.M., Taylor S.E., Nevill A.M., Indusekhar R., Nayak A.U. Hemoglobin A1c in early postpartum screening of women with gestational diabetes. World J Diabetes. 2013;4:76–81. doi: 10.4239/wjd.v4.i3.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim C., Herman W.H., Cheung N.W., Gunderson E.P., Richardson C. Comparison of hemoglobin A1c with fasting plasma glucose and 2-h postchallenge glucose for risk stratification among women with recent gestational diabetes mellitus. Diabetes Care. 2011;34:1949–1951. doi: 10.2337/dc11-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Noctor E., Crowe C., Carmody L.A., Avalos G.M., Kirwan B., Infanti J.J. ATLANTIC DIP: simplifying the follow-up of women with previous gestational diabetes. Eur J Endocrinol. 2013;169:681–687. doi: 10.1530/EJE-13-0491. [DOI] [PubMed] [Google Scholar]
- 23.Claesson R., Ekelund M., Berntorp K. The potential impact of the new diagnostic criteria on the frequency of diabetes mellitus in Sweden. Acta Obstet Gynecol Scand. 2013;92:1223–1226. doi: 10.1111/aogs.12209. [DOI] [PubMed] [Google Scholar]
- 24.Herman W.H., Ma Y., Uwaifo G., Haffner S., Kahn S.E., Horton E.S. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care. 2007;30:2453–2457. doi: 10.2337/dc06-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hopper I., Billah B., Skiba M., Krum H. Prevention of diabetes and reduction in major cardiovascular events in studies of subjects with prediabetes: meta-analysis of randomised controlled clinical trials. Eur J Cardiovasc Prev Rehabil. 2011;18:813–823. doi: 10.1177/1741826711421687. [DOI] [PubMed] [Google Scholar]
- 26.Burnett R.W., D'Orazio P., Fogh-Andersen N., Kuwa K., Kulpmann W.R., Larsson L. IFCC recommendation on reporting results for blood glucose. Clin Chim Acta. 2001;307:205–209. doi: 10.1016/s0009-8981(01)00431-4. [DOI] [PubMed] [Google Scholar]