Abstract
Objective
Thyroid hormones as modulators of adaptive thermogenesis can potentially contribute to development of obesity. The purpose of our study is to observe a relationship between TSH and BMI, blood lipids, BP and HbA1c in type 2 diabetic subjects with euthyroidism.
Methods
A total of 120 subjects with type 2 diabetes were recruited for this study from November 2012 to June 2014. Subjects were included in the study with TSH values between 0.4 and 4.5 mU/l, who did not take any thyroid medication and had a similar iodine diet. Subjects were weighed and anthropometric indices, lipid parameters, fasting plasma glucose, HbA1c, eGFR, blood pressure (BP) were documented. TSH was measured by an electrochemiluminescence immunoassay. Statistical analysis was performed by using SPSS 18(P value <0.05 was considered significant).
Results
The mean age of the participants was 60.6 ± 11.6 years with a BMI of 25.3 ± 3.1 kg/m2. Serum TSH levels were significantly and positively associated with BMI, systolic and diastolic BP, serum triglyceride and HbA1c levels, whereas negatively with eGFR. Subjects with a TSH in a higher normal range (2.5–4.5 mU/I, n = 58) had a significantly higher BMI (26.7 ± 3 vs. 24.1 ± 2.7) and this relation remained significant adjusted for age and sex (P < 0.001). When TSH was in low normal range, the number of patients with glycemic goal (HbA1c > 7%) decreased from 27.5% to 12.5% (P = 0.02, adjusted for age and sex).
Conclusion
In type 2 diabetic subjects with biochemical euthyroidism we found significant association between high normal TSH levels and components of metabolic syndrome. High normal TSH levels were associated with more number of subjects with glycemic goal (HbA1c >7%).
Keywords: Metabolic syndrome, Insulin resistance, Thyroid hormones, Body mass index, Obesity
Abbreviations: BMI, body mass index; HbA1c, glycated hemoglobin; FPG, fasting plasma glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; TG, triglyceride; FT3, free triiodothyronine; MetS, metabolic syndrome
Highlights
-
•
We report TSH levels association with MetS components in type 2 diabetic subjects.
-
•
Diabetes control was somehow complicated in subjects with high-normal TSH levels.
-
•
We suggest low normal TSH concentrations in insulin-resistant subjects.
Introduction
Sharply rising abdominal adiposity is probably the main cause of MetS. In obese subjects, in the setting of insulin resistance, insulin is unable to properly suppress accelerated free fatty acids (FFAs) mobilization (lipolysis) from stored adipose tissue triglyceride 1, 2. Increased FFAs [3] also appear to cause insulin resistance. Both, insulin resistance and increased fatty acids increase the sympathetic nervous system activity and themselves contribute to vasoconstriction 4, 5 by triggering hypertension.
It is generally well accepted that hypothyroidism, with its accompanying dyslipidemia and hypertension, similar to the components of MetS is associated with CVD 6, 7. However, most cardiovascular events occur in subjects with normal thyroid function, and, little information is available about weight changes and insulin resistance when thyroid functions are in the normal range. Some investigators have suggested the existence of partially bio inactive TSH in obese subjects [8]. Others display that there may be certain thyroid hormone resistance. Ideally, early TH (thyroid hormone) therapy and lowing high-normal TSH levels should increase fat loss by stimulating thermogenesis and basal metabolic rate. Such therapy is also justified in diabetic patients as FT3 has an anti-apoptotic and protective effect on the pancreatic beta cells and higher T3 levels induce kinase activity of the TR beta1-associated PI3 kinase and stimulates insulin secretion [9].
The aim of the study was to establish TSH levels association with MetS components in insulin resistance subjects. It was further to compare MetS components association with low normal and high normal TSH levels in type 2 diabetic patients and to assess diabetes control in patients with high-normal TSH levels.
Subjects and methods
In our study, subjects, who met the inclusion criteria, were 120 Caucasian patients with type 2 diabetes mellitus. Patients who met metabolic syndrome by WHO criteria [10], who were seeking care to improve their diabetes control from November 2012 to June 2014 were evaluated for inclusion into this study. The exclusion criteria were autoimmune conditions (Hashimoto thyroiditis, Grave's disease, type 1 diabetes or positive diabetes-related auto-antibodies), medications for thyroid disease and medications, such as steroids, dopamine, iodine, amiodarone. The inclusion criteria were nonsmokers with euthyroidism (TSH values between 0.4 and 4.5 mU/l) and negative for thyroid autoantibodies (thyroid peroxidase and thyroglobulin antibodies). Subjects recruited for this study had a similar iodine diet. All the participants were given questionnaires to assess dietary habits. In addition, they were from the same iodine deficiency zone. The study subjects were weighed and their anthropometric indices were taken. Total cholesterol (TC), TG, HDL-C, LDL-C, fasting plasma glucose (after 12 h fasting), HbA1c, eGFR, SBP, DBP were documented, and TSH, FT4, FT3 were measured by an electrochemiluminescence immunoassay. The laboratory reference ranges were 0.4–4.5 mU/l for TSH. In our study we used BMI (weight divided by the squared value of height) of 25.0 kg/m2 as cut-off for overweight and normal weight individuals.
Statistical analysis
All correlation analyses were performed with partial correlation analyses using TSH as a continuous variable. Adjustments were made for age and gender. P < 0.05 was considered to indicate statistical significance. Statistical analysis was performed on a personal computer using a statistical software package SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
Ethics
The study was approved by the regional ethics committee. All subjects gave their written informed consent.
Results
Characteristics of study subjects
The mean age of the participants was 60.6 ± 11.6 years with a BMI of 25.3 ± 3.1 kg/m2. TSH, FT4, and TT3 levels were not significantly different between male and female participants. Spearman rank correlation coefficient was used to determine associations of variables with serum levels of TSH, FT4 and TT3. We found no significant association between FT4, TT3 and MetS components. Thereby we focused on TSH for further analysis. Subjects with a TSH in a higher normal range (2.5–4.5 mU/I, n = 58) had a strong association with higher BMI (26.7 ± 3 vs. 24.1 ± 2.7) and HbA1c levels (HbA1c > 7) (Table 1). This association remained significant in linear regression analysis after adjustment for sex and age (P < 0.001). TSH was also associated with TG, SBP, DBP (P < 0.01), but neither with eGFR and FPG (P > 0.05).
Table 1.
Clinical characteristics in subjects with low and higher normal TSH levels
| Variables | All subjects | Subjects with |
T-test | P value (2-tailed) | |
|---|---|---|---|---|---|
| Low normal TSH levels (0.4–2.49 mI/ml) |
Higher normal* TSH levels (2.5–4.5 mI/ml) |
||||
| No (M/F) | 120 (62/58) | 62 (35/27) | 58 (27/31) | N/A | N/S |
| Age | 60.6 ± 11.6 | 59.8 ± 11.6 | 61.2 ± 11.6 | 0.577 | N/S (0.565) |
| BMI | 25.3 ± 3.1 | 24.1 ± 2.7 | 26.7 ± 3 | 4.957 | <0.001 |
| SBP | 124.8 ± 15.8 | 120.3 ± 13.9 | 129.4 ± 16.5 | 3.252 | <0.01 |
| DBP | 75.7 ± 11 | 73 ± 10.2 | 78.6 ± 11.3 | 2.797 | <0.01 |
| TG | 137.8 ± 84.4 n/p | 117.6 ± 75.4 | 159.4 ± 88.5 | 2.788 | <0.01 |
| HDL-C | 52.3 ± 16.3 n/p | 54 ± 17.2 | 50.3 ± 15.3 | −1.292 | N/S (0.199) |
| LDL-C | 97.6 ± 31.8 | 95.3 ± 32.1 | 99.4 ± 31.5 | 0.600 | N/S (0.550) |
| FPG | 137.7 ± 39.4 n/p | 134.1 ± 39.8 | 141.6 ± 38.7 | 1.052 | N/S (0.295) |
| HBA1C | 7 ± 1.3 n/p | 6.6 ± 1 | 7.5 ± 1.3 | 3.827 | <0.001 |
| eGFR | 77.7 ± 20.8 | 82 ± 19.8 | 73 ± 21.2 | −2.424 | N/S (>0.05) |
*P value <0.05.
HbA1c
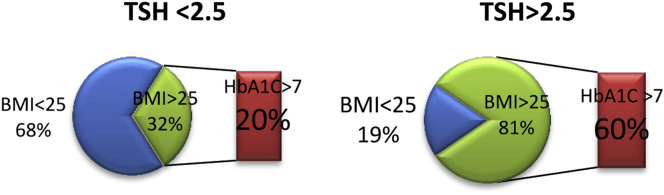
In the group with high-normal TSH levels, subjects with overweight or obesity (BMI – 25kg/m2 or higher) had about three times higher prevalence of HbA1c > 7 levels (Fig. 1). The research shows, that when TSH is in low normal range, the number of patients with glycemic goal (HbA1c > 7%) decreases from 27.5% to 12.5% (P = 0.02, adjusted for age and sex).
Fig. 1.
Comparison of diabetes control in euthyroid MetS subjects with low normal and high normal TSH levels.
MetS prevalence
The prevalence of MetS was 26% in this study population. Subjects with high normal TSH levels (2.5–4.0 mIU/L) demonstrated a significantly higher prevalence of MetS than those with low normal TSH levels (0.4–2.5 mIU/L) at a rate of 37.93% vs. 14.51% (P < 0.01).
Lipids
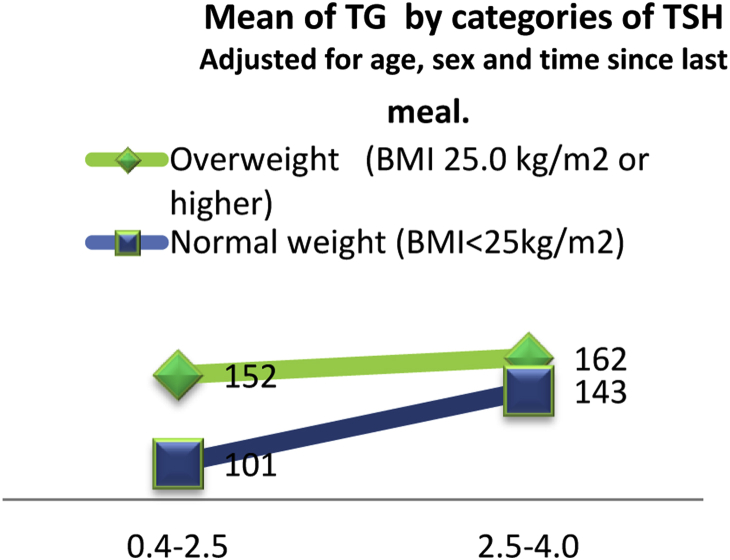
Among lipids only triglycerides were significantly associated with TSH levels (P < 0.05). Triglycerides were significantly higher in subjects in the upper normal TSH range compared with those in the low normal TSH range (159.4 ± 88.5 vs. 117.6 ± 75.4, P = 0.006). Fig. 2 displays that mean of TG by categories of TSH is more obviously increased in subjects with normal weight than in overweight/obese subjects (adjusted for age, sex and time since last meal).
Fig. 2.
Mean TG by categories of TSH.
Medication and TSH levels
There were no associations between TSH levels and medications used for the treatment of diabetes, dyslipidemia and hypertension (Table 2).
Table 2.
Regression analyses
| Medications | Number of subjects | P value |
|---|---|---|
| Diet only | 23 | N/S (−0.074) |
| OHAs | 76 | N/S (−0.188) |
| Insulin | 11 | N/S (−0.084) |
| Insulin + OHAs | 10 | N/S (0.192) |
| Anti-hypertensive drugs | (−) 94 (+) 26 |
N/S (0.241) |
| Lipid-lowering drugs | (−) 80 (+) 40 |
N/S (0.969) |
Discussion
Insulin sensitivity and TSH
There are many reports about the association between thyroid hormones and MetS in non-diabetic subjects. But reports about the relationship between thyroid hormones and MetS components in type 2 diabetic patients are very rare. In our study, we found not only a positive association between TSH levels and MetS components in diabetic subjects, but we also could show that diabetes control was somehow complicated when subjects had a high-normal range of TSH. Some studies described decreased insulin sensitivity in hypothyroidism 11, 12, while others did not 13, 14. The study of Jackson et al. even reported increased insulin sensitivity [15]. Recently, an experimental study in an animal model has demonstrated that the mutation in the α-isoform of the thyroid hormone receptor caused insulin resistance and thyroid hormone resistance [16]. In addition, studies investigating thyroid hormone receptors in MetS subjects (mostly in obese individuals) demonstrated a decrease of thyroid hormone receptor density 17, 18.
In our study we found no significant association between FT4 and total T3 levels with Mets components in diabetic subjects. This may be explained that T3 levels in the normal range do not have very strong physiological activity and do not reflect in peripheral tissue, which are known to actually exert the metabolic effects. There are also differences in expression of thyroid hormone receptors on central and peripheral tissues and can, therefore, also play a role in inducing a discrepancy of thyroid hormones effects in central and peripheral tissues [19].
BMI and TSH
Our data revealed positive association between TSH and BMI. This association could be affected by a third factor. To control for the effect of age, age was included as an independent variable in the multiple regression analysis. Furthermore, the positive association between TSH and BMI was also significant in those above the age of 50. Age was therefore an unlikely explanation for the association between elevated TSH and BMI. Moreover, this association could be causal. Many reports have shown that after reducing body weight or after bariatric surgery 20, 21, there was a significant decrease in TSH levels. The mechanism could be explained by the leptin levels secreted by adipose tissue, which is directly correlated to the amount of adipose tissue. In experimental stage, leptin has been reported to stimulate the biosynthesis of TSH [22]. On the other hand, we could not ignore the possible role of hyperglycemia on TSH-depend BMI levels.
Lipids and TSH
Among MetS components TSH was correlated with triglyceride levels. In 1980s: it was demonstrated that LDL receptor activity was regulated by thyroid hormone [23]. Increased level of triglycerides in high-normal TSH subjects could be caused by a reduced activity of lipoprotein lipase, or impaired clearance of lipoproteins dependent on LDL receptor function. LDL-C clearance is different between subjects with high normal and subjects with low normal TSH levels [24]. In our study this difference remained unnoticed, probably, because of insulin resistance. In the liver of insulin-resistant patients, triglyceride synthesis and storage are increased, and excess triglyceride is secreted as VLDL [25]. However, LDL-C concentrations remain essentially unchanged in insulin-resistant states because of a decrease in cholesterol content per LDL particle, resulting in higher concentrations of small dense LDL particles 10, 26, 27. This positive association between TSH levels and TG could have long-term harmful effects on cardiovascular disease.
Study limitation
The main limitation of our study is the relatively small size and regional homogeneity of the population visiting the aforementioned Hospital. This data does not reflect that of the general population. Further larger randomized trials are necessary to evaluate increased risk of early stage of thyroid dysfunction and to display the potential benefit for the earlier stage management of thyroid dysfunction.
Conclusion
In summary, we found a significant association between high normal TSH levels and components of metabolic syndrome in type 2 diabetic subjects with biochemical euthyroidism. High normal TSH levels were associated with more diabetic subjects with glycemic goal (HbA1c > 7%).
Conflicts of interest/financial disclosure
None.
Acknowledgments
Nothing to declare.
Footnotes
Author contributions: Kyu Yeon Hur; manuscript reviewer, statistic department of Samsung Medical Center; statistical analysis.
References
- 1.Ginsberg H.N. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106:453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn B.B., Flier J.S. Obesity and insulin resistance. J Clin Invest. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 4.Tooke J.E., Hannemann M.M. Adverse endothelial functionand the insulin resistance syndrome. J Intern Med. 2000;247:425–431. doi: 10.1046/j.1365-2796.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 5.Tripathy D., Mohanty P., Dhindsa S., Syed T., Ghanim H., Aljada A. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]
- 6.Benvenga S., Robbins J. Lipoprotein-thyroid hormone interactions. Trends Endocrinol Metab. 1993;4:194–198. doi: 10.1016/1043-2760(93)90116-v. [DOI] [PubMed] [Google Scholar]
- 7.Loeb J.N., Werner S.C., Ingbar S.H. 7th ed. Lippincott-Raven; Philadelphia: 1996. Metabolic changes in hypothyroidism. A fundamental and clinical text; p. 858. [Google Scholar]
- 8.Reinehr T. Obesity and thyroid function. J Mol Cell Endocrinol. 2010;316:165–171. doi: 10.1016/j.mce.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Verga Falzacappa C., Petrucci E., Patriarca V., Michienzi S., Stigliano A., Brunetti E. Thyroid hormone receptor TRbeta1 mediates Akt activation by T3 in pancreatic beta cells. J Mol Endocrinol. 2007;38:221–233. doi: 10.1677/jme.1.02166. [DOI] [PubMed] [Google Scholar]
- 10.Cornier M., Dabelea D., Hernandez T., Lindstrom R., Steig A., Stob N. The metabolic syndrome. J Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanicka S., Vondra K., Pelikanova T., Vlcek P., Hill M., Zamrazil V. Insulin sensitivity and counter-regulatory hormones in hypothyroidism and during thyroid hormone replacement therapy. Clin Chem Lab Med. 2005;43:715–720. doi: 10.1515/CCLM.2005.121. [DOI] [PubMed] [Google Scholar]
- 12.Rochon C., Tauveron I., Dejax C., Benoit P., Capitan P., Fabricio A. Response of glucose disposal to hyperinsulinaemia in human hypothyroidism and hyperthyroidism. Clin Sci. 2003;104:7–15. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 13.Harris P.E., Walker M., Clark F., Home P., Alberti K. Forearm muscle metabolism in primary hypothyroidism. Eur J Clin Invest. 1993;23:585–588. doi: 10.1111/j.1365-2362.1993.tb00970.x. [DOI] [PubMed] [Google Scholar]
- 14.Owecki M., Nikisch E., Sowinski J. Hypothyroidism has no impact on insulin sensitivity assessed with HOMA-IR in totally thyroidectomized patients. Acta Clin Belg. 2006;61:69–73. doi: 10.1179/acb.2006.013. [DOI] [PubMed] [Google Scholar]
- 15.Jackson I.M., Prentice C.R., McKiddie M.T. The effect of hypothyroidism on glucose tolerance and insulin metabolism. J Endocrinol. 1970;47:257–258. doi: 10.1677/joe.0.0470257. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y.Y., Schultz J.J., Brent G.A. A thyroid hormone receptor alpha gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J Biol Chem. 2003;278:38913–38920. doi: 10.1074/jbc.M306120200. [DOI] [PubMed] [Google Scholar]
- 17.Burman K.D., Latham K.R., Djuh Y.Y., Smallridge R., Tseng Y., Lukes Y. Solubilized nuclear thyroid hormone receptors in circulating human mononuclear cells. J Clin Endocrinol Metab. 1980;51:106–116. doi: 10.1210/jcem-51-1-106. [DOI] [PubMed] [Google Scholar]
- 18.Escobar-Morreale H.F., Obregon M.J., Escobar del Rey F., Morreale de Escobar G. Replacement therapy for hypothyroidism with thyroxine alone does not ensure euthyroidism in all tissues, as studied in thyroidectomized rats. J Clin Invest. 1995;96:2828–2838. doi: 10.1172/JCI118353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peeters R., van Toor H., Klootwijk W., de Rijke Y., Kuiper G., Uitterlinden A. Polymorphisms in thyroid hormone pathway genes are associated with plasma TSH and iodothyronine levels in healthy subjects. J Clin Endocrinol Metab. 2003;88:2880–2888. doi: 10.1210/jc.2002-021592. [DOI] [PubMed] [Google Scholar]
- 20.Sari R., Balci M.K., Altunbas H., Karayalcin U. The effect of body weight and weight loss on thyroid volume and function in obese women. Clin Endocrinol. 2003;59:258–262. doi: 10.1046/j.1365-2265.2003.01836.x. [DOI] [PubMed] [Google Scholar]
- 21.Moulin de Moraes C.M., Mancini M.C., de Melo M.E., Figueiredo D.A., Villares S.M., Rascovski A. Prevalence of subclinical hypothyroidism in a morbidly obese population and improvement after weight loss induced by Roux-en-Y gastric bypass. Obes Surg. 2005;15:1287–1291. doi: 10.1381/096089205774512537. [DOI] [PubMed] [Google Scholar]
- 22.Ortiga-Carvalho T.M., Oliveira K.J., Soares B.A., Pazos-Moura C.C. The role of leptin in the regulation of TSH secretion in the fed state: in vivo and in vitro studies. J Endocrinol. 2002;174:121–125. doi: 10.1677/joe.0.1740121. [DOI] [PubMed] [Google Scholar]
- 23.Thompson G.R., Soutar A.K., Spengel F.A., Jadhav A., Gavigan S., Myant N. Defects of receptor-mediated low density lipoprotein catabolism in homozygous familial hypercholesterolemia and hypothyroidism in vivo. Proc Natl Acad Sci U S A. 1981;78(4):2591–2595. doi: 10.1073/pnas.78.4.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayashi H., Mizushima N., Yoshinaga H., Kawamitsu H., Matsuda S., Tanoue M. The relationship between lipoprotein(a) and low density lipoprotein receptors during the treatment of hyperthyroidism. Horm Metab Res. 1996;28:384–387. doi: 10.1055/s-2007-979821. [DOI] [PubMed] [Google Scholar]
- 25.Ginsberg H.N., Zhang Y.L., Hernandez-Ono A. Regulation of plasma triglycerides in insulin resistance and diabetes. Arch Med Res. 2005;36:232–240. doi: 10.1016/j.arcmed.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 26.Howard B.V. Insulin resistance and lipid metabolism. Am J Cardiol. 1999;84:28J–32J. doi: 10.1016/s0002-9149(99)00355-0. [DOI] [PubMed] [Google Scholar]
- 27.Syvanne M., Taskinen M.R. Lipids and lipoproteins as coronary risk factors in non-insulin-dependent diabetes mellitus. Lancet. 1997;350(Suppl. 1):SI20–SI23. doi: 10.1016/s0140-6736(97)90024-6. [DOI] [PubMed] [Google Scholar]