Abstract
Background
Clinically, percutaneous vertebroplasty (PVP) is frequently applied to treat osteoporotic vertebral compression fracture (OVCF). It is believed that new compression fractures are more likely to occur adjacent to the PVP-treated segment, typically within 1 month after PVP. The purpose of this study was to investigate risk factors for adjacent vertebral compression fractures (AVCF) after PVP in patients with OVCF after menopause.
Material/Methods
Between Jun 2012 and Dec 2016, 412 patients were initially identified. We enrolled 390 patients in this study, and 22 were lost to follow-up. The medical records of the patients were retrospectively collected. Patients were followed up for at least 6 months, with an average follow-up period of 18 months. The potential risk factors investigated in this study included age, duration of menopause (DoM), preoperative vertebral compression, number of preoperative vertebral fractures (NPVF), bone mineral density (BMD), surgical approach (unilateral or bilateral), anesthesia methods, bone cement dose, complications (including COPD), and anti-osteoporosis treatment. Logistic regression analysis was used to determine the risk factors.
Results
Sixty-eight patients were observed to have suffered from AVCF after PVP at the last follow-up. Univariate analysis showed that age, DoM, NPVF, BMD, COPD, and anti-osteoporosis treatment were the potential variables associated with the onset of AVCF (all P<0.05). Binary logistic regression analysis showed that the logistic regression equation was as follows: logit P=−3.10−1.07×X2+0.99×X3+2.15×X4 (where X2=BMD; X3=DoM; X4=NPVF), and “logit P” stands for the likelihood of developing an AVCF following PVP.
Conclusions
A long duration of menopause and preoperative multi-level vertebral fractures were the risk factors for AVCF in patients following PVP after menopause, while a high-level BMD acted in a protective role for AVCF development.
MeSH Keywords: Osteoporosis, Postmenopausal; Osteoporotic Fractures; Risk Factors; Spinal Fractures; Vertebroplasty
Background
With the continued aging of the population, the incidence of osteoporosis and osteoporotic vertebral compression fracture (OVCF) is increasing around the world each year [1]. OVCF is now believed to be a common disorder, and it particularly affects elderly patients. OVCF frequently causes persistent back pain, significantly impairing mobility and quality of life [2]. Since such injuries greatly reduce quality of life and influence the daily life activities of the elderly, it is urgent to develop methods for preventing osteoporosis and OVCF [3,4].
Over the past few years, as a minimally invasive technique, percutaneous vertebroplasty (PVP), has been widely used to treat painful OVCF throughout the world [5,6]. Although PVP is increasingly being used in clinical practice as a treatment for OVCF, the increasing risk of AVCF postoperatively has been reported by some authors [7–11]. However, some other authors have stated that there is no convincing evidence that PVP results in such poor outcomes [1,12,13]. In addition, others have suggested that PVP might reduce the incidence of AVCF [14]. Thus, controversy still exists regarding whether PVP can increase the incidence of AVCF during the follow-up period. Although some clinical studies have compared PVP to conservative treatment [6,7,12–15], it is still unclear whether new AVCF is caused by PVP or is simply due to the natural development of osteoporosis.
Therefore, the aim of this study was to investigate whether the occurrence of AVCF is increased following PVP surgery and, at the same time, to explore the risk factors related to AVCF after PVP in menopausal women.
Material and Methods
Ethics statement
This study was approved by Ethics Committee of the Third Hospital of Hebei Medical University. The approval number is K2017-01-005.
Patients
Between Jun 2012 and Dec 2016, 412 patients were initially identified in our Spinal Department. All patients were diagnosed as having OVCF during menopause. The fractured vertebrae in the thoracolumbar level ranged from T10 to L2. Patients had undergone PVP surgery without cement leakage and trauma during the follow-up period. Patients were divided into 2 groups based on the occurrence of adjacent vertebral compression fracture (AVCF). The medical records of the patients were retrospectively collected. Patients who did not have regular follow-up visits and those who had systemic disorders were excluded. All patients were routinely asked to return to the hospital for a checkup every half year after PVP.
Evaluation of risk factors
The potential risk factors investigated in this study included age, duration of menopause (DoM), preoperative vertebral compression, number of preoperative vertebral fractures (NPVF), bone mineral density (BMD), QCT measurement (<80 mg/cm3 for osteoporosis [16]), surgical approach (unilateral or bilateral), anesthesia method, bone cement dose, complications (including COPD), and anti-osteoporosis treatment (AOT).
Statistical analyses
Statistical analyses were performed using SPSS for Windows, version 18.0 (SPSS, Inc., USA). All of the measurement data are presented as the mean ±SD (standard deviation) when the data satisfied the criteria for normality with p>0.10. Otherwise, they are presented as the median (interquartile range, IQR). When the data satisfied the criteria for normality and homogeneity of variance, statistical analysis between groups was performed using the t test. For the count data, the chi-square test was used for data analysis. Binary logistic regression analysis was used to determine the risk factors. Values of p<0.05 for two-tailed tests were regarded as being statistically significant.
Results
Distribution of vertebral fractures
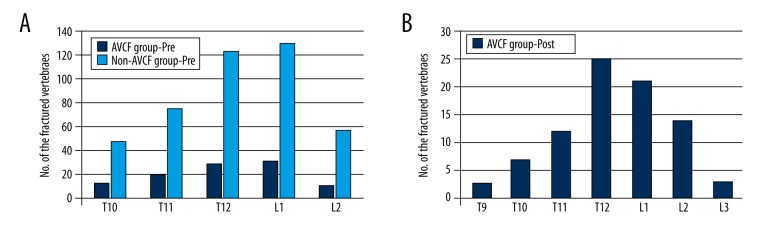
The distribution of preoperative vertebral fractures for all patients, including those of the AVCF group (102 fractured vertebrae) and those of the non-AVCF group (432 fractured vertebrae), is shown in Figure 1A. In addition, as shown in Figure 1B, 68 patients were observed to have suffered from AVCF after PVP, with 84 new fractured vertebrae.
Figure 1.
The distribution of OVCF in spinal vertebral bodies. (A) OVCFs before PVP surgery are shown. (B) AVCFs after PVP surgery are shown.
Comparison of age
We enrolled 390 patients in this study, and 22 were lost to follow-up. Patients were followed up for at least 6 months, with an average follow-up of 18 months. The mean age of patients in the AVCF group (n=68) was 71.8±6.7 years, and that of patients in the non-AVCF group (n=322) was 69.5±7.2 years. There was a significant difference in the age between the 2 groups (P=0.016). There were no significant differences regarding the anesthesia methods, surgical approaches, bone cement dose, and preoperative degree of vertebral compression, as well as for the other complications (hypertension, DM, and heart diseases) (all P>0.05).
Comparisons of BMD and DoM
As shown in Table 1, BMD between the AVCF group and non-AVCF group showed a significant difference (P=0.033). BMD less than 60 mg/cm3 was observed for more than half of AVCF patients, while it was observed for one-third of those in the non-AVCF group. As shown in Table 2, there was a significant difference regarding the DoM between the AVCF group and non-AVCF group (P=0.019). The proportion of patients with a DoM >10 years in the AVCF group was higher than that in the non-AVCF group.
Table 1.
Comparison regarding BMD.
| BMD (mg/cm3) | 60≤ BMD <80 | 40≤ BMD <60 | 20≤ BMD <40 | <20 |
|---|---|---|---|---|
| AVCF* (n=68) | 33 | 26 | 7 | 2 |
| Non-AVCF (n=322) | 214 | 85 | 20 | 3 |
P=0.033, compared with non-AVCF group, by chi-squared test.
AVCF – adjacent vertebral compression fracture; BMD – bone mineral density.
Table 2.
Comparison regarding duration of menopause.
| Duration of menopause (years) | ≤10 | 10< X <20 | ≥20 |
|---|---|---|---|
| AVCF* (n=68) | 19 | 38 | 11 |
| Non-AVCF (n=322) | 128 | 172 | 22 |
P=0.019, compared with non-AVCF group, by chi-squared test.
AVCF – adjacent vertebral compression fracture.
Comparisons of NPVF, COPD, and AOT
As shown in Table 3, there was a significant difference regarding NPVF between the AVCF group and non-AVCF group (P=0.039). The proportion of patients with NPVF ≥2 in the AVCF group was higher than that in the non-AVCF group. As shown in Table 4, there was a significant difference regarding COPD between the AVCF group and non-AVCF group (P=0.003). The proportion of patients with COPD in the AVCF group was higher than that in the non-AVCF group. As shown in Table 5, there was a significant difference regarding AOT between the AVCF group and non-AVCF group (P=0.001). The proportion of patients with AOT in the AVCF group was lower than that in the non-AVCF group.
Table 3.
Comparison regarding the number of preoperative vertebral fractures.
| N of vertebral fractures | 1 | 2 | 3 |
|---|---|---|---|
| AVCF* (n=68) | 37 | 28 | 3 |
| Non-AVCF (n=322) | 225 | 84 | 13 |
P=0.039, compared with non-AVCF group, by chi-squared test.
AVCF – adjacent vertebral compression fracture.
Table 4.
Comparison regarding concurrent COPD.
| COPD | Non-COPD | |
|---|---|---|
| AVCF* (n=68) | 24 | 44 |
| Non-AVCF (n=322) | 61 | 261 |
P=0.003, compared with non-AVCF group, by chi-squared test.
AVCF – adjacent vertebral compression fracture; COPD – chronic obstructive pulmonary disease.
Table 5.
Comparison regarding postoperative anti-osteoporosis treatment.
| Yes | No | |
|---|---|---|
| AVCF* (n=68) | 31 | 37 |
| Non-AVCF (n=322) | 217 | 105 |
P=0.001, compared with non-AVCF group, by chi-squared test.
AVCF – adjacent vertebral compression fracture.
Logistic regression analysis
The conditions of regression were as follows: Backward (LR), probability for stepwise (Entry 0.10, Removal 0.15). As shown in Table 6, the binary logistic regression analysis showed that the logistic regression equation was as follows: logit P=−3.10−1.07×X2+0.99×X3+2.15×X4 (X2=BMD; X3=DoM; X4=NPVF), and “logit P” stands for the likelihood of developing an AVCF following PVP. The equation was statistically significant using the Pearson chi-square test (P<0.001).
Table 6.
Binary logistic regression analysis regarding AVCF.
| No. | Items | B | Exp(B) | p-value | 95% CI for Exp(B) |
|---|---|---|---|---|---|
| X1 | Age | 0.43 | 1.53 | 0.071 | (0.85, 4.12) |
| X2 | BMD | −1.07 | 0.34 | <0.001 | (0.15, 0.74) |
| X3 | DoM | 0.99 | 2.68 | 0.001 | (1.64, 3.35) |
| X4 | NPVF | 2.15 | 8.60 | 0.001 | (4.85, 12.52) |
| X5 | COPD | 0.09 | 1.10 | 0.523 | (0.75, 4.32) |
| X6 | AOT | −1.40 | 0.25 | 0.344 | (0.07, 2.57) |
| X0 | Constant | −3.10 | 0.04 | 0.000 | – |
AVCF – adjacent vertebral compression fracture; BMD – bone mineral density; DoM – duration of menopause; NPVF – the number of preoperative vertebral fractures; COPD – chronic obstructive pulmonary disease; AOT – anti-osteoporosis treatment.
Discussion
Osteoporosis, a systemic disorder, is characterized by a lower BMD, which causes bone fragility and leads to a high risk of fracture [17,18]. Due to osteoporosis, OVCF is usually seen in the spinal vertebral bodies, which commonly leads to back pain and disability, affecting nearly 25% of the elderly population who are older than 50 years [19]. Conservative treatment, including staying in bed, lumbar bracing, and taking pain-killers, may relieve pain for a short time, such as several weeks or months. However, for elderly patients, a long-term lack of activity may contribute to serious complications, such as pneumonia, bedsores, deep vein thrombosis, and pulmonary embolism, and can even lead to death. Invasive surgery is a treatment option, but it is not the best treatment method, usually because of the poor body status of elderly patients [20]. Currently, as minimally invasive methods, procedures of bone cement augmentation, such as PVP, are widely used to treat elderly patients with OVCF [21–23].
Although PVP is increasingly being used in clinical practice as a treatment for OVCF, an increased risk for AVCF postoperatively has been reported [7–11]. It is believed that new compression fractures are more likely to occur adjacent to the PVP-treated segment, typically within 1 month after PVP [24]. However, other authors have stated that there is no convincing evidence that PVP results in such poor outcomes [1,12,13]. In addition, others have suggested that PVP might reduce the incidence of AVCF [14]. Thus, controversy still exists regarding whether PVP can increase the incidence of AVCF during the follow-up period. Although some clinical studies have compared PVP to conservative treatment [6,7,12–15], it is still unclear whether new AVCF is caused by PVP or is simply due to the natural development of osteoporosis.
In a recent retrospective study including 61 postmenopausal female patients [24], an advanced age and decreased lumbar and hip BMD scores were found to be most strongly indicative of a risk for AVCF within the first month after PVP surgery. However, as the authors stated, the main limitations of their study were its retrospective design and small sample size (n=61). It was obvious that strict statistical analyses had been performed to compensate for the shortcoming of the small sample size. Thus, their findings are believed to be applicable to the treatment of OVCF using PVP surgery.
Our study results are partially consistent with the results of that study. In our study, the mean age of patients in the AVCF group (n=68) was 71.8±6.7 years and that of the patients in the non-AVCF group (n=322) was 69.5±7.2 years. There was a significant difference in the age between the 2 groups (P=0.016). In this single-factor analysis, advanced age was identified as a risk factor for new AVCF following PVP, although it was excluded from binary logistic analysis. Moreover, lower spinal BMD was identified as a risk factor, meaning that high-level BMD protected against AVCF development following PVP. This observation is also in line with the results of the study mentioned above. According to the International Society for Clinical Densitometry and the International Osteoporosis Foundation, one of the key clinical risk factors for osteoporosis is a diminished lumbar BMD [25]. In the present study, the strongest risk factor for new AVCF was lumbar BMD.
The merits of our study have overcome the shortcomings existing in the study of Takahara et al. [24]. Compared to that study, the sample size used in our study is larger, as 390 patients were enrolled, with a much longer follow-up period. Patients were followed up for at least 6 months, with an average follow-up of 18 months. Additionally, all of the patients recruited in the previous study were postmenopausal women, but the duration of menopause was not included as a potential risk factor. In our study, a long duration of menopause was observed to increase the risk of new AVCF following PVP. No patients with multiple spinal vertebral fractures were recruited during the study period in the study of Takahara et al. [24]. However, in our study, preoperative multi-level vertebral fractures were investigated as potential risk factors and were observed to increase the risk for AVCF development following PVP after menopause.
The present study has produced findings with great clinical significance. However, this work also has some limitations. First, as a retrospective single-center comparative study, it lacks extensive representativeness. Second, we did not apply blinding methods throughout the study. Therefore, future research should strive to overcome these shortcomings and provide more reliable clinical research data. A large-sample, prospective, multicenter, randomized, controlled study with blinding methods applied is needed.
Conclusions
In summary, a long duration of menopause and preoperative multi-level vertebral fractures were risk factors for AVCF in patients following PVP after menopause, while high-level BMD protected against development of AVCF.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Zhang H, Xu C, Zhang T, et al. Does percutaneous vertebroplasty or balloon kyphoplasty for osteoporotic vertebral compression fractures increase the incidence of new vertebral fractures? A meta-analysis. Pain Physician. 2017;20:E13–E28. [PubMed] [Google Scholar]
- 2.Nishimura A, Akeda K, Kato K, et al. Osteoporosis, vertebral fractures and mortality in a Japanese rural community. Mod Rheumatol. 2014;24:840–43. doi: 10.3109/14397595.2013.866921. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki T, Yoshida H. Low bone mineral density at femoral neck is a predictor of increased mortality in elderly Japanese women. Osteoporos Int. 2010;21:71–79. doi: 10.1007/s00198-009-0970-6. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki N, Ogikubo O, Hansson T. Previous vertebral compression fractures add to the deterioration of the disability and quality of life after an acute compression fracture. Eur Spine J. 2010;19:567–74. doi: 10.1007/s00586-009-1162-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rousing R, Hansen KL, Andersen MO, et al. Twelve-months follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty: A clinical randomized study. Spine (Phila Pa 1976) 2010;35:478–82. doi: 10.1097/BRS.0b013e3181b71bd1. [DOI] [PubMed] [Google Scholar]
- 6.Klazen CA, Lohle PN, de Vries J, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): An open-label randomised trial. Lancet. 2010;376:1085–92. doi: 10.1016/S0140-6736(10)60954-3. [DOI] [PubMed] [Google Scholar]
- 7.Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine. 2011;14:561–69. doi: 10.3171/2010.12.SPINE10286. [DOI] [PubMed] [Google Scholar]
- 8.Pflugmacher R, Kandziora F, Schroeder RJ, et al. Percutaneous balloon kyphoplasty in the treatment of pathological vertebral body fracture and deformity in multiple myeloma: A one-year follow-up. Acta Radiol. 2006;47:369–76. doi: 10.1080/02841850600570425. [DOI] [PubMed] [Google Scholar]
- 9.Pflugmacher R, Schroeder RJ, Klostermann CK. Incidence of adjacent vertebral fractures in patients treated with balloon kyphoplasty: Two years’ prospective follow-up. Acta Radiol. 2006;47:830–40. doi: 10.1080/02841850600854928. [DOI] [PubMed] [Google Scholar]
- 10.Uppin AA, Hirsch JA, Centenera LV, et al. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology. 2003;226:119–24. doi: 10.1148/radiol.2261011911. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–23. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 12.Yi X, Lu H, Tian F, et al. Recompression in new levels after percutaneous vertebroplasty and kyphoplasty compared with conservative treatment. Arch Orthop Trauma Surg. 2014;134:21–30. doi: 10.1007/s00402-013-1886-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HK, Lu K, Liang CL, et al. Comparing clinical outcomes following percutaneous vertebroplasty with conservative therapy for acute osteoporotic vertebral compression fractures. Pain Med. 2010;11:1659–65. doi: 10.1111/j.1526-4637.2010.00959.x. [DOI] [PubMed] [Google Scholar]
- 14.Movrin I. Adjacent level fracture after osteoporotic vertebral compression fracture: A nonrandomized prospective study comparing balloon kyphoplasty with conservative therapy. Wien Klin Wochenschr. 2012;124:304–11. doi: 10.1007/s00508-012-0167-4. [DOI] [PubMed] [Google Scholar]
- 15.Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361:569–79. doi: 10.1056/NEJMoa0900563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelke K, Adams JE, Armbrecht G, et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: The 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–62. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee S, Yeh J, Ellamushi H. Pain and functional outcomes following vertebroplasty for vertebral compression fractures – A tertiary centre experience. Br J Neurosurg. 2016;30:57–63. doi: 10.3109/02688697.2015.1096901. [DOI] [PubMed] [Google Scholar]
- 18.Nas ÖF, İnecikli MF, Hacıkurt K, et al. Effectiveness of percutaneous vertebroplasty in patients with multiple myeloma having vertebral pain. Diagn Interv Radiol. 2016;22:263–68. doi: 10.5152/dir.2016.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun ZY, Li XF, Zhao H, et al. Percutaneous balloon kyphoplasty in treatment of painful osteoporotic occult vertebral fracture: A retrospective study of 89 cases. Med Sci Monit. 2017;23:1682–90. doi: 10.12659/MSM.903997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark W, Bird P, Gonski P, et al. Safety and efficacy of vertebroplasty for acute painful osteoporotic fractures (VAPOUR): A multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1408–16. doi: 10.1016/S0140-6736(16)31341-1. [DOI] [PubMed] [Google Scholar]
- 21.Barr JD. Randomized controlled trial of vertebroplasty versus kyphoplasty in the treatment of vertebral compression fractures. J Neurointerv Surg. 2016;8:765–66. doi: 10.1136/neurintsurg-2016-012279. [DOI] [PubMed] [Google Scholar]
- 22.Awwad A, Le JI, Kumaran M, Sosin MD. A rock in a hard place: Cement pulmonary emboli after percutaneous vertebroplasty. Int J Cardiol. 2016;208:162–63. doi: 10.1016/j.ijcard.2016.01.176. [DOI] [PubMed] [Google Scholar]
- 23.Bornemann R, Jansen TR, Otten LA, et al. Comparison of radiofrequency kyphoplasty and balloon kyphoplasty in combination with posterior fixation for the treatment of vertebral fractures. J Back Musculoskelet Rehabil. 2017;30:591–96. doi: 10.3233/BMR-140224. [DOI] [PubMed] [Google Scholar]
- 24.Takahara K, Kamimura M, Moriya H, et al. Risk factors of adjacent vertebral collapse after percutaneous vertebroplasty for osteoporotic vertebral fracture in postmenopausal women. BMC Musculoskelet Disord. 2016;17:12. doi: 10.1186/s12891-016-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22:2395–411. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]