Abstract
Background
To explore the theoretical basis for protecting the brain from ischemic stroke with tetramethylpyrazine, we studied whether and how tetramethylpyrazine could protect neurons against the oxygen-glucose deprivation (OGD)-induced death and whether transient receptor potential cation channel, subfamily C, member 6 (TRPC6) was involved.
Material/Methods
Primary rat cortical neurons were cultured and an OGD model was established in the presence or absence of tetramethylpyrazine. Neuronal death was assessed by measuring the uptake of membrane-impermeable PI. Western blot analysis was used to determine the protein expressions of TRPC6 and caspase-3. The involvement of TRPC6 was tested via RNAi against TRPC6.
Results
OGD-induced neuronal death was decreased by tetramethylpyrazine in a concentration-dependent manner. The expression of TRPC6 protein was decreased by OGD. Furthermore, downregulating TRPC6 by RNA interfering mimicked the effect of OGD in neuronal death. Tetramethylpyrazine attenuated OGD-induced TRPC6 downregulation in a tetramethylpyrazine concentration-dependent manner. However, these effects of tetramethylpyrazine on attenuating OGD-induced neuronal death were abolished by TRPC6 RNAi.
Conclusions
Tetramethylpyrazine can protect neurons from oxygen-glucose deprivation-induced death, possibly via TRPC6.
MeSH Keywords: Niemann-Pick Disease, Type A; NK Cell Lectin-Like Receptor Subfamily C; Satellite Cells, Perineuronal
Background
Ischemic stroke can occur after cardiac arrest or cerebral arterial occlusion. It can lead to devastating complications and is a global health problem. Severe outcomes of ischemic stroke, such as severe cognitive and motor dysfunction, neurodegenerative diseases, and even sudden death, are becoming a great burden on patients’ families and society [1–3]. Neuronal death is a key pathological event during ischemia-induced defects-progression [2,3]. Hence, many studies focus on elucidating the mechanisms underlying ischemia-induced neuronal death and related interventions. Among them, traditional Chinese herbal medicine has been receiving increasing attention.
Tetramethylpyrazine is a biologically active alkaloid extracted from Ephedra sinica [4], which has been widely used in Chinese herbal medicines for various purposes including treating cardiovascular and cerebrovascular defects, anti-oxidation, anti-fibrosis, anti-nociception, anti-inflammatory, and anti-neoplastic activity [5]. Treating brain disorders with tetramethylpyrazine has also been reported. It can promote production of BDNF and bFGF after severe brain injury and exhibited curative effects [6]. In an ischemia model, it also showed anti-inflammatory effects [7]. Most importantly, tetramethylpyrazine has been used in ischemic patients and shows curative effects [8]. However, although tetramethylpyrazine was reported to protect neurons during OGD, possibly via Cx32 [9], the relationship between tetramethylpyrazine and the classic ischemia-induced signaling pathway is not clear.
TRPC6 (transient receptor potential cation channel, subfamily C, member 6) is a kind of non-selective cation channels permeable to Ca2+ and has been found in many cell types, including neurons [10–13]. It can be activated by molecules showing protective effects during ischemia, including G protein-coupled receptors (GPCR) and receptor tyrosine kinases [14–17]. In vivo and in vitro experiments both found that TRPC6 downregulation was involved in ischemic neuronal cell death, and suppression of such downregulation prevents neuronal death and ischemic brain damage [18]. Thus, we studied whether tetramethylpyrazine can influence TRPC6 and protect neurons from ischemia-induced death.
In this study, we characterized whether and how tetramethylpyrazine could protect neurons from ischemia-induced death. We found tetramethylpyrazine can protect neurons from OGD-induced death, possibly via TRPC6. Our study provides a theoretical basis for treating ischemia with tetramethylpyrazine in clinical practice.
Material and Methods
Primary rat cortical neuron culture and TRPC6 RNA interfering
Primary rat cortical neuron culture was performed as described previously [19]. Briefly, primary cortical neurons were isolated from embryonic day 15 Sprague-Dawley rats. Meninges-free cortices were dissociated and seeded in 25-cm2 culture plates with equal cell numbers (1–2×106 cells/ml). The neurons were maintained in neurobasal medium (NBM) with 2% B27 and 0.3 mM glutamine (Invitrogen) under humidified 95% air/5% CO2 at 37°C. Medium was changed every 3–4 days. We added 5 μM Cytarabine (ara-C) to kill glial cells.
Transfection was carried out following the manufacture’s protocol (Nucleofector Kit, Amaxa Biosystems). Briefly, after culture medium was removed, cells were seeded at 1×105 cells/well in 6-wells plates for 24 h prior to transfection. Transfection mixes were set up with 100 pmol of siRNA in 250 μl of Opti-MEM and 5 μl of Lipofectamine 2000 in 250 μl of Opti-MEM (per well). A nonsense sequence was selected as a control. Transfection mixes were added to the cells for 5 h, subsequently removed, and replaced by 2 ml of new medium. The result of gene silencing was evaluated by immunoblotting.
Oxygen-glucose deprivation (OGD) and tetramethylpyrazine treatments
OGD was performed as previously described [20]. Culture medium was replaced by glucose-free Earle’s balanced salt solution (Sigma) gassed with nitrogen (5% CO2 and 95% N2) for 10 min. Cells were then transferred for 2 h into an anaerobic chamber filled with 5% CO2 and 95% N2 and switched back to normal culture conditions for OGD-termination [18]. Tetramethylpyrazine (95162, Sigma) was dissolved in DMSO and added into medium at different concentrations (low, 50 μg/ml; medium, 100 μg/ml; and high, 150 μg/ml) 30 min before OGD onset and until further treatments such as PI/DAPI staining and Western blot.
Assessment of cell death
Cell death was assessed by measuring the uptake of membrane-impermeable PI. Briefly, cells were stained with PI (10 μM) and Hoechst 33258 (10 μM; for nuclear counter-staining) for 20 min and observed and imaged with a fluorescence microscope (Eclipse 80i, Nikon). The percentage of PI-positive cells relative to DAPI-positive cells per field was counted with use of Image-J software.
Western blot
Prior to lysis, cells were washed with 0.01M PBS 3 times. Lysis buffer was then added. Homogenates were incubated in ice for 30 min and centrifuged at 12 000 rpm for 10 min at 4°C. Supernatants were collected and stored at −80°C for long-term use. Protein concentration was quantified by measuring OD value after incubating with Coomassie blue (G250, Life Technoledge) and the loading amount was adjusted. Samples were electrophoresed with SDS PAGE and transferred to polyvinyl difluoride membranes (PVDF, Merck Millipore). The membranes were blocked in 5% milk for 60 min at room temperature, incubated overnight at 4°C in the primary antibodies against TRPC6 (1: 10000, Ab81111, Abcam), Caspase-3 (1: 1000, 8G109665, Cell Signaling) or GAPDH (1: 1000, Cw0266, CW Biotech), and then, after washing, in horseradish peroxidase-conjugated anti-rabbit (1: 5000, BA1054, BOSTER LTD) or anti-mouse (1: 5000, BA1050, BOSTER LTD) secondary antibodies (BOSTER Biosciences) for 60 min at room temperature. The membranes were washed with TBST 3 times for 15 min each after antibody incubation. Proteins were visualized using the BeyoECLPlus ECL system.
Statistical analysis
Data are represented as mean ± standard error of the mean (SEM). GraphPad Prism software was used for data analysis. One-way ANOVA and post hoc analysis was applied to compare the significance among groups. Significance was accepted at P<0.05.
Results
Tetramethylpyrazine rescued OGD-induced neuronal apoptosis
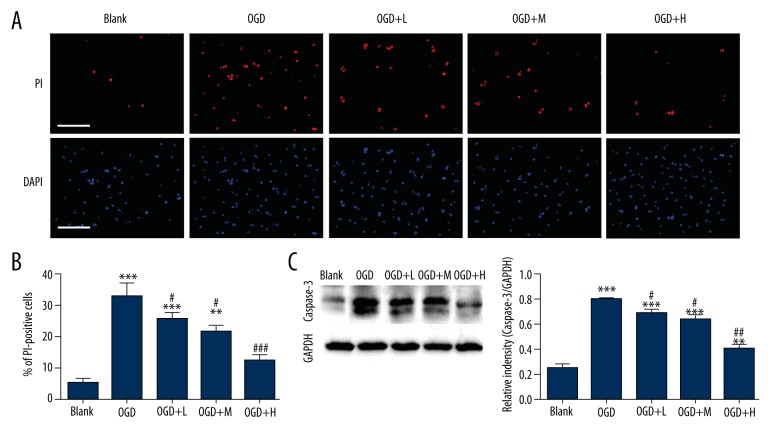
We first examined whether tetramethylpyrazine can protect neurons from death after OGD, in an in vitro ischemic model, by treating neurons with tetramethylpyrazine at different concentrations. PI staining reporting neuronal death revealed that OGD-induced neuronal death (5.33±1.45% and33.0±3.79%, P<0.0001, Figure 1A, 1B) could be significantly rescued by tetramethylpyrazine in a dose-dependent manner (P<0.05 between OGD and OGD+Tetramethylpyrazine, Figure 1A, 1B). We also characterized the expression of caspase-3, a well-known apoptotic protein, with Western blot analysis. Consistent with the effect in reducing neuronal death, tetramethylpyrazine significantly decreased the expression of caspase-3 induced by OGD (0.26±0.030 in control and 0.80±0.019 in OGD, P<0.0001 and P<0.05 between OGD and OGD+Tetramethylpyrazine, Figure 1C), indicating that tetramethylpyrazine protected neurons from OGD-induced apoptosis.
Figure 1.
Tetramethylpyrazine rescued OGD-induced neuronal death and apoptosis. (A, B) Representative imaging (A) and statistics (B) showing PI and DAPI staining of neurons receiving different treatments. L, M, and H mean low-, medium-, and high-dose tetramethylpyrazine, respectively. Blank means without any treatment. Bar=100 μm. (C) Western blot to assay the expression of Caspase-3. ** P<0.01, *** P<0.001, vs. Blank; # P<0.05, ### P<0.001, vs. OGD, n=6 mice/group.
Tetramethylpyrazine attenuated the downregulating of TRPC6 induced by OGD
Western blot analysis showed that OGD significantly reduced the expression of TRPC6 compared with the blank control group. However, this reduction was significantly attenuated by tetramethylpyrazine in a dose-dependent manner (0.72±0.019 in control and 0.28±0.026 in OGD, P<0.0001 and P<0.05 between OGD and OGD+Tetramethylpyrazine groups, Figure 2).
Figure 2.
Tetramethylpyrazine rescued OGD-induced TRPC6 downregulation. H means high-dose tetramethylpyrazine. Blank means without any treatment. Western blot to assay the expression of TRPC6 protein in neurons with different treatment. ** P<0.01, *** P<0.001, vs. Blank; # P<0.05, ##, P<0.01, vs. OGD, n=6 mice/group.
Efficacy of TRPC6 RNA interfering
As TRPC6 was reported to mediate OGD-induced neuronal death, we further tested whether downregulating TRPC6 attenuates the effect of tetramethylpyrazine. To accomplish this, we first tested the efficacy of an RNA interfering (RNAi) against TRPC6 with Western blot assay and found that TRPC6 protein decreased significantly after interfering (0.55±0.03 in control and 0.32±0.03 in RNAi, P<0.01, Figure 3). The amount of TRPC6 was not significantly different between the control group and blank group (0.55±0.03 in control and 0.54±0.05 in blank transfection, P>0.05, Figure 3).
Figure 3.
The efficacy of TRPC6 RNA Interfering. Western blot to test the effect of TRPC6 RNA interfering on the expression of TRPC6 protein in neurons. ** P<0.01, vs. Blank or Control, n= 6 mice/group.
TRPC6 downregulation abolished the protective effect of tetramethylpyrazine
We used TRPC6 RNAi to investigate whether TRPC6 is required in the protective effect of tetramethylpyrazine after OGD. Compared with the OGD group, the percentage of PI-positive cells (Figure 4A, 4B, 31.67±2.03% in OGD and 12±1.73% in OGD+Tetramethylpyrazine, P<0.05). The expression of caspase-3 (Figure 4C, 0.80±0.02 in OGD and 0.42 ±0.03 in OGD+Tetramethylpyrazine, P<0.001) was significantly reduced in the presence of tetramethylpyrazine. Such reductions were blocked after downregulating TRPC6 with TRPC6 RNAi as compared with OGD+H, and the percentage of PI-positive cells (Figure 4A, 4B. 12.00±1.73% in OGD+H and 30.00±2.31% in OGD+H+RNAi, P<0.0001) and the expression of caspase-3 protein in the OGD+H+RNAi group increased significantly (Figure 4C. 0.42±0.029 in OGD+H and 0.78±0.042 inOGD+H+RNAi, P<0.0001).
Figure 4.
Effects of TRPC6 RNAi on neuronal death and apoptosis induced by OGD. (A, B) Representative imaging (A) and statistics (B) showing PI and DAPI staining of neurons receiving different treatments. H means high-dose tetramethylpyrazine. Blank means without any treatment. Bar=100 μm. (C) Western blot assay of the expression of caspase-3. * P<0.05, ** P<0.01, *** P<0.001, vs. Blank; $$$, P<0.001, vs. OGD+H+RNAi, n=6 mice/group.0
Discussion
During ischemia and stroke, oxygen-deficiency can induce neuronal death and lead to brain damage [2,3]. Using an in vitro assay, we showed that tetramethylpyrazine protects neurons from OGD-induced death (Figure 1). We then found, consistent with a previous study, that downregulating TRPC6 mimics the effect of OGD (Figure 4). We further found that tetramethylpyrazine inhibits TRPC6 degradation during OGD and rescues OGD-induced TRPC6 downregulation in a dose-dependent manner. Finally, we found that the protective effect of tetramethylpyrazine can be abolished after downregulating TRPC6. In light of the well-documented role of TRPC6 in OGD-induced neuronal death, our data show that tetramethylpyrazine protects neurons from OGD-induced death via attenuating TRPC6 degradation. Thus, our results suggest that tetramethylpyrazine can be used as a potent treatment of ischemia and stroke.
Our study demonstrated that tetramethylpyrazine could protect neurons from OGD-induced neuronal death. Such finding is consistent with previous work and in line with the notion that tetramethylpyrazine is beneficial for brain after injury or ischemia [9,21–23]. As previous studies have suggested multiple mechanisms mediating the neuroprotective effect of tetramethylpyrazine, our finding uncovered an unknown pathway mediating protective effect of tetramethylpyrazine which may be upstream of effects observed in other study since TRPC6 is a calcium channel directly related to calcium signaling modulating multiple cellular processes including survival [24,25] and neurotropic factor production [26,27].
TRPC6 is non-selective cation channel. Its dynamics has been shown to be essential for ischemic brain injury both in vivo and in vitro. Firstly, TRPC6 level was greatly reduced before the onset of ischemia-induced neuronal death. Secondly, ischemia-induce neuronal death can be enhanced and inhibited by blocking and activating TRPC6, respectively. Exogenous peptide blocking TRPC6 degradation can reduce infarct size and improved behavioral performance [18]. Similar effects were also observed for OGD-induced neuronal death. Thus, TRPC6 may be a more direct and central mechanism for ischemic neuronal death. Based on these findings, our results provide new knowledge for understanding the mechanism involved in treating ischemia with tetramethylpyrazine.
In summary, our work demonstrates that tetramethylpyrazine can protect neurons from OGD-induced neuronal death via TRPC6. Since we did not identify how tetramethylpyrazine influences TRPC6, future studies are required to explore this question. Our work provides a theoretical basis for treating ischemia with tetramethylpyrazine.
Conclusions
Tetramethylpyrazine can protect neurons from oxygen-glucose deprivation-induced death. Such protection is possibly mediated via TRPC6.
Footnotes
Conflict of Interest
None.
Source of support: This work was supported by grants from the Youth Science Foundation of Heilongjiang Province (QC2014C102)
References
- 1.Fisher M, Saver JL. Future directions of acute ischaemic stroke therapy. Lancet Neurol. 2015;14:758–67. doi: 10.1016/S1474-4422(15)00054-X. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2016 doi: 10.1016/j.pneurobio.2016.01.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 3.Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: A systematic review and meta-analysis. Lancet Neurol. 2015;14:377–87. doi: 10.1016/S1474-4422(15)70027-X. [DOI] [PubMed] [Google Scholar]
- 4.Yeom GG, Min S, Kim SY. 2,3,5,6-Tetramethylpyrazine of Ephedra sinica regulates melanogenesis and inflammation in a UVA-induced melanoma/keratinocytes co-culture system. Int Immunopharmacol. 2014;18:262–69. doi: 10.1016/j.intimp.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Ran X, Ma L, Peng C, et al. Ligusticum chuanxiong Hort: A review of chemistry and pharmacology. Pharm Biol. 2011;49:1180–89. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- 6.Ma L, Liu WK, Zhang YK, Ju Y. [Impacts of tetramethylpyrazine on BDNF, bFGF expression and neuron-protection in severe brain injury tissue of rat]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2008;39:207–10. [in Chinese] [PubMed] [Google Scholar]
- 7.Kao TK, Chang CY, Ou YC, et al. Tetramethylpyrazine reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. Exp Neurol. 2013;247:188–201. doi: 10.1016/j.expneurol.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Li QA, Fang M, Qi JJ. Clinical research of protective effect of Tetramethylpyrazine on cerebral ischemia/reperfusion injury. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2011;20:1177–78. [Google Scholar]
- 9.Gong G, Yuan L, Cai L, et al. Tetramethylpyrazine suppresses transient oxygen-glucose deprivation-induced connexin32 expression and cell apoptosis via the ERK1/2 and p38 MAPK pathway in cultured hippocampal neurons. PLoS One. 2014;9:e105944. doi: 10.1371/journal.pone.0105944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wes PD, Chevesich J, Jeromin A, et al. TRPC1, a human homolog of a Drosophila store-operated channel. Proc Natl Acad Sci USA. 1995;92:9652–56. doi: 10.1073/pnas.92.21.9652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X, Chu PB, Peyton M, Birnbaumer L. Molecular cloning of a widely expressed human homologue for the Drosophila trp gene. FEBS Lett. 1995;373:193–98. doi: 10.1016/0014-5793(95)01038-g. [DOI] [PubMed] [Google Scholar]
- 12.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–66. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 13.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell. 2002;108:595–98. doi: 10.1016/s0092-8674(02)00670-0. [DOI] [PubMed] [Google Scholar]
- 14.Justicia C, Planas AM. Transforming growth factor-alpha acting at the epidermal growth factor receptor reduces infarct volume after permanent middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 1999;19:128–32. doi: 10.1097/00004647-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Vennekens R, Voets T, Bindels RJ, et al. Current understanding of mammalian TRP homologues. Cell Calcium. 2002;31:253–64. doi: 10.1016/s0143-4160(02)00055-6. [DOI] [PubMed] [Google Scholar]
- 16.Nilius B, Voets T. Diversity of TRP channel activation. Novartis Found Symp. 2004;258:140–49. discussion 149–59, 263–66. [PubMed] [Google Scholar]
- 17.Saarelainen T, Lukkarinen JA, Koponen S, et al. Transgenic mice overexpressing truncated trkB neurotrophin receptors in neurons show increased susceptibility to cortical injury after focal cerebral ischemia. Mol Cell Neurosci. 2000;16:87–96. doi: 10.1006/mcne.2000.0863. [DOI] [PubMed] [Google Scholar]
- 18.Du W, Huang J, Yao H, et al. Inhibition of TRPC6 degradation suppresses ischemic brain damage in rats. J Clin Invest. 2010;120:3480–92. doi: 10.1172/JCI43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Yu Z, Guo S, et al. Effects of neuroglobin overexpression on mitochondrial function and oxidative stress following hypoxia/reoxygenation in cultured neurons. J Neurosci Res. 2009;87:164–70. doi: 10.1002/jnr.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu Z, Zhang Y, Liu N, et al. Roles of neuroglobin binding to mitochondrial complex III subunit cytochrome c1 in oxygen-glucose deprivation-induced neurotoxicity in primary neurons. Mol Neurobiol. 2016;53(5):3249–57. doi: 10.1007/s12035-015-9273-4. [DOI] [PubMed] [Google Scholar]
- 21.Ho WK, Wen HL, Lee CM. Tetramethylpyrazine for treatment of experimentally induced stroke in Mongolian gerbils. Stroke. 1989;20:96–99. doi: 10.1161/01.str.20.1.96. [DOI] [PubMed] [Google Scholar]
- 22.Lin JB, Zheng CJ, Zhang X, et al. Effects of tetramethylpyrazine on functional recovery and neuronal dendritic plasticity after experimental stroke. Evid Based Complement Alternat Med. 2015;2015:394926. doi: 10.1155/2015/394926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang CY, Kao TK, Chen WY, et al. Tetramethylpyrazine inhibits neutrophil activation following permanent cerebral ischemia in rats. Biochem Biophys Res Commun. 2015;463:421–27. doi: 10.1016/j.bbrc.2015.05.088. [DOI] [PubMed] [Google Scholar]
- 24.Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol. 2003;5:1041–43. doi: 10.1038/ncb1203-1041. [DOI] [PubMed] [Google Scholar]
- 25.Harr MW, Distelhorst CW. Apoptosis and autophagy: Decoding calcium signals that mediate life or death. Cold Spring Harb Perspect Biol. 2010;2:a005579. doi: 10.1101/cshperspect.a005579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finkbeiner S. Calcium regulation of the brain-derived neurotrophic factor gene. Cell Mol Life Sci. 2000;57:394–401. doi: 10.1007/PL00000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang J, Ye Z, Hu X, et al. Electrical stimulation induces calcium-dependent release of NGF from cultured Schwann cells. Glia. 2010;58:622–31. doi: 10.1002/glia.20951. [DOI] [PubMed] [Google Scholar]