Highlights
-
•
No relationship was found between depression and PIR.
-
•
Prevalence of PIR was 47.2% in insulin-naïve patients and 8.7% in insulin users.
-
•
PIR etiology is likely multifactorial in Hong Kong primary care population.
-
•
PIR was associated with multiple social factors.
Keywords: Type 2 diabetes mellitus, Hong Kong, Psychological insulin resistance, Insulin Treatment Appraisal Scale
Abstract
Aim
Patients with diabetes mellitus (DM) often delay the initiation of insulin treatment and titration due to psychological factors. This phenomenon is called psychological insulin resistance (PIR). The insulin treatment appraisal scale (ITAS) that was initially developed for Western populations has been translated and validated to measure PIR in Taiwanese populations (C-ITAS). This study aims to estimate the prevalence of PIR in primary care patients with DM in Hong Kong and to examine the relationship between PIR and psychosocial factors.
Method
402 DM patients from a government-funded general outpatient clinic completed the C-ITAS and a health questionnaire (the Patient Health Questionnaire-9, PHQ-9). Patient demographics were recorded and associations among C-ITAS scores, PHQ-9 scores and demographic data were evaluated.
Results
There was no relationship between the presence of depression and PIR. Furthermore, the prevalence of PIR was 47.2% in insulin-naive patients but only 8.7% in current insulin users. Tools such as the C-ITAS may help clinicians understand the etiology of PIR, which this study suggests is likely the result of multiple risk factors. Factors associated with a lower prevalence of PIR included current insulin use, a family history of insulin use, a high education level, male sex, and having received counseling from a physician about insulin within the previous 6 months.
Introduction
Type 2 diabetes mellitus (DM) is a prevalent and increasingly common disease worldwide [1]. Ten percent of the population of Hong Kong (HK) (approximately 700,000 people) is estimated to have DM [2]. Treatment to lower and achieve early good control of glycosylated hemoglobin (HbA1c) can lead to good long-term HbA1c control (known as the ‘legacy effect’). Achieving good DM control early in the disease course can reduce DM-induced microvascular complications and may reduce macrovascular complications [3], [4]. Tight DM control after a long duration of hyperglycemia has not shown such beneficial results; it may even result in mortality [5], [6], [7]. Therefore, achieving early tight HbA1c control through lifestyle changes and the use of medications, including insulin, is important.
Due to the progressive nature of DM, most patients will eventually require insulin [8]. Despite robust evidence of the benefits of early strict HbA1c control, patients often delay insulin initiation and titration. In a UK study, 50% of DM patients delayed insulin initiation despite suboptimal control for 5 years, regardless of the presence of complications [9]. The reluctance to initiate insulin use [9], [10], [11], as well as to its subsequent titration [12], is termed “psychological insulin resistance (PIR)”. The prevalence of PIR was estimated to be higher in Singapore (70.6%) [10] than in Western countries (approximately 20–40%) [11]. A questionnaire study in Hong Kong involving 97 subjects reported a similarly high PIR prevalence of 72.1% [13]. Previous studies conducted in Western countries have identified several factors that can lead to PIR [10], [11], [12]. However, the reasons for PIR may differ in Asian countries [14], [15]. Recently, a local primary care research group developed a scale (Ch-ASIQ) to identify barriers to insulin initiation in insulin-naive DM patients [15]. These investigators found that Asian patients may be more affected by the availability of social support and that cultural differences may also play a role. For example, Chinese patients are more likely to combine Western medical treatments with traditional Chinese medicine [16], and they may believe that hypoglycemic agents cause renal toxicity [17].
Depression, a common co-morbid condition among DM patients, is known to worsen clinical outcomes [18], [19], [20], [21]. Depression has been shown to affect patient decision-making [17]. Behaviors such as poor drug compliance may be associated with low motivation and drive, which are central to the clinical presentation of depression [20], [21]. Alternatively, depression may have a direct biological effect through the stimulation of the sympathetic nervous system, increasing inflammatory and platelet aggregation responses [21]. In addition, depression has been shown to correlate with PIR in Western studies with a variance of 3.8% [11] and a correlation factor of 0.2 [19].
This study aimed to estimate the prevalence of PIR in a clinical setting and to examine the relationship between PIR and psychosocial factors, including depression.
Methods
The Research Ethics Committee, Kowloon West Cluster, Hospital Authority approved this research on 25 April 2013.
Participants
Participants were recruited from a government-funded primary care general outpatient clinic in HK from July to September 2013. Patients who fulfilled the following criteria were recruited: (1) diagnosis of type 2 DM as defined by the World Health Organization [22] for ≥6 months; (2) age of 30 years or over; (3) Chinese ethnicity; (4) ability to communicate effectively in Cantonese or Mandarin; and (5) the mental capacity to provide informed written consent. The exclusion criteria were severe sensory deficits and severe mental illnesses (dementia, psychosis and mental retardation) or any other health conditions that compromised the patient's ability to comprehend and complete the questionnaire. Potential subjects were sampled from the clinical appointment database using a random method.
The required sample size was calculated from the estimated prevalence rate of PIR in the primary care setting. With a type I error set at 0.05, a power set at 0.80, and an estimated 70% prevalence of PIR among patients with diabetes in public primary care settings [10], [13], the required sample size was 312 people. To compensate for the predicted 20% dropout rate, at least 390 patients were needed.
Demographic data, including age, sex, marital status, employment status, education level, family history of insulin use and general attitudes toward insulin use, were also recorded using a standardized questionnaire. Case records were retrieved for HbA1c measures, the presence of diabetic complications, the presence of treatments for depression and the types of diabetic treatments used (oral agents and/or insulin).
Patients were encouraged to complete the questionnaires on their own, as the C-ITAS and PHQ-9 are self-administered instruments. Because the majority of the patients who attend public primary care clinics are of lower socio-economic statuses and education levels, patients who had difficulty completing the questionnaires were assisted by research assistants who were trained by the principal investigator (PI).
Instruments
Insulin Treatment Appraisal Scale (ITAS)
The ITAS is a 20-item instrument that contains 16 negative and 4 positive statements that provide information regarding a patient's appraisal of insulin treatment. Each statement is ranked on a 5-point Likert-type scale. Positive scores are reversed to allow for summation. The total possible score ranges from 0 to 80. A higher score indicates a more negative appraisal of insulin. The ITAS was developed to measure PIR for clinical use [23]. However, there was no cut-off score for diagnosing PIR.
Patient Health Questionnaire-9 (PHQ-9)
Unlike similar studies that used the Center for Epidemiologic Studies Depression Scale (CES-D) [11], [19], the PHQ-9 was used in the present study because it is internationally validated and widely used locally. Furthermore, evidence suggests that higher response rates can be obtained for shorter questionnaires such as the PHQ-9 [24], and the PHQ-9 has been used extensively in many research and clinical settings [24], [25].
The PHQ-9 questionnaire contains 9 items. Each item is ranked from 0 (not at all) to 3 (nearly every day), and the total possible score ranges from 0 to 27 [20]. The original group that developed the scale suggested cut-off scores of 5, 10, 15, and 20 to represent mild, moderate, moderately severe, and severe depression [20]; at a cutoff score of 10, the questionnaire exhibits high sensitivity and specificity values of approximately 80–90% for identifying depressive disorders, with reference to the Structured Clinical Interview for DSM-IV [24], [25].
Analysis
Patients were classified as having PIR if they responded ‘strongly unacceptable’ or ‘unacceptable’ to the question, “Will you agree to start or titrate insulin treatment if advised by your case doctor?”
Differences in demographic data, clinical data, and scores on the PHQ-9 and ITAS in patients with and without PIR were detected using the t-test for continuous variables and the chi-square test for categorical variables, with a significance level set at p < 0.05. Each ITAS item was dichotomized. The individual ITAS item responses among patients with and without PIR were compared using the chi-square test.
Results
Participants
A total of 399 insulin-naive DM patients were randomly selected from the clinical database and approached by the research team. Forty-two patients were excluded for the following reasons: 2 because they were incorrectly diagnosed with DM; 27 because they had severely impaired hearing not compensated for with the use of hearing aids; 3 because they only spoke languages other than Cantonese and Mandarin; 8 due to known severe psychiatric illnesses, such as dementia, psychosis and mental retardation; 1 due to leaving at the beginning of the interview when called into the consultation room; and 1 due to submitting an invalid questionnaire (all of the boxes in the questionnaire were checked).
In addition to insulin-naive DM patients, current insulin users (47 patients) were invited to participate in this study and were interviewed by phone. In the group of patients who used insulin, three subjects were excluded for the following reasons: 1 for not being able to speak Cantonese or Mandarin; 1 who was not in Hong Kong during the interview period; and 1 whose questionnaire was invalidated due to a missing entry for the subject's case number.
The overall response rate was 89.8% (89.6% for the insulin-naive patients and 90.9% for insulin users). Most respondents had a household income of less than HKD $10,000 per month (69.1%) and were elderly (mean 67.7, median 69, range 39–91) and female (60.8%). Many responders had a household income of less than HKD $5000 (40.2%). Most responders had up to a primary school level of education (71%); only 4.9% had an education at the tertiary level or above, and 23.8% had no formal education. The majority of the patients were married (67.7%) and retired (76.6%). Fully 45.3% of the patients had diabetes for more than 10 years; 47.1% had an HbA1c level lower than 7% (53 mmol/mol), and 16% had an HbA1c level higher than 8% (63.9 mmol/mol). Most patients (59.9%) had LDL levels of less than 2.7 mmol/L, and 12.0% had LDL levels equal to or higher than 3.5 mmol/L. Sixteen percent of patients had microalbuminuria, 20% exhibited renal impairment with an estimated glomerular filtration rate of 60 or less using the Modification of Diet in Renal Disease (MDRD) equation, 13.2% had retinopathy that required referral to an eye specialist, and 4.2% had evidence of diabetic foot neuropathy with impaired results when tested for vibration. A total of 12.5% of our subjects were placed on insulin. Patients with HbA1c >7% (53 mmol/mol) (22.3%) were more likely to be on insulin treatment than those with HbA1c <7% (53 mmol/mol) (4.1%) (p ≤ 0.001). HbA1c levels were not significantly associated with the presence of DM complications in the current study.
Non-responders were significantly older (mean age 72.32 years versus 67.17 years, p = 0.0002), were less likely to agree to titrate insulin (for current insulin users) and were less educated (91.7% educated up to the primary level versus 68.9% for responders; p = 0.004). The differences in other demographic data, including the DM complication rate, insulin use status, marital status, employment status, family income, and sex, were not statistically significant.
Relationship between psychosocial factors and PIR
The PIR prevalence was 47.2% in insulin-naive patients but only 8.7% in current insulin users.
Insulin-naive participants with PIR displayed significantly different attitudes toward insulin use. Such participants were more likely to respond “agree” to the following C-ITAS statements: Q4, insulin will make others perceive greater sickness (64.8% to 49.3%, p = 0.013); Q5, insulin will make life less flexible (from 72.5% to 46.4%, p ≤ 0.001); Q6, fear of needle injection (from 84.6% to 54.8%, p ≤ 0.001); Q7, insulin will increase the risk of hypoglycemia (from 25.4% to 19.7%, p = 0.003); Q9, insulin will cause weight gain (from 19.9% to 11.5%; p = 0.002); Q10, insulin will be difficult to administer (from 65% to 29.4%, p ≤ 0.001); Q11, insulin means I have to give up activities that I enjoy (from 39% to 17.4%, p ≤ 0.001); Q12, insulin means my health will deteriorate (from 46.8% to 31.3%, p ≤ 0.001); Q13, injecting insulin is embarrassing (from 53.9% to 25.0%; p ≤ 0.001); Q14, injecting insulin is painful (from 68.5% to 42.9%, p ≤ 0.001); Q15, it is difficult to inject insulin correctly every time (from 75.4% to 43.6%, p ≤ 0.001); and Q16, insulin makes it difficult to fulfill my responsibilities (from 51.4% to 20.9%, p ≤ 0.001). Conversely, insulin-naive participants with PIR were more likely to disagree with the following statements: Q3, insulin will improve my prognosis (29.1 to 72.1%, p ≤ 0.001); Q8, insulin will improve my health (38.7 to 58.2%, p = 0.001); Q17, insulin helps to maintain good control of blood glucose (43.7 to 73.5%, p ≤ 0.001); and Q19, insulin helps to improve my energy levels (28.2 to 35.5%, p = 0.011).
Among insulin-naive subjects, total C-ITAS scores were higher in those with PIR (from 42.47% to 34.41%, p = 0.005). Current insulin users exhibited lower total C-ITAS scores than insulin-naive patients (29.62 to 38.93; p ≤ 0.001).
C-ITAS scores were lower in those who had a family history of insulin use (33.09 to 38.31, p ≤ 0.001) or who had received counseling from a physician about insulin in the previous 6 months (34.13 to 38.2, p = 0.005).
Those who had a family history of insulin use were less likely to agree with the following statements: Q2, diabetes has gotten worse (71.7 to 84.4%, p = 0.034); Q6, fear of injecting with a needle (56.5 to 74.4%, p = 0.012); Q9, causes weight gain (54.3 to 69%, p = 0.048); and Q15, difficulty injecting it correctly each time (47.8 to 71.9%, p = 0.001).
Those who had received a physician's advice were less likely to agree with the following statements: Q2, diabetes has gotten worse (68.1 to 84.7%, p = 0.005); Q4, perceived by others as more sick (58.3 to 72.4%, p = 0.047); Q5, life less flexible (53.2 to 71.8%, p = 0.01); Q15, difficulty injecting it correctly each time (51.1 to 71.2%, p = 0.006); and Q16, it prevents me from fulfilling my responsibilities (p = 31.9 to 57.4%, p = 0.001).
Patients with different sexes, education levels, and DM complications also displayed significant differences in their attitudes toward insulin use, as presented in Table 1.
Table 1.
Relationship of psychosocial factors with reasons for psychological resistance
| On insulin Tx? | Willing to start insulin if advised? | Having insulin using relatives? | Did doctor recommend to start/titrate insulin? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | Yes | No | p | Yes | No | p | ||
| ITAS total scores | Agree or neutral (%) | 29.62 | 38.93 | 0 | 34.41 | 42.47 | 0.005 | 33.09 | 38.31 | 0 | 34.13 | 38.2 | 0.005 |
| Q2 Diabetes has gotten worse | 66 | 85.2 | 0.001 | 71.7 | 84.4 | 0.034 | 68.1 | 84.7 | 0.005 | ||||
| Q3 Prevent complications | 91.9 | 83.2 | 0.012 | ||||||||||
| Q4 Perceived by others as more sick | 65.9 | 78.2 | 0.012 | 58.3 | 72.4 | 0.047 | |||||||
| Q5 Life less flexible | 44.7 | 73.3 | 0 | 60.9 | 83.1 | 0 | 53.2 | 71.8 | 0.01 | ||||
| Q6 Fear of injection with needle | 36.2 | 77.8 | 0 | 62.6 | 87.4 | 0 | 56.5 | 74.4 | 0.012 | ||||
| Q7 Risk of hypoglycemia | 38.3 | 75.7 | 0 | 64.2 | 81 | 0.001 | |||||||
| Q8 Improves health | 89.1 | 79.6 | 0.012 | ||||||||||
| Q9 Causes weight gain | 36.2 | 71.8 | 0 | 60.6 | 77.3 | 0.001 | 54.3 | 69 | 0.048 | ||||
| Q10 Takes time and energy | 19.1 | 65.3 | 0 | 45.9 | 79.7 | 0 | |||||||
| Q11 Give up activities I enjoy | 17 | 51.1 | 0 | 36.1 | 63.1 | 0 | |||||||
| Q12 My health will deteriorate | 31.9 | 67.2 | 0 | 54.4 | 75.2 | 0 | |||||||
| Q13 Injecting is embarrassing | 29.8 | 51.3 | 0.006 | 40 | 61.7 | 0 | |||||||
| Q14 Injecting is painful | 63.5 | 81.1 | 0 | ||||||||||
| Q15 Difficult to always inject correctly | 27.7 | 74.9 | 0 | 59.1 | 83.8 | 0 | 47.8 | 71.9 | 0.001 | 51.1 | 71.2 | 0.006 | |
| Q16 Difficult to fulfill responsibilities | 10.6 | 61 | 0 | 42.3 | 73.2 | 0 | 31.9 | 57.4 | 0.001 | ||||
| Q17 Helps to control blood glucose | 89.4 | 75.8 | 0.038 | 94.5 | 83.8 | 0.001 | |||||||
| Q18 Family/friends more concerned | 85.8 | 64.8 | 0 | ||||||||||
| Q19 Helps to improve energy levels | 83.6 | 70.4 | 0.003 | ||||||||||
| Q20 More dependent on doctor | 46.8 | 66.8 | 0.008 | ||||||||||
| Education level less than or at primary school? | Sex is male? | Presence of microalbuminuria? | (Insulin user) will agree to titrate insulin? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | Yes | No | p | Yes | No | p | ||
| Q1 Failed on pre-insulin therapy | Agree or neutral (%) | 84.4 | 73.4 | 0.015 | 85.7 | 33.3 | 0.022 | ||||||
| Q2 Diabetes has gotten worse | 86.9 | 76.1 | 0.012 | ||||||||||
| Q11 Give up activities I enjoy | 50.4 | 38.5 | 0.039 | 61.4 | 43.9 | 0.015 | |||||||
| Q13 Injecting is embarrassing | 44.9 | 57 | 0.036 | ||||||||||
| Q15 Difficult to always inject correctly | 72.8 | 61.5 | 0.032 | ||||||||||
| Q18 Family/friends more concerned | 63.8 | 80.2 | 0.006 | ||||||||||
| Q20 More dependent on doctor | 74.5 | 57.2 | 0.001 | ||||||||||
| HbA1c > 7% | >7 year DM? | Married? | Income < $1000 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | Yes | No | p | Yes | No | p | ||
| ITAS total score | Agree or neutral (%) | 36.83 | 39.42 | 0.014 | |||||||||
| Q2 Diabetes has gotten worse | 78.3 | 86.4 | 0.043 | ||||||||||
| Q3 Prevent complications | 92.1 | 84.5 | 0.026 | ||||||||||
| Q4 Perceived by others as more sick | 65.2 | 76.6 | 0.019 | ||||||||||
| Q9 Causes weight gain | 78.6 | 61.7 | 0.001 | ||||||||||
| Q11 Give up activities I enjoy | 57.3 | 41.5 | 0.005 | 50.2 | 38.9 | 0.048 | |||||||
Relationship between depression and PIR
Approximately 12% of the participants were screened positive for depression (i.e., PHQ-9 ≥ 10). More female participants (14.8% vs. 7.6%; p = 0.032) and more patients with a past depressive episode (16.7% vs. 3.7%; p = 0.001) had a PHQ score at or above the cutoff of 10.
Insulin-naive participants with a positive screen for depression did not differ in their willingness to start using insulin if suggested (from 59.5% to 58.3%, p = 0.878). The total C-ITAS scores of the participants who screened positive for depression did not differ significantly from those who screened negative (from 38 to 37.63; p = 0.285).
There was no association between the presence of depression and other psychosocial factors, including the HbA1c level, LDL level, presence of DM complications, insulin use status, family income, marital status, employment status, and number of years on insulin.
The published literature suggests that a loss of energy or fatigue may be associated with PIR [20], but a post-hoc analysis revealed no association between individual PHQ-9 item scores and the presence of PIR.
Discussion
In comparison to other published data, in which 30–40% and 25.5–37% [26] of patients attained their HbA1c and LDL targets, respectively, more patients in the public primary care clinic in Hong Kong achieved satisfactory glycemic control and LDL levels. A similar control rate has been observed in other government-funded clinics. In the year 2013, approximately 56% and 56.9% of all DM patients who received follow-up care in government-funded clinics had HbA1c levels below 7% (53 mmol/mol) and LDL below 2.7 mmol/L. The prevalence of depression in DM patients in our study was similar to the prevalences observed in other studies [27]. Despite the low education levels among the participants in this study, 89.8% were able to finish the C-ITAS questionnaire.
Presence of depression and its relationship with other variables
Although multiple psychosocial reasons for PIR were identified, depression was not found to be a significant factor. There may be several reasons for this finding. First, a substantial proportion of the participants who were screened positive for depression on the PHQ did not complete the C-ITAS. Further study with a larger sample size to verify the relationship between depression and PIR is merited, as there was a higher incompletion rate in this subgroup in this study. Second, a previous similar study suggested that the relationship could be weak after adjusting for DM-related psychological distress [19]. In another study, the variance explained by the model was only 3.8% [11]. A recent study reported no association between depression and the delay of insulin initiation [20]. Taking other studies' results into account, depression does not appear to display a strong relationship with patients' psychological resistance to insulin therapy.
The presence of depression was not associated with the presence of DM complications. This finding may be due to our definitions of DM complications, which included a glomerular filtration rate (eGFR) < 60, the presence of microalbuminuria, the presence of retinopathy on retinal photos requiring referral, and possible neuropathy with impaired vibration sensation (VPT). These complications did not signify the presence of symptoms; therefore, patients may have been unaware of these complications and/or were not distressed by them.
Psychological insulin resistance and its relationship with psychosocial variables
Psychological resistance in current insulin users was very low at 8.7%. This result could either be due to a low PIR at baseline (i.e., these patients did not resist the initiation of insulin therapy) or a genuine drop in resistance after the initiation of insulin therapy, as suggested in a qualitative study [28]. One longitudinal study involving 44 insulin-naive patients who switched to insulin therapy reported decreased negative attitudes toward insulin therapy after insulin was started [29]. The ITAS scores in that study were higher than the scores in the current study (ITAS mean score of 50), although that study involved a relatively small number of patients. Therefore, further longitudinal studies may be needed to confirm the hypothesis that PIR changes over the course of treatment. In our analysis, insulin users differed significantly from insulin-naive patients in their opinions of insulin (Table 1 attached).
The most common barriers in patients with high psychological resistance were ‘afraid to inject myself with a needle’ (83.6%), ‘it is difficult to inject the right amount of insulin correctly at the right time everyday’ (75.4%) and ‘taking insulin makes life less flexible’ (72.5%), in contrast to what doctors may commonly assume is the predominant fear (i.e., ‘injecting insulin is painful’ (68.5%)).
Patients who had received the advice of a physician regarding starting or titrating insulin exhibited lower total C-ITAS scores. The patients who received a physician's advice may have had a clearer understanding of insulin initiation and were thus less concerned about injection techniques, difficulty in undertaking the task or the implication that they were sicker. These results suggest that advice from physicians is effective in reducing psychological resistance. The importance of counseling was also reflected by the fact that patients with a lower education level (at or below the primary school level) were more likely to believe that insulin was difficult to administer, painful and embarrassing, and they were more anxious about self-injections.
A family history of insulin use was protective against PIR (Table 1), which may be due to the low incidence of complications among relatives (12.5%). However, the PIR rate between those who had relatives with and without complications while on insulin was not statistically significant (p = 0.713). As previously mentioned, patients may indirectly learn from the experiences of relatives and thus have a clearer understanding of the nature of insulin use, which suggests that positive role models can influence PIR.
The above finding suggests that participants' opinions of insulin use are strongly influenced by social interactions, such as those with their doctors and families, perhaps even more than the actual severity of the disease and the presence of complications. Counseling strategies have been suggested [30], [31], but further study may be needed to identify which counseling elements effectively ameliorate PIR. The importance of social interactions or family support was also identified by another study conducted in Hong Kong [15] that suggested a correlation between perceived family support and health behaviors in Chinese patients. To the author's knowledge, no study has yet been conducted to evaluate the effects of interventions, such as counseling, on ITAS scores or to use ITAS scores to detect reasons for PIR. However, such studies are feasible because the ITAS questionnaire was designed to detect changes in a patient's perception of insulin treatment [23].
As social interactions had significant effects on PIR, effects of advice from friends, other health professionals (e.g., nursing staff), and patient support groups can be examined in future research.
Comparison of ITAS scores with those from other studies
An interesting observation in the current study was that the mean total C-ITAS score in insulin-naive patients was generally lower (38.9%) than the scores obtained in other studies, which range from 50 [28] to 62.9 [14]. This is in contrast with the fact that, as mentioned previously, there is a higher prevalence of PIR in the local community. Further studies may be needed to re-examine the validity of the Chinese ITAS questionnaire and to seek an explanation for this observation.
Strengths and weaknesses
The strengths of our study include the relatively large sample size, the use of random sampling and the very high response rate. To the author's knowledge, this is the first local study using an internationally validated questionnaire (the ITAS) to examine the relationship between depression and PIR in the primary care setting.
The results of this study supporting the proposed use of insulin in patients are tentative. For example, the estimated psychological resistance rate may have decreased because patients perceived that their condition had deteriorated and that further intervention was needed.
Although this study was conducted in a major government-funded clinic in Hong Kong, the demographics of the participants were similar to those of other government clinics (Fig. 1), and the results of the current study are largely comparable to similar studies conducted in Istanbul [19] and Amsterdam [11], the extent to which the results can be generalized to other countries and to other social classes (e.g., wealthy patients attending private primary clinics) is not known.
Figure 1.
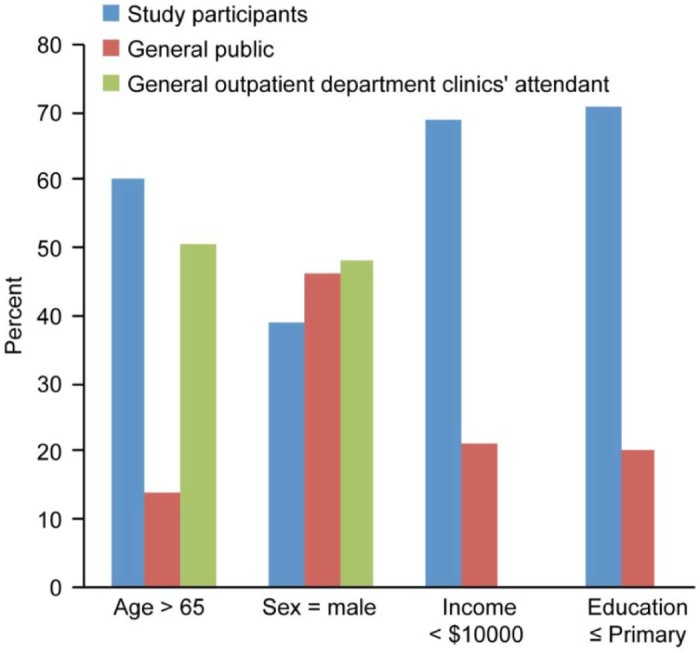
Comparison of demographic data.
Conclusion
Depression was identified as a common co-morbid condition of DM. Multiple psychosocial dimensions were associated with PIR, but depression was not. These results suggest that PIR is influenced by numerous factors, including information, adequate counseling and positive role models, such as family members.
This study suggested that the ITAS is a valid tool for understanding reasons underlying patients' PIR, and it could, therefore, help doctors provide specific and patient-centered counseling. In the Chinese population, psychosocial factors, rather than presence of complications, may play a more important role in determining PIR. This suggests that certain interventions (e.g., patient support groups) may be more effective in this population.
Further study may be needed to find ways to ameliorate PIR and to see if PIR changes over the course of DM treatments.
Funding
None.
Conflict of interest
The authors declare they have no conflicts of interest.
Acknowledgment
The author wishes to thank Prof Sandra Chan and Prof Samuel Wong for their help with research support and Dr. Fu SN and Dr Yiu YK who provided the site and assistants for the research.
References
- 1.International Diabetes Federation IDF diabetes atlas. 2015. http://www.diabetesatlas.org/ accessed 16.11.15.
- 2.Hong Kong Department of Health Hong Kong reference framework for diabetes care for adults in primary care settings. 2013. http://www.pco.gov.hk/english/resource/professionals_diabetes_pdf.html accessed 30.06.15.
- 3.Genuth S., Eastman R., Kahn R., Klein R., Lachin J., Lebovitz D. Implications of the United Kingdom prospective diabetes study. Diabetes Care. 2003;26:S28–32. doi: 10.2337/diacare.26.2007.s28. [DOI] [PubMed] [Google Scholar]
- 4.Timothy M.E.D. The continuing legacy of the United Kingdom prospective diabetes study. Med J Aust. 2004;180:104–105. doi: 10.5694/j.1326-5377.2004.tb05828.x. [DOI] [PubMed] [Google Scholar]
- 5.The ADVANCE Collaborative Group. Patel A., MacMahon S., Chalmers J., Neal B., Billot L. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 6.The Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein H.C., Miller M.E., Byington R.P., Goff D.C., Bigger J.T. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The ACCORD Study Group. Gerstein H.C., Miller M.E., Genuth S., Ismail-Beigi F., Buse J.B. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med. 2011;364:818–828. doi: 10.1056/NEJMoa1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner R.C., Cull C.A., Frighi V., Holman R.R. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005–2012. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 9.Rubino A., McQuay L.J., Gough S.C., Kvasz M., Tennis P. Delayed initiation of subcutaneous insulin therapy after failure of oral glucose-lowering agents in patients with Type 2 diabetes: a population-based analysis in the UK. Diabet Med. 2007;24:1412–1418. doi: 10.1111/j.1464-5491.2007.02279.x. [DOI] [PubMed] [Google Scholar]
- 10.Wong S., Lee J., Ko Y., Chong M.F., Lam C.K., Tang W.E. Perceptions of insulin therapy amongst Asian patients with diabetes in Singapore. Diabet Med. 2011;28:206–211. doi: 10.1111/j.1464-5491.2010.03195.x. [DOI] [PubMed] [Google Scholar]
- 11.Woudenberg Y.J., Lucas C., Latour C., Scholte op Reimer W.J. Acceptance of insulin therapy: a long shot? Psychological insulin resistance in primary care. Diabet Med. 2012;29:796–802. doi: 10.1111/j.1464-5491.2011.03552.x. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins N., Hallowell N., Farmer A.J., Holman R.R., Lawton J. Participants' experiences of intensifying insulin therapy during the treating to target in type 2 diabetes (4-T) trial: qualitative interview study. Diabet Med. 2011;28:543–548. doi: 10.1111/j.1464-5491.2010.03200.x. [DOI] [PubMed] [Google Scholar]
- 13.Yiu M.P., Cheung K.L., Chan K.W. A questionnaire study to analyze the reasons of insulin refusal of DM patients on maximum dose of oral hypoglycemic agents (OHA) among 3 GOPC in Kowloon West Cluster. 2010. http://www.ha.org.hk/haconvention/hac2010/proceedings/pdf/Poster/spp-p5-38.pdf accessed 30.06.15.
- 14.Chen C.C., Chang M.P., Hsieh M.H., Huang C.Y., Liao L.N., Li T.C. Evaluation of perception of insulin therapy among Chinese patients with type 2 diabetes mellitus. Diabetes Metab. 2011;37:389–394. doi: 10.1016/j.diabet.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 15.Fu S.N., Chin W.Y., Wong C.K., Yeung V.T., Yiu M.P., Tsui H.Y. Development and validation of the Chinese attitudes to starting insulin questionnaire (Ch-ASIQ) for primary care patients with type 2 diabetes. PLoS ONE. 2013;8:e78933. doi: 10.1371/journal.pone.0078933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas H., Matthew H., Gareth S.O. Depression and decision-making capacity for treatment or research: a systemic review. BMC Med Ethics. 2013;14:54. doi: 10.1186/1472-6939-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makine C., Karşidağ C., Kadioğlu P., Ilkova H., Karşidağ K., Skovlund S.E. Symptoms of depression and diabetes-specific emotional distress are associated with a negative appraisal of insulin therapy in insulin-naïve patients with type 2 diabetes mellitus. A study from the European Depression in Diabetes [EDID] Research Consortium. Diabet Med. 2009;26:28–33. doi: 10.1111/j.1464-5491.2008.02606.x. [DOI] [PubMed] [Google Scholar]
- 18.Ali S., Stone M.A., Peters J.L., Davies M.J., Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–1173. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 19.Peyrot M., Rubin R.R., Lauritzen T., Snoek F.J., Matthews D.R., Skovlund S.E. Psychosocial problems and barriers to improved diabetes management: results of the Cross-National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabet Med. 2005;22:1379–1385. doi: 10.1111/j.1464-5491.2005.01644.x. [DOI] [PubMed] [Google Scholar]
- 20.Nefs G., Pop V.J., Denollet J., Pouwer F. The longitudinal association between depressive symptoms and initiation of insulin therapy in people with type 2 diabetes in primary care. PLoS ONE. 2013;8:e78865. doi: 10.1371/journal.pone.0078865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bahrmann A., Abel A., Zeyfang A., Petrak F., Kubiak T., Hummel J. Psychological insulin resistance in geriatric patients with diabetes mellitus. Patient Educ Couns. 2014;94:417–422. doi: 10.1016/j.pec.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization About diabetes. 2015. http://www.who.int/diabetes/action_online/basics/en/index1.html accessed 30.06.15.
- 23.Snoek F.J., Skovlund S.E., Pouwer F. Development and validation of the insulin treatment appraisal scale (ITAS) in patients with type 2 diabetes. Health Qual Life Outcomes. 2007;5:69. doi: 10.1186/1477-7525-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung A., Fung F., Yu S.C., Vorono S., Ly M., Wu S. Validation of the patient health questionnaire-9 for depression screening among Chinese Americans. Compr Psychiatry. 2008;49:211–217. doi: 10.1016/j.comppsych.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Löwe B., Schenkel I., Carney-Doebbeling C., Göbel C. Responsiveness of the PHQ-9 to psychopharmacological depression treatment. Psychosomatics. 2006;47:62–67. doi: 10.1176/appi.psy.47.1.62. [DOI] [PubMed] [Google Scholar]
- 26.Chan J.C., Gagliardino J.J., Baik S.H., Chantelot J.M., Ferreira S.R., Hancu N. Multifaceted determinants for achieving glycemic control: the International Diabetes Management Practice Study (IDMPS) Diabetes Care. 2009;32:227–233. doi: 10.2337/dc08-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park M., Katon W.J., Wolf F.M. Depression and risk of mortality in individuals with diabetes: a meta-analysis and systematic review. Gen Hosp Psychiatry. 2013;35:217–225. doi: 10.1016/j.genhosppsych.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris J., Povey R., Street C. Experiences of people with type 2 diabetes who have changed from oral medication to self-administered insulin injections. Pract Diabetes Int. 2005;22:239–243. [Google Scholar]
- 29.Norbert H., Mahr M., Kulzer B., Skovlund S.E., Haak T. Barriers towards insulin therapy in type 2 diabetic patients: results of an observational longitudinal study. Health Qual Life Outcomes. 2010;8:113. doi: 10.1186/1477-7525-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peyrot M., Rubin R.R., Khunti K. Addressing barriers to initiation of insulin in patients with type 2 diabetes. Primary Care Diabetes. 2010;4:S11–18. doi: 10.1016/S1751-9918(10)60004-6. [DOI] [PubMed] [Google Scholar]
- 31.Wang H.F., Yeh M.C. Psychological resistance to insulin therapy in adults with type 2 diabetes: mixed-method systematic review. J Adv Nurs. 2012;68:743–757. doi: 10.1111/j.1365-2648.2011.05853.x. [DOI] [PubMed] [Google Scholar]


