Abstract
Aims
To examine the factors associated with diabetes, a late diabetes diagnosis, and whether these factors are different for males and females.
Methods
Cross-sectional study including 7101 individuals aged ≥25 years in Newfoundland and Labrador, Canada (466 with diabetes; 332 diagnosed late). Logistic regression analysis was used to determine the factors associated with a diabetes diagnosis and late diabetes diagnosis.
Results
For males, overweight/obesity (HR, 1.35; 95% CI, 1.06–1.72) was positively associated with diabetes while being a regular/occasional drinker (HR, 0.53; 95% CI, 0.32–0.88) was inversely associated with diabetes. Living in a rural area (HR, 1.47; 95% CI, 1.01–2.15), receiving social assistance (HR, 2.80; 95% CI, 1.52–5.15), having poor self perceived health (HR, 2.06; 95% CI, 1.32–3.21), and considering most days stressful (HR, 1.45; 95% CI, 1.01–2.10) were positively associated with diabetes for females. No factors were significantly associated with a late diabetes diagnosis for males. Having a low education (OR, 0.33; 95% CI, 0.11–0.99) was inversely associated with a late diabetes diagnosis for females.
Conclusions
Different factors are associated with diabetes for males and females. Disadvantaged females appear to be at the greatest risk. The factors associated with a late diabetes diagnosis were also different for males and females. Females with lower education levels are diagnosed with diabetes earlier than females with higher education levels. Certain risk factors appear to impact males and females differently and more research is needed on how males and females develop diabetes and when they are diagnosed.
Keywords: Diabetes, Late diabetes diagnosis, Sex differences, Canadian Community Health Survey
Introduction
Worldwide, there are approximately 366 million people with diabetes and it is estimated that 552 million will be affected by 2030 [1]. In Canada, the prevalence of diabetes was 6.8% in 2008/09, with more men having diabetes than women (7.2% versus 6.4%) [2].
A challenge with type 2 diabetes is diagnosing the disease early in an effort to prevent progression to complications. About 183 million people, or half of those who have diabetes, are unaware they have the disease [1]. Furthermore, type 2 diabetes can be present for 9–12 years before being diagnosed and, as a result, complications are often present at the time of diagnosis [3]. However, the potential does exit to prevent or at least delay the onset of type 2 diabetes as several randomized control trials have shown that both lifestyle and pharmacologic interventions in adults are effective [4], [5], [6], [7]. In addition to preventing diabetes, it is also possible to reduce diabetes related complications through intensive blood glucose control [5], [6], [7].
In most countries, even though females have lower mortality rates than males, they experience poorer health [8]. Diabetes tends to affect males more than females since more males are diagnosed with diabetes [2]. In addition, males are diagnosed at lower BMI levels than females [9]. Even though more males have diabetes, females with diabetes have a greater risk of mortality and hospitalizations [10], [11], [12], [13], [14], [15], [16], [17], [18], [19].
Various factors are associated with type 2 diabetes including older age, overweight or obesity, physical inactivity, marital status, smoking, lower education and low income [1], [2]. Early detection of type 2 diabetes is critical as effective and active management is essential for those newly diagnosed patients who have not developed complications. As the rates of diabetes increase it is important to study the factors associated with late diagnosis of diabetes and whether these determinants differ for males and females. Therefore, the objectives of this study are to examine the factors associated with diabetes and whether these factors differ for males and females. In addition, the factors associated with a late diabetes diagnosis will be explored and whether these factors differ for males and females.
Subjects, materials and methods
This cross-sectional study utilized administrative and survey data in Newfoundland and Labrador, Canada. Databases included were: (1) the Canadian Chronic Disease Surveillance System (CCDSS), 1999–2005; (2) the Canadian Community Health Survey (CCHS), 2000/01, 2003, 2005; (3) the Clinical Database Management System (CDMS), 1998–2006; and, (4) the MCP Fee-For-Service Physician Claims Database, 1998–2006. Ethical approval for this study was granted by the Health Research Ethics Authority of Newfoundland and Labrador.
The CCDSS is a collaborative network of provincial and territorial surveillance systems and uses a nationally validated case definition to identify individuals with diabetes. One hospitalization or two or more fee-for-service physician claims with a diagnosis of diabetes within a 2-year period is required to be considered a diabetes case. Cases remain in the CCDSS until a record of their death is received or they leave the province. The case definition used for the CCDSS has 86% sensitivity and 98% specificity for identifying individuals who had diabetes recorded in their primary care charts [20]. The CCHS is a national cross-sectional survey conducted by Statistics Canada which collects information related to health determinants, health status and health system utilization for 130,000 Canadians. Three cycles of the CCHS (2000/01, 2003, 2005) were combined to increase the sample size and to decrease variation in the estimates [21]. The CDMS is the provincial hospital separation database that captures demographic, clinical and interventional information for patients admitted to all acute health care facilities and surgical day care in the province. The MCP system contains information related to services provided by fee-for-service physicians under the provincial Medical Care Plan (MCP).
Diabetes and early and late diagnosis status
The dependent variables used in this study were diabetes status and early and late diabetes diagnosis status. CCHS respondents' aged 25 years and older in Newfoundland and Labrador, who consented to share their data, were linked to the CCDSS via MCP number (provincial health insurance number) to verify their diabetes status according to the CCDSS case definition. All incident diabetes cases, as determined through the CCDSS, comprised the diabetes study sample. Individuals reporting that they were not diagnosed with diabetes at age 25 years or older, who participated in the CCHS, and who consented to share their data were eligible for the non-diabetes group. These cases were linked to the CCDSS via MCP number to verify whether or not they had diabetes according to the CCDSS case definition.
Individuals with diabetes were classified as being diagnosed ‘early’ or ‘late’ depending on when diabetes related comorbidities or complications developed. Individuals early on in the disease course would not have any diabetes related comorbidities or complications around the time of their case date. On the contrary, a late diagnosed diabetes patient would have conditions related to diabetes around the time they were diagnosed. Since type 2 diabetes can be present for 9–12 years before being diagnosed, complications are often present at the time of diagnosis [3]. Insulin resistance and beta-cell dysfunction are largely responsible for the development of diabetes and its related complications and both are present very early in the natural history of diabetes [22]. The progression of diabetes from pre-diabetes to complications is different for each patient. In some individuals complications may develop early at lower glucose concentrations or during increases in glucose rather than after thresholds for a diagnosis are reached and remain consistent [23]. In fact, diabetes may be initially detected at the same time diabetes complications are being diagnosed [24]. The UKPDS found that 50% of patients had diabetes related tissue damage at the time of diagnosis [25]. (UKPDS, VIII).
In an effort to identify when comorbidities or complications develop for individuals with diabetes, a series of definitions ranging from specific to very broad (6 months–2 years, before/after diagnosis) were developed and sample sizes were determined. Since there was little change in the sample distribution across definitions, the range of 6 months before and after diagnosis was used to define early and late diabetes diagnosis. To classify individuals diagnosed early and late, records for those with diabetes were linked to the MCP and CDMS data to identify when hospital and physician visits for diabetes related comorbidities or complications occurred and these were compared to the diabetes case dates. Incident diabetes patients without any diabetes related comorbidities or complications within 6 months before or after the diabetes case date were classified as early diagnosed while those with a late diagnosis were defined as incident diabetes patients with at least one diabetes related comorbidities or complications within 6 months before or after diagnosis. The diabetes related conditions that were used to define early and late status are listed in Table 1.
Table 1.
Conditions used to identify early and late diabetes diagnoses
| Conditions | ICD 9 codes | ICD-10-CA codes |
|---|---|---|
| Cardiovascular disease | 390–448 | I00–I78 |
| Ischemic heart disease | 410–414 | I20–I25 |
| Hypertensive disease | I10–I13, I15 | |
| Acute myocardial infarction | 410 | I21–I22 |
| Heart failure | 428 | I50 |
| Stroke | 430–438 | I60–I69 |
| Renal disease | 585–586 | N18–N19 |
| Atherosclerosis | 440 | |
| Amyloidosis | 277.3 | E85 |
| Other peripheral vascular diseases | 443 | I73 |
| Other and unspecified hyperlipidemia | 372.4 | |
| Other proliferative retinopathy: Proliferative vitreo-retinopathy | H35.2 | |
| Chorioretinal scars | 363 | H31.0 |
| Atherosclerotic retinopathy | I70.8 H36.8 | |
| Other disorders of optic nerve and visual pathways | H47 | |
| Other retinal disorders | 362 | H35 |
| Nephritis and nephropathy, not specified as acute or chronic | 583 | |
| Acute renal failure | 584 | |
| Disorder of kidney and ureter, unspecified | N28.9 | |
| Other renal tubulo-interstitial diseases | N15 | |
| Acute nephritic syndrome | N00 | |
| Unspecified nephritic syndrome | N05 | |
| Isolated proteinuria with specified morphological lesion | N06 | |
| Neuromuscular dysfunction of bladder, not elsewhere classified | N31 | |
| Other polyneuropathies | G62 | |
| Nerve root and plexus disorders | 353 | G54 |
| Other mononeuropathies | G58 | |
| Mononeuropathies of lower limb | G57 | |
| Mononeuropathies of upper limb | G56 | |
| Facial nerve disorders | 351 | G51 |
| Disorders of autonomic nervous system | 337 | G90 |
| Inflammatory polyneuropathy | G61 | |
| Radiculopathy | M54 | |
| Mononeuritis of upper limb and mononeuritis multiplex | 354 | |
| Mononeuritis of lower limb | 355 | |
| Neuralgia, neuritis, and radiculitis, unspecified | 729.2 | |
| Lower limb amputations | 96.11, 96.12, 96.13, 96.14, 96.15, 96.2 |
1VC93LA, 1VG93LA, 1VQ93LA, 1WA93LA, 1WE93LA, 1WJ93LA, 1WL93LA, 1WM93LA, 1WV59LA |
Covariates
A number of independent variables were explored in this study. The demographic variables included age, sex and marital status. Individuals who were married or common law were classified as partnered while individuals who were single, widowed, separated or divorced were considered unpartnered. The socioeconomic variables included education level, social assistance and region of residence. Low education was defined as less than secondary or completed secondary education while high education was defined as post-secondary education or completed post-secondary education. Urban region of residence was defined in the CCHS as an area with a population concentration of 1000 or more and a population density of 400 or more per square kilometer based on census counts. Lifestyle variables included leisure time physical activity (active/moderately active, inactive), body mass index (BMI), smoking status (former smoker/never smoked, occasionally/daily smoker) and alcohol consumption (former/non-drinker, regular/occasional drinker). Physical activity level was derived from total energy expenditure during leisure time, which uses the frequency and duration of respondents' reported leisure time activities in the previous 3 months. BMI was calculated from self-reported height and weight and classified as normal (18 ≤ BMI ≥ 24.99 kg/m2) and overweight/obese (BMI ≥ 25 kg/m2). Other factors that were assessed include regular medical doctor, high blood pressure, self perceived health, life stress (most days considered stressful/not stressful), sense of community belonging, employed in the past 12 months, exposed to second hand smoke at home and self perceived unmet health care needs.
Statistical analysis
Characteristics of the study population are presented as weighted percentages and compared between individuals with and without diabetes and those diagnosed early and late with diabetes using chi-square tests and t-tests. To determine the factors associated with a diabetes diagnosis and late diabetes diagnosis logistic regression analysis was used to calculate odds ratios (OR). Since the CCHS utilized a complex survey design, coefficients of variation and 95% confidence intervals (CI) were estimated using Statistics Canada's Bootvar program [26]. Analyses were conducted separately for males and females. All statistical analysis was performed using SAS version 9.2 (SAS Institute, Cary, NS) software.
Results
The study sample consisted of 7101 individuals, and mean age was 48.4 years (SD, 14.8 years). Forty eight percent of the sample were male and 52% were female. Characteristics of the study sample by diabetes status and sex are presented in Table 2. Overall, 6.7% of the study sample had diabetes; 7.6% of males had diabetes compared to 5.9% of females. Males and females with diabetes were more likely to be older, live in a rural area and have less education than those without diabetes (P < 0.01). Females with diabetes were more likely to receive social assistance compared to females without diabetes (P < 0.01), whereas no difference was found for males. Males and females with diabetes were more likely to be overweight/obese and physically inactive compared to those without diabetes (P < 0.01). Males without diabetes were more likely to be smokers compared to males with diabetes (P < 0.01), while females had similar smoking rates regardless of diabetes status.
Table 2.
Characteristics of the study sample by diabetes status and sex
| Males (n = 3144) |
Females (n = 3957) |
Total (n = 7101) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Diabetes (n = 224) |
No diabetes (n = 2920) |
P valuea | Diabetes (n = 242) |
No diabetes (n = 3715) |
P valuea | Diabetes (n = 466) |
No diabetes (n = 6635) |
P valuea | |
| Age (years), mean (SD) | 58.9 (12.7) | 47.5 (14.3) | 0.000 | 59.2 (12.1) | 47.8 (14.9) | 0.000 | 59.1 (12.5) | 47.6 (14.6) | 0.000 |
| Has high blood pressure, % (n) | 43.5% (92) | 16.3% (536) | 0.000 | 46.3% (127) | 19.6% (850) | 0.000 | 44.8% (219) | 18.0% (2808) | 0.000 |
| Female, % (n) | – | – | – | – | – | – | 6.7% (242) | 93.3% (3715) | 0.000 |
| Low education, % (n) | 54.8% (133) | 42.8% (1314) | 0.000 | 65.9% (169) | 44.8% (1801) | 0.000 | 59.9% (302) | 43.9% (3115) | 0.000 |
| Rural place of residence, % (n) | 43.3% (93) | 41.1% (1259) | 0.000 | 44.4% (106) | 37.0% (1459) | 0.000 | 43.8% (199) | 38.9% (2718) | 0.000 |
| Receives social assistance, % (n) | F | 6.4% (193) | 0.517 | 16.8% (31)E | 8.6% (332) | 0.000 | 11.1% (43)E | 7.6% (525) | 0.000 |
| Poor/Fair self perceived health, % (n) | 29.0% (63)E | 12.0% (398) | 0.000 | 30.8% (71) | 11.0% (445) | 0.000 | 29.8% (134) | 11.5% (843) | 0.000 |
| Body mass index (BMI), % (n) | |||||||||
| Normalb | 14.8% (44)E | 30.0% (882) | 0.000 | 20.3% (50) | 43.5% (176) | 0.000 | 17.3% (94) | 37.0% (2348) | 0.000 |
| Overweightc/Obesed | 85.2% (178) | 70.0% (2012) | 79.7% (176) | 56.5% (2080) | 82.7% (354) | 63.0% (4092) | |||
| Physical activity, % (n) | |||||||||
| Active/Moderately | 32.6% (67)E | 44.4% (1225) | 0.000 | 25.4% (64) | 35.8% (1331) | 0.000 | 29.2% (131) | 39.8% (2556) | 0.000 |
| Inactive | 67.4% (140) | 55.6% (1583) | 74.6% (176) | 64.2% (2352) | 70.8% (316) | 60.2% (3935) | |||
| Daily/Occasional smoker, % (n) | 13.3% (36)E | 28.5% (867) | 0.000 | 24.8% (52) | 25.6% (970) | 0.090 | 18.5% (88) | 27.0% (1837) | 0.000 |
| Regular/Occasional drinker, % (n) | 70.3% (157) | 85.2% (2438) | 0.000 | 52.8% (118) | 75.1% (2658) | 0.000 | 62.3% (275) | 79.9% (5096) | 0.000 |
| Life stress, % (n) | |||||||||
| Not stressful | 48.1% (117) | 43.6% (1361) | 0.000 | 35.4% (104) | 38.7% (1501) | 0.000 | 42.3% (221) | 41.0% (2862) | 0.000 |
| Stressful | 51.9% (106) | 56.4% (1554) | 64.6% (138) | 61.3% (2213) | 57.7% (244) | 59.0% (3767) | |||
| Marital status, % (n) | |||||||||
| Partnered | 83.5% (170) | 81.3% (2185) | 0.000 | 64.3% (129) | 73.8% (2474) | 0.000 | 74.7% (299) | 77.4% (4659) | 0.000 |
| Unpartnered | 16.5% (54)E | 18.7% (733) | 35.7% (113) | 26.2% (1241) | 25.3% (167) | 22.6% (1974) | |||
| Weak sense of belonging, % (n) | 20.0% (38)E | 20.1% (512) | 0.785 | 20.2% (48)E | 21.5% (727) | 0.006 | 20.1% (86) | 20.9% (1239) | 0.000 |
| Unemployed in the last 12 months, % (n) | 41.3% (90) | 21.6% (673) | 0.000 | 65.8% (148) | 35.8% (1324) | 0.000 | 53.0% (238) | 29.1% (1997) | 0.000 |
| Exposed to second hand smoke at home, % (n) | 12.8% (21)E | 20.5% (389) | 0.000 | 25.9% (31)E | 22.3% (526) | 0.000 | 18.4% (52)E | 21.5% (915) | 0.000 |
| Self perceived unmet health care needs, % (n) | 10.8% (24)E | 10.7% (306) | 0.763 | 11.0% (28)E | 13.0% (482) | 0.000 | 10.9% (52)E | 11.9% (788) | 0.000 |
| Has regular medical doctor, % (n) | 94.2% (206) | 82.3% (2248) | 0.000 | 96.3% (231) | 89.8% (3201) | 0.000 | 95.2% (437) | 86.2% (5449) | 0.000 |
Data with a coefficient of variation (CV) from 16.6% to 33.3% are identified as follows: (E) use with caution; Data with a coefficient of variation (CV) greater than 33.3% were suppressed due to extreme sampling variability and are identified as follows: (F) too unreliable to be published.
Significance level = 0.05.
BMI = 18.5–24.9.
BMI = 25.0–29.9.
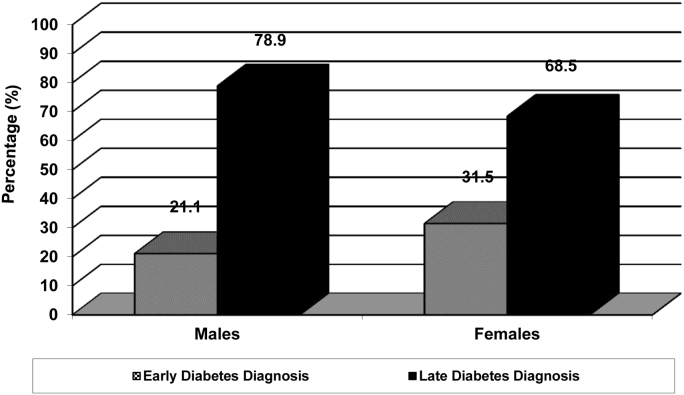
Characteristics of the diabetes sample by early and late diagnosis status and sex are presented in Table 3. For individuals with diabetes, 25.8% were diagnosed early and 74.2% were diagnosed late. For males, 21.1% were diagnosed with diabetes early and 78.9% were diagnosed late, whereas 31.5% of females were diagnosed with diabetes early and 68.5% were diagnosed late (Figure 1). Both males and females with late diagnoses were older than those with early diagnoses (P < 0.01). Males diagnosed late with diabetes were more likely to live in a rural area compared to early diagnosed males (P < 0.01), whereas no difference was found for females. Similarly, males with late diagnoses were more likely to be overweight/obese compared to early diagnosed males (P < 0.01), while no difference was found for females. On the other hand, females with a late diabetes diagnosis were more likely to be physically inactive compared to females diagnosed early (P < 0.01). Physical activity level for males was similar for those diagnosed early and late with diabetes.
Table 3.
Characteristics of the study sample by early and late diabetes diagnosis statusa and sex
| Males (n = 224) |
Females (n = 242) |
Total (n = 466) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Early (n = 58) | Late (n = 166) | P valueb | Early (n = 76) | Late (n = 176) | P valueb | Early (n = 134) | Late (n = 332) | P valueb | |
| Age (years), mean (SD) | 56.1 (11.3) | 59.7 (13.0) | 0.000 | 56.7 (13.2) | 60.4 (11.4) | 0.000 | 56.4 (12.4) | 60.0 (12.3) | 0.000 |
| Female, % (n) | – | – | – | – | 31.5% (76) | 68.5% (166) | 0.000 | ||
| Low education, % (n) | 52.6% (33)E | 55.4% (100) | 0.022 | 76.8% (56) | 60.9% (113) | 0.000 | 66.1% (89) | 57.7% (213) | 0.000 |
| Rural place of residence, % (n) | 31.9% (17)E | 46.3% (76) | 0.000 | 45.6% (37)E | 43.9% (69) | 0.147 | 39.5% (54)E | 45.3% (145) | 0.000 |
| Receives social assistance, % (n) | F | F | F | 15.5% (22)E | F | 10.9% (33)E | 0.249 | ||
| Poor/Fair self perceived health, % (n) | 22.2% (12)E | 30.8% (51)E | 0.000 | 26.0% (19)E | 33.0% (52)E | 0.000 | 24.3% (31)E | 31.8% (103) | 0.000 |
| Body mass index (BMI), % (n) | |||||||||
| Normalc | 18.8% (12)E | 13.7% (32)E | 0.000 | 21.3% (14)E | 19.8% (36)E | 0.113 | 20.2% (26)E | 16.3% (68) | 0.000 |
| Overweightd/Obesee | 81.2% (45)E | 86.3% (133) | 78.7% (57) | 80.2% (119) | 79.8% (102) | 83.7% (252) | |||
| Physical activity, % (n) | |||||||||
| Active/Moderately | 32.7% (20)E | 32.6% (47)E | 0.912 | 31.6% (22)E | 22.6% (42)E | 0.000 | 32.1% (42)E | 28.1% (89) | 0.000 |
| Inactive | 67.3% (36)E | 67.4% (104) | 68.4% (54) | 77.4% (122) | 67.9% (90) | 71.9% (226) | |||
| Daily/Occasional smoker, % (n) | 22.9% (15)E | 10.7% (21)E | 0.000 | 32.3% (23)E | 21.3% (29)E | 0.000 | 28.2% (38)E | 15.2% (50)E | 0.000 |
| Regular/Occasional drinker, % (n) | 81.4% (44)E | 67.3% (113) | 0.000 | 57.0% (42)E | 50.9% (76) | 0.000 | 67.9% (86) | 60.4% (189) | 0.000 |
| Life stress, % (n) | |||||||||
| Not stressful | 40.6% (29)E | 50.1% (88) | 0.000 | 31.7% (28)E | 37.1% (76) | 0.000 | 35.7% (57) | 44.7% (164) | 0.000 |
| Stressful | 59.4% (29)E | 49.9% (77) | 68.3% (48)E | 62.9% (90) | 64.3% (77) | 55.3% (167) | |||
| Marital status, % (n) | |||||||||
| Partnered | 88.1% (43) | 82.2% (127) | 0.000 | 67.5% (47)E | 62.7% (82) | 0.000 | 76.7% (90) | 74.0% (209) | 0.000 |
| Unpartnered | 11.9% (15)E | 17.8% (39)E | 32.5% (29)E | 37.3% (84) | 23.3% (44)E | 26.0% (123) | |||
| Weak sense of belonging, % (n) | F | F | 23.9% (18)E | 18.5% (30)E | 19.7% (27)E | 20.3% (59) | 0.439 | ||
| Unemployed in the last 12 months, % (n) | 36.4% (20)E | 42.8% (70) | 0.000 | 63.0% (44)E | 67.1% (104) | 0.000 | 51.5% (64) | 53.6% (174) | 0.018 |
| Exposed to second hand smoke at home, % (n) | F | 10.5% (15)E | 25.6% (13)E | 26.0% (18)E | 0.787 | 24.1% (19)E | 16.5% (33)E | 0.000 | |
| Self perceived unmet health care needs, % (n) | F | 9.5% (15)E | 14.4% (11)E | 9.5% (17)E | 0.000 | 15.1% (20)E | 9.5% (32)E | 0.000 | |
| Has regular medical doctor, % (n) | 96.1% (54) | 93.7% (152) | 0.000 | 98.4% (73) | 95.4% (158) | 0.000 | 97.4% (127) | 94.4% (310) | 0.000 |
Data with a coefficient of variation (CV) from 16.6% to 33.3% are identified as follows: (E) use with caution; Data with a coefficient of variation (CV) greater than 33.3% were suppressed due to extreme sampling variability and are identified as follows: (F) too unreliable to be published.
BMI = ≥30.0; % = weighted percentages; n = unweighted numbers.
Conditions used to identify early and late diabetes diagnoses are listed in Table 1.
Significance level = 0.05.
BMI = 18.5–24.9.
BMI = 25.0–29.9.
BMI = ≥30.0; % = weighted percentages; n = unweighted numbers.
Figure 1.
Percentage of males and females diagnosed with diabetes early and late.
Adjusted odds ratios (OR) and 95% confidence intervals (CI) for factors associated with diabetes are presented in Table 4. High blood pressure was positively associated with having diabetes for both males (HR, 1.81; 95% CI, 1.15–2.85) and females (HR, 1.58; 95% CI, 1.03–2.42). Being unemployed in the last 12 months and not having a regular doctor were inversely associated with diabetes for both males and females. For males only, being overweight or obese (HR, 1.35; 95% CI, 1.06–1.72) was positively associated with diabetes while being a regular or occasional drinker (HR, 0.53; 95% CI, 0.32–0.88) was inversely associated with diabetes. In contrast, living in a rural area (HR, 1.47; 95% CI, 1.01–2.15), receiving social assistance (HR, 2.80; 95% CI, 1.52–5.15), having poor self perceived health (HR, 2.06; 95% CI, 1.32–3.21), and considering most days stressful (HR, 1.45; 95% CI, 1.01–2.10) were positively associated with diabetes for females.
Table 4.
Adjusted odds ratios (OR) and 95% confidence intervals (CI) for factors associated with diabetes
| Males |
Females |
Total |
|
|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Age | 1.07 (1.04–1.09)** | 1.07 (1.05–1.09)** | 1.07 (1.05–1.08)** |
| Sex | |||
| Male | – | – | 1.00 |
| Female | – | – | 0.68 (0.51–0.91)* |
| High blood pressure | |||
| Yes | 1.81 (1.15–2.85)** | 1.58 (1.03–2.42)* | 1.70 (1.24–2.34)** |
| No | 1.00 | 1.00 | 1.00 |
| Education | |||
| High | 1.00 | 1.00 | 1.00 |
| Low | 0.99 (0.64–1.55) | 1.11 (0.71–1.73) | 1.04 (0.78–1.40) |
| Region | |||
| Urban | 1.00 | 1.00 | 1.00 |
| Rural | 0.94 (0.88–1.54) | 1.47 (1.01–2.15)* | 1.14 (0.86–1.52) |
| Social assistance | |||
| Yes | 0.80 (0.27–2.34) | 2.80 (1.52–5.15)** | 1.93 (1.15–3.23)* |
| No | 1.00 | 1.00 | 1.00 |
| Self perceived health | |||
| Good/Very good/Excellent | 1.00 | 1.00 | 1.00 |
| Poor/Fair | 1.68 (0.92–3.07) | 2.06 (1.32–3.21)** | 1.80 (1.25–2.60)** |
| Body mass index (BMI) | |||
| Normal | 1.00 | 1.00 | 1.00 |
| Overweight/Obese | 1.35 (1.06–1.72)* | 1.10 (0.98–1.22) | 1.17 (1.07–1.28)** |
| Physical activity | |||
| Active/Moderately active | 1.00 | 1.00 | 1.00 |
| Inactive | 1.52 (0.98–2.37) | 1.12 (0.71–1.75) | 1.32 (0.95–1.81) |
| Smoking status | |||
| Non smoker | 1.00 | 1.00 | 1.00 |
| Daily/Occasional | 0.60 (0.33–1.08) | 1.12 (0.68–1.86) | 0.83 (0.58–1.18) |
| Drinking status | |||
| Former/Non drinker | 1.00 | 1.00 | 1.00 |
| Regular/Occasional | 0.53 (0.32–0.88)* | 0.71 (0.48–1.06) | 0.61 (0.44–0.85)** |
| Life stress | |||
| Not stressful | 1.00 | 1.00 | 1.00 |
| Stressful | 1.08 (0.74–1.59) | 1.45 (1.01–2.10)* | 1.25 (0.96–1.67) |
| Marital status | |||
| Partnered | 1.00 | 1.00 | 1.00 |
| Unpartnered | 0.89 (0.55–1.44) | 0.97 (0.64–1.46) | 0.93 (0.68–1.26) |
| Sense of belonging | |||
| Strong | 1.00 | 1.00 | 1.00 |
| Weak | 1.19 (0.72–1.98) | 0.97 (0.62–1.52) | 1.09 (0.78–1.52) |
| Employed in the last 12 months | |||
| Yes | 1.00 | 1.00 | 1.00 |
| No | 0.75 (0.64–0.88)** | 0.77 (0.68–0.88)** | 0.76 (0.68–0.84)** |
| Exposed to second hand smoke | |||
| Yes | 1.04 (0.93–1.16) | 1.08 (0.99–1.19) | 1.06 (0.99–1.14) |
| No | 1.00 | 1.00 | 1.00 |
| Self-perceived unmet health care needs | |||
| Yes | 1.09 (0.54–2.21) | 0.88 (0.52–1.48) | 0.95 (0.61–1.46) |
| No | 1.00 | 1.00 | 1.00 |
| Has a regular medical doctor | |||
| Yes | 1.00 | 1.00 | 1.00 |
| No | 0.50 (0.25–0.99)** | 0.30 (0.11–0.83)* | 0.40 (0.24–0.67)** |
‘–’: odds ratios not calculated for these indicators; **P < 0.01; *P < 0.05.
Adjusted ORs and 95% CIs for factors associated with a late diabetes diagnosis are presented in Table 5. For males, no factors were significantly associated with an early or late diabetes diagnosis. However, for females, having a low education (OR, 0.33; 95% CI, 0.11–0.99) was inversely associated with a late diabetes diagnosis. No other factors were significantly associated with a late diabetes diagnosis in females.
Table 5.
Adjusted odds ratios (OR) and 95% confidence intervals (CI) for factors associated with a late diabetes diagnosis
| Males |
Females |
Total |
|
|---|---|---|---|
| Adjusted OR (95% CI) | Adjusted OR (95% CI) | Adjusted OR (95% CI) | |
| Age | 1.02 (0.98–1.07) | 1.04 (0.98–1.09) | 1.02 (0.99–1.06) |
| Sex | |||
| Male | – | – | 1.00 |
| Female | – | – | 0.65 (0.37–1.14) |
| Education | |||
| High | 1.00 | 1.00 | 1.00 |
| Low | 0.71 (0.28–1.84) | 0.33 (0.11–0.99)* | 0.49 (0.25–0.94)* |
| Region | |||
| Urban | 1.00 | 1.00 | 1.00 |
| Rural | 1.84 (0.64–5.28) | 1.05 (0.46–2.39) | 1.32 (0.70–2.47) |
| Self-perceived health | |||
| Good/Very good/Excellent | 1.00 | 1.00 | 1.00 |
| Poor/Fair | 2.33 (0.73–7.46) | 1.13 (0.44–2.87) | 1.66 (0.87–3.16) |
| Body mass index (BMI) | |||
| Normal | 1.00 | 1.00 | 1.00 |
| Overweight/Obese | 2.14 (0.62–7.35) | 0.99 (0.36–2.71) | 1.30 (0.59–2.85) |
| Physical activity | |||
| Active/Moderately Active | 1.00 | 1.00 | 1.00 |
| Inactive | 1.19 (0.47–2.99) | 1.38 (0.57–3.34) | 1.26 (0.69–2.33) |
| Smoking status | |||
| Non smoker | 1.00 | 1.00 | 1.00 |
| Daily/Occasional | 0.46 (0.12–1.70) | 0.72 (0.26–1.99) | 0.64 (0.29–1.42) |
| Drinking status | |||
| Former/Non drinker | 1.00 | 1.00 | 1.00 |
| Regular/Occasional | 0.47 (0.16–1.35) | 0.78 (0.32–1.95) | 0.68 (0.37–1.24) |
| Life stress | |||
| Not stressful | 1.00 | 1.00 | 1.00 |
| Stressful | 0.62 (0.23–1.64) | 0.92 (0.37–2.32) | 0.79 (0.41–1.52) |
| Marital status | |||
| Partnered | 1.00 | 1.00 | 1.00 |
| Unpartnered | 1.58 (0.42–6.02) | 0.98 (0.38–2.53) | 1.10 (0.51–2.35) |
| Employed in the last 12 months | |||
| Yes | 1.00 | 1.00 | 1.00 |
| No | 0.94 (0.55–1.63) | 0.92 (0.64–1.34) | 0.96 (0.73–1.26) |
| Exposed to second hand smoke | |||
| Yes | – | – | 0.99 (0.84–1.16) |
| No | – | – | 1.00 |
| Self perceived unmet health care needs | |||
| Yes | – | – | 0.47 (0.21–1.05) |
| No | – | – | 1.00 |
| Has a regular medical doctor | |||
| Yes | – | – | 1.00 |
| No [1] | – | – | 2.18 (0.36–13.07) |
‘–’: odds ratios not calculated for these indicators; **P < 0.01; *P < 0.05.
Discussion
This study found that for males and females, high blood pressure was positively associated with diabetes. This finding is consistent with Meisinger et al. [27] who also found that hypertension was strongly associated with diabetes in both males and females. This study also found males and females who do not have a regular doctor are less likely to be diagnosed with diabetes than those who do. This could be due to the fact that those who have a doctor have an increased opportunity to discuss symptoms and to be screened for diabetes.
Being overweight or obese was associated with diabetes for males only in this study. Similarly, Njolstad et al. [28] also found that BMI was positively associated with diabetes; however, after controlling for other factors BMI was a stronger predictor in men. Previous research has found that men are diagnosed at lower BMI levels than females, which suggest males are more susceptible to diabetes than females [9]. In addition, abdominal fat is associated with higher risk of diabetes and males usually carry weight in their abdominal region while females tend to carry weight in their hips and thighs [29].
For males only, being a regular or occasional drinker was inversely associated with diabetes. A U-shaped relationship has been found between diabetes risk and alcohol consumption [30], [31]. A meta-analysis conducted in 2005 found that moderate alcohol consumption is associated with a 30% reduced risk of type 2 diabetes in males and females [30]. The findings of this study are consistent with a recent study by Rasouli et al. [32] which found that moderate alcohol consumption is protective for type 2 diabetes in males but not in females The authors suggest that females could be more sensitive to the negative effects of alcohol compared to males or that females are more likely than males to underreport their alcohol intake.
This study found that living in a rural area was associated with diabetes for females only. The prevalence of diabetes is higher for individuals living in rural areas compared to urban areas [33], [34]. In general, diabetes prevalence is higher in males than females [2]. Johnson et al. [35] found diabetes prevalence was highest in rural men; however, mortality rates declined slightly for rural men and did not change for rural women between 1995 and 2006. Individuals living in rural areas are also more likely than urban residents to visit an emergency room or be admitted to hospital for the management of diabetes [36]. These findings highlight the differences in diabetes outcomes for individuals living in rural areas, especially females.
Receiving social assistance was associated with diabetes for females only in this study. Lysy et al. [37] found that diabetes risk is higher for lower income groups compared to higher income groups. In addition, risk was higher for lower income females compared to males. Dinca-Panaitescu et al. [33] also found an association between diabetes and income for both males and females, and the odds ratios were higher for females.
Having poor self perceived health was positively associated with diabetes for females only. Unden et al. [38] found that females with diabetes reported having a worse health situation than males, and were more likely to rate their health as poor compared to males. They conclude that diabetes may be experienced differently for males and females. In addition, Badawi et al. [39] also found that females were less likely to rate their health as excellent compared with males. They also found that self-rated health was significantly associated with diabetes complications. One explanation for the discrepancy is that men and women use different information when making assessments about their health. Women have been found to base their health ratings on both serious and mild diseases, while men base them on serious illness only [40].
Previous research has also found that stress increases the risk of diabetes [41], [42]. This study found that considering most days stressful was positively associated with diabetes for females but not for males. In addition, being unemployed in the last 12 months was inversely associated with diabetes for both males and females. This is interesting since work stress has been found to increase the risk of diabetes for females while the risk in men was decreased by high work demands [43].
This study found that females with a low education level, defined as less than secondary or completed secondary education, were less likely to be diagnosed late with diabetes compared to those with a higher level of education. Research investigating the association between early and late diabetes diagnosis and educational attainment has not been previously explored; however, research has been conducted on education level as a risk factor for diabetes. Most research suggests that individuals with a low level of education have a higher risk of diabetes and that the association is stronger in females [44], [45], [46]. However, Chien et al. [47] found that higher education levels were significantly associated with developing pre-diabetes or type 2 diabetes and this finding was significant for females only. When comparing literature from other conditions, Sobrino-Vegas et al. [48] found that females with a high education level were more likely to have a delayed HIV diagnosis than those with a low education level. The opposite was observed in males. The authors suggest that females with low education levels and males believe they are at higher risk for HIV as do their health care providers. As a result, they are offered routine HIV testing more than females with high education levels. HIV and diabetes are very different conditions since HIV is associated with social stigma and discrimination. However, previous research has found that patients with a low education level have more consultation time spent on physical examination and nutritional counseling compared to higher educated patients [49]. In addition, Piette et al. [50] examined general communication processes and diabetes-specific communication. Patients with lower education levels reported better general and diabetes-specific communication than patients with higher education levels. Health care providers may spend more time counseling patients that they perceive are in need of extra attention or explanation [50]. Physicians may pay particular attention to individuals with lower education levels in an effort to diagnosis type 2 diabetes earlier and since females visit their doctors more than males [51], females with lower education levels may be diagnosed with diabetes earlier than females with higher education levels or males.
Another possible explanation is that demands on an individuals' time may negatively affect their health. Adults with diabetes who are the primary caregivers for children or the elderly may not have time to obtain health care for themselves [52]. Females are usually the primary caregivers for their families and often have to balance their families' needs with caring for an aging or sick relative and working outside the home. When asked the reasons for delaying or going without care, one in five women state that lack of time is a barrier [53].
Finally, this finding could also be explained by other factors that were not available in the data, such as genetics, family history, A1C, and blood glucose levels.
Limitations
There are also several limitations that need to be addressed. Firstly, this was a cross-sectional study and therefore not as strong as a cohort or intervention study. Secondly, the covariates in this study are based on self reported responses from the CCHS. Self reported information can be affected by recall bias and social desirability bias. In addition, the CCDSS diabetes case definition does not differentiate between type 1 and type 2 diabetes. However, since most adults are diagnosed with type 2 diabetes [1] it's unlikely to have a major impact on the results. Furthermore, the CCDSS diabetes case definition uses physician claims data. In NL, one-third of the province's physicians are paid on a salary basis and these physicians are not required to submit medical claims so information on these visits is not captured. As a result, the sample of diabetes cases may be less than the true number of incident cases. In addition, some misclassification could have occurred as individuals with diabetes could have been classified as not having diabetes because a salaried physician provided most of their care. Also, early and late diabetes diagnosis was determined by linking records for those with diabetes to the MCP and CDMS data to identify when hospital and physician visits for diabetes related comorbidities or complications occurred and these were compared to the diabetes case dates. The range of 6 months before and after diagnosis was used to define early and late diabetes diagnosis. Some misclassification could have occurred as comorbidities or complications could have developed outside the 6 month range. Comorbidities such as cardiovascular disease, stroke and coronary artery disease have similar risk factors as diabetes and could be diagnosed at the same time or before diabetes is diagnosed. Since many definitions of early and late diabetes diagnosis were tested and there was little change in the sample distribution across definitions, we feel that the range of 6 months before or after diagnosis is a good definition of early and late diabetes diagnosis. However, more research into when comorbidities and complications of diabetes develop is needed. Finally, even though the problem of small sample sizes is reduced by combining CCHS cycles, it is not completely eliminated.
In conclusion, the results of this study suggest that different factors are associated with diabetes for males and females. Disadvantaged females, including those living in a rural area, receiving social assistance, having poor self-perceived health, and considering most days stressful appear to be at the greatest risk. The factors associated with a late diabetes diagnosis were also different for males and females. Females with lower education levels are diagnosed with diabetes earlier than females with higher education levels. Certain risk factors appear to impact males and females differently and more research is needed on how males and females develop diabetes and when they are diagnosed.
Acknowledgments
There are no potential conflicts of interest relevant to this article to disclose. M.R. conceptualized the study, acquired the data, performed data analysis, interpreted the results and wrote the manuscript. P.W. contributed to data analysis plan/discussion and reviewed/edited the manuscript. M.R. is the guarantor of this work, had access to all study data and takes responsibility for the data analysis and contents of the article.
Parts of this study were presented in abstract form at the 2008 American Public Health Association, San Diego, California, October 25–29, 2008. Partial funding for this work was provided by the Public Health Agency of Canada.
Footnotes
This is an open access article under the CC BY-NC-SA license (http://creativecommons.org/licenses/by-nc-sa/3.0/).
References
- 1.International Diabetes Federation . 5th ed. International Diabetes Federation; Brussels: 2011. The diabetes atlas. [Google Scholar]
- 2.Public Health Agency of Canada . 2011. Diabetes in Canada: facts and figures from a public health perspective. Ottawa. [Google Scholar]
- 3.Harris M.I., Klein R., Welborn T.A., Knuiman M.W. Onset of NIDDM occurs at least 4–7 years before clinical diagnosis. Diabetes Care. 1992;15:815–819. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
- 4.Pan X.R., Li G.W., Hu Y.H., Wang J.X., Yang W.Y., An Z.X. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 5.Tuomilehto J., Lindstrom J., Eriksson J.G., Valle T.T., Hamalainen H., Ilanne-Parikka P. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 6.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A. Reduction in the incidence of type 2 diabetes with lifestyle interventions or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 8.Malmusi D., Artazcoz L., Benach J., Borrell C. Perception or real illness? How chronic conditions contribute to gender inequalities in self-rated health. Eur J Public Health. 2012;22(6):781–786. doi: 10.1093/eurpub/ckr184. [DOI] [PubMed] [Google Scholar]
- 9.Logue J., Walker J.J., Colhoun H.M., Leese G.P., Lindsay R.S., McKnight J.A. Do men develop type 2 diabetes at lower body mass indices than women? Diabetologia. 2011;54(12):3003–3006. doi: 10.1007/s00125-011-2313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orchard T.J. The impact of gender and general risk factors on the occurrence of atherosclerotic vascular disease in non-insulin-dependant diabetes mellitus. Ann Med. 1996;28(4):323–333. doi: 10.3109/07853899608999089. [DOI] [PubMed] [Google Scholar]
- 11.Howard B.V., Cowan L.D., Go O., Welty T.K., Robbins D.C., Lee E.T. Adverse effects of diabetes on multiple cardiovascular disease risk factors in women: the Strong Heart Study. Diabetes Care. 1998;21(8):1258–1265. doi: 10.2337/diacare.21.8.1258. [DOI] [PubMed] [Google Scholar]
- 12.Lee W.L., Cheung A.M., Cape D., Zinman B. Impact of diabetes on coronary artery disease in women and men: a meta-analysis of prospective studies. Diabetes Care. 2000;23(7):962–968. doi: 10.2337/diacare.23.7.962. [DOI] [PubMed] [Google Scholar]
- 13.Becker A., Bos G., de Vegt F., Kostense P.J., Dekker J.M., Nijpels G. Cardiovascular events in type 2 diabetes: comparison with nondiabetic individuals without and with prior cardiovascular disease; 10-year follow-up of the Hoorn Study. Eur Heart J. 2003;24(15):1406–1413. doi: 10.1016/s0195-668x(03)00197-0. [DOI] [PubMed] [Google Scholar]
- 14.Natarajan S., Liao Y., Cao G., Lipsitz S.R., McGee D.L. Sex differences in risk for coronary heart disease mortality associated with diabetes and established coronary heart disease. Arch Intern Med. 2003;163(14):1735–1740. doi: 10.1001/archinte.163.14.1735. [DOI] [PubMed] [Google Scholar]
- 15.Juutilainen A., Kortelainen S., Letho S., Ronnemaa T., Pyorala K., Laakso M. Gender differences in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care. 2004;27(12):2898–2904. doi: 10.2337/diacare.27.12.2898. [DOI] [PubMed] [Google Scholar]
- 16.Huxley R., Barzi F., Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Booth G.L., Kapral M.K., Fung K., Tu J.V. Relation between age and cardiovascular disease in men and women with diabetes compared with non-diabetic people: a population-based retrospective cohort study. Lancet. 2006;368(9529):29–36. doi: 10.1016/S0140-6736(06)68967-8. [DOI] [PubMed] [Google Scholar]
- 18.Jeerakathil T., Johnson J.A., Simpson S.H., Majumdar S.R. Short-term risk for stroke is doubled in persons with newly treated type 2 diabetes compared with persons without diabetes: a population-based cohort study. Stroke. 2007;38(6):1739–1743. doi: 10.1161/STROKEAHA.106.481390. [DOI] [PubMed] [Google Scholar]
- 19.Barnett K.N., Ogston S.A., McMurdo M.E.T., Morris A.D., Evans J.M.M. A 12-year follow-up study of all cause and cardiovascular mortality among 10 532 people newly diagnosed with Type 2 diabetes in Tayside, Scotland. Diabet Med. 2010;27(10):1124–1129. doi: 10.1111/j.1464-5491.2010.03075.x. [DOI] [PubMed] [Google Scholar]
- 20.Hux J.E., Ivis F., Flintoft V., Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–516. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 21.Thomas S., Wannell B. Combining cycles of the Canadian community health survey. Health Rep. 2009;20(1):53–58. [PubMed] [Google Scholar]
- 22.Kahn S.E. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 23.Stolar M. Glycemic control and complications in type 2 diabetes mellitus. Am J Med. 2010;123:S3–S11. doi: 10.1016/j.amjmed.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Ruigomez A., Garcia Rodriguez L.A. Presence of diabetes related complication at the time of NIDDM diagnosis: an important prognostic factor. Eur J Epidemiol. 1998;14(5):439–445. doi: 10.1023/a:1007484920656. [DOI] [PubMed] [Google Scholar]
- 25.UK Prospective Diabetes Study (UKPDS) VIII. Study design, progress and performance. Diabetologia. 1991;34(12):877–890. [PubMed] [Google Scholar]
- 26.Statistics Canada. Bootvar software. http://www.statcan.gc.ca/rdc-cdr/bootvar_sas-eng.htm.
- 27.Meisinger C., Thorand B., Schneider A., Stieber J., Doring A., Lowel H. Sex differences in risk factors for incident type 2 diabetes mellitus: the MONICA Augsburg cohort study. Arch Intern Med. 2002;162(1):82–89. doi: 10.1001/archinte.162.1.82. [DOI] [PubMed] [Google Scholar]
- 28.Njolstad I., Arnesen E., Lund-Larsen P.G. Sex differences in risk factors for clinical diabetes mellitus in a general population: a 12-year follow-up of the Finnmark Study. Am J Epidemiol. 1998;147(1):49–58. doi: 10.1093/oxfordjournals.aje.a009366. [DOI] [PubMed] [Google Scholar]
- 29.Karastergiou K., Fried S.K., Xie H., Lee M.J., Divoux A., Rosencrantz M.A. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab. 2013;98(1):362–371. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson S., Hammar N., Grill V. Alcohol consumption and type 2 diabetes: meta-analysis of epidemiological studies indicates a U-shaped relationship. Diabetologia. 2005;48(6):1051–1054. doi: 10.1007/s00125-005-1768-5. [DOI] [PubMed] [Google Scholar]
- 31.Baliunas D.O., Taylor B.J., Irving H., Roerecke M., Patra J., Mohapatra S. Alcohol as a risk factor for type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2009;32(11):2123–2132. doi: 10.2337/dc09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasouli B., Ahlbom A., Andersson T., Grill V., Midthjell K., Olsson L. Alcohol consumption is associated with reduced risk of Type 2 diabetes and autoimmune diabetes in adults: results from the Nord-Trondelag health study. Diabet Med. 2012;30(1):56–64. doi: 10.1111/j.1464-5491.2012.03713.x. [DOI] [PubMed] [Google Scholar]
- 33.Dinca-Panaitescu S., Dinca-Panaitescu M., Bryant T., Daiski I., Pilkington B., Raphael D. Diabetes prevalence and income: results of the Canadian community health survey. Health Policy. 2011;99(2):116–123. doi: 10.1016/j.healthpol.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 34.O'Connor A., Wellenius G. Rural-urban disparities in the prevalence of diabetes and coronary heart disease. Public Health. 2012;126(10):813–820. doi: 10.1016/j.puhe.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Johnson J.A., Balko S.U., Hugel G., Low C., Svenson L.W. Increasing incidence and prevalence with limited survival gains among rural Albertans with diabetes: a retrospective cohort study, 1995-2006. Diabet Med. 2009;26(10):989–995. doi: 10.1111/j.1464-5491.2009.02805.x. [DOI] [PubMed] [Google Scholar]
- 36.Booth G.L., Hux J.E., Fang J., Chan B.T. Time trends and geographic disparities in acute complications of diabetes in Ontario, Canada. Diabetes Care. 2005;28(5):1045–1050. doi: 10.2337/diacare.28.5.1045. [DOI] [PubMed] [Google Scholar]
- 37.Lysy Z., Booth G.L., Shah B.R., Austin P.C., Luo J., Lipscombe L.L. The impact of income on the incidence of diabetes: a population-based study. Diabetes Res Clin Pract. 2013;99(3):372–379. doi: 10.1016/j.diabres.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Unden A.L., Elofsson S., Andreasson A., Hillered E., Eriksson I., Brismar K. Gender differences in self-rated health, quality of life, quality of care, and metabolic control in patients with diabetes. Gend Med. 2008;5(2):162–180. doi: 10.1016/j.genm.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Badawi G., Gariepy G., Page V., Schmitz N. Indicators of self-rated health in the Canadian population with diabetes. Diabet Med. 2012;29(8):1021–1028. doi: 10.1111/j.1464-5491.2012.03571.x. [DOI] [PubMed] [Google Scholar]
- 40.Benyamini Y., Leventhal E.A., Leventhal H. Gender differences in processing information for making self-assessments of health. Psychosom Med. 2000;62(3):354–364. doi: 10.1097/00006842-200005000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Pouwer F., Kupper N., Adriaanse M.C. Does emotional stress cause type 2 diabetes mellitus? A review from the European Depression in Diabetes (EDID) Research Consortium. Discov Med. 2010;9(45):112–118. [PubMed] [Google Scholar]
- 42.Mommersteeg P.M., Herr R., Zijlstra W.P., Schneider S., Pouwer F. Higher levels of psychological distress are associated with a higher risk of incident diabetes during 18 year follow-up: results from the British household panel survey. BMC Public Health. 2012;12:1109. doi: 10.1186/1471-2458-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eriksson A.K., van den Donk M., Hilding A., Ostenson C.G. Work stress, sense of coherence, and risk of type 2 diabetes in a prospective study of middle-aged Swedish men and women. Diabetes Care. 2013;36(9):2683–2689. doi: 10.2337/dc12-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dasgupta K., Khan S., Ross N.A. Type 2 diabetes in Canada: concentration of risk among most disadvantaged men but inverse social gradient across groups in women. Diabet Med. 2010;27(5):522–531. doi: 10.1111/j.1464-5491.2010.02982.x. [DOI] [PubMed] [Google Scholar]
- 45.Agardh E., Allebeck P., Hallqvist J., Moradi T., Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40(3):804–818. doi: 10.1093/ije/dyr029. [DOI] [PubMed] [Google Scholar]
- 46.Muller G., Hartwig S., Greiser K.H., Moebus S., Pundt N., Schipf S. Gender differences in the association of individual social class and neighbourhood unemployment rate with prevalent type 2 diabetes mellitus: a cross-sectional study from the DIAB-CORE consortium. BMJ Open. 2013;3(6):1–11. doi: 10.1136/bmjopen-2013-002601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chien L., Li T.C., Lin C.C., Liu C.S., Li C.I., Chen C.C. The 3-Year incidence of pre-diabetes or type 2 diabetes in a Taiwanese Metropolitan general population aged 40 and over. Diabetes. 2010;59(Suppl. 1) http://professional.diabetes.org/Abstracts_Display.aspx?TYP=1&CID=81250 Accessed 13.12.13. [Google Scholar]
- 48.Sobrino-Vegas P., Rodriguez-Urrego J., Berenguer J., Caro-Murillo A.M., Blanco J.R., Viciana P. Educational gradient in HIV diagnosis delay, mortality, antiretroviral treatment initiation and response in a country with universal health care. Antivir Ther. 2012;17(1):1–8. doi: 10.3851/IMP1939. [DOI] [PubMed] [Google Scholar]
- 49.Fiscella K., Goodwin M.A., Stange K.C. Does patient educational level affect office visits to family physicians? J Natl Med Assoc. 2002;94(3):157–165. [PMC free article] [PubMed] [Google Scholar]
- 50.Piette J.D., Schillinger D., Potter M.B., Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med. 2003;18(8):624–633. doi: 10.1046/j.1525-1497.2003.31968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bertakis K.D., Azari R., Helms L.J., Callahan E.J., Robbins J.A. Gender differences in the utilization of health care services. J Fam Pract. 2000;49(2):147–152. [PubMed] [Google Scholar]
- 52.Brown A.F., Ettner S.L., Piette J., Weinberger M., Gregg E., Shapiro M.F. Socioeconomic position and health among persons with diabetes mellitus: a conceptual framework and review of the literature. Epidemiol Rev. 2004;26:63–77. doi: 10.1093/epirev/mxh002. [DOI] [PubMed] [Google Scholar]
- 53.Salganicoff A., Ranji U.R., Wyn R. The Henry J. Kaiser Family Foundation; July 2005. Women and health care: a national Profile. [Google Scholar]