Abstract
Objective:
Breast planning with volumetric modulated arc therapy (VMAT) has been explored, especially for left-sided breast treatments, with the primary intent of lowering the heart dose and improving target dose homogeneity. As a trade-off, larger healthy tissue volumes would receive low dose levels, with the potential risk of increasing late toxicities and secondary cancer induction, although no clinical data are available today to confirm the risk level. The scope of this work is to explore the dosimetric trade-offs using two different VMAT plans.
Methods:
Two planning strategies for dual-partial-arc VMAT, RA_avoid and RA_full, with and without avoidance sectors, were explored in a cohort of 20 patients, for whole left breast irradiation for 40.05 Gy to the mean target dose in 15 fractions. Common dose objectives included a stringent dose homogeneity, mean dose to the heart <5 Gy, ipsilateral lung (Ilung) <8 Gy, contralateral lung (Clung) <2 Gy and contralateral breast (Cbreast) <3 Gy.
Results:
RA_full showed a better dose conformity, lower high-dose spillage in the healthy tissue and lower skin dose. RA_avoid presented a reduction of the mean doses for all critical structures: 51% to the heart, 12% to the Ilung, 81% to the Clung and 73% to the Cbreast. All differences were significant with p < 0.0001.
Conclusion:
The adaptation of VMAT options to planning objectives reduced significantly the healthy tissue dose levels at the price of some high-dose spillage. Evaluation of the trade-offs for application to the different critical structures should drive in improving the usage of the VMAT technique for breast cancer treatment.
Advances in knowledge:
Different planning strategies in the same VMAT technique could give significant variations in dose distributions. The choice of the trade-offs would affect the possible future late toxicity and secondary cancer induction risk.
INTRODUCTION
Radiotherapy treatment is an integral part of the standard of care of patients with breast cancer after breast-conserving surgery. Different fractionation schemes have been applied, from the conventional 50 Gy in 25 fractions to hypofractionated regimes like 40.05 Gy delivered in 15 fractions, as proposed by the START (UK Standardisation of Breast Radiotherapy) Trial B.1
The most traditional treatment technique is the use of conventional tangential fields. Some more conformal approaches were proposed2 to improve the ipsilateral lung (Ilung) sparing. With no nodal involvement, this provided excellent results in the vast majority of patients and has been the basis of the large randomized trials showing the relevance of radiotherapy.3,4
Nevertheless, selected subgroups of patients, presenting with challenging anatomical features or requiring specific dosimetric objectives, induced the investigation of the role of intensity modulation [intensity-modulated radiotherapy (IMRT)] and volumetric modulated arc therapy (VMAT). Many dosimetric studies explored these techniques,5–8 proving that, as a trade-off of improved target dose distributions, larger volumes of the surrounding tissues would receive a more or less pronounced low-dose bath. This was not an unexpected feature but many institutions refrained from the systematic use of advanced techniques for breast cancer treatments. However, there are still no clinical results demonstrating the detrimental effect of the low-dose bath with respect to the two-tangential beam dose delivery. In the absence of such data, a good approach is to drive the inverse optimization processes of IMRT and VMAT, decrease the dose to all the critical structures as much as possible and maximize the target dose homogeneity. This can be primarily achieved by means of an adequate beam arrangement and by highly restrictive planning objectives, more restrictive than the clinical need. The reduction of unintended tissue involvement is more compelling for patients with left-sided breast cancer owing to the unavoidable partial irradiation of the heart. The risk of cardiac toxicity that mainly manifests its effects at a late stage (more than 10 years) was recently well addressed by Darby et al9 and Boekel et al.10 To mitigate the risk of radiation-induced heart damage, some geometrical precautions can be applied. The most popular is the use of respiratory gating in deep inspiration breath-hold (DIBH), as illustrated by Korreman et al11,12 and Aznar et al.13 In this case, the distance between the heart and chest wall is increased with the consequent reduction of the heart irradiation.
The clinical use of VMAT for the hypofractionated treatment of patients with early-stage breast cancer was already reported in ref. 14. The aim of the present study was to evaluate some of the possible trade-offs in breast VMAT planning, exploring the degree of achievable dosimetric sparing of different organs at risk (OARs) by using two similar VMAT plan settings. The comparison of the dosimetric differences was considered a surrogate for evaluating the relative risk of long-term toxicity and might guide the clinical implementation of VMAT.
METHODS AND MATERIALS
Patient data set
Planning CT scans of 20 patients presenting early-stage left breast cancer, after breast-conserving surgery, were used for this planning study. Data were acquired with patients in the supine position with both arms above the head. DIBH was applied. CT acquisition was with 2-mm-thick adjacent slices.
For each patient, the clinical target volume was delineated by a specialized radiation oncologist following the Radiation Therapy Oncology Group recommendations published in the RTOG breast contouring atlas.15 A 5-mm clinical target volume was added to the planning target volume (PTV) margin. The PTV was then cropped 5 mm inside the patient outline. The PTVs varied between 300 and 1800 cm3 (mean 1035 ± 415 cm3). The following OARs were contoured: Ilung and contralateral lung (Clung), heart, left anterior descending artery (LAD) and contralateral breast (Cbreast). The heart and LAD were delineated according to the guidelines described by Feng et al;16 the LAD was expanded by a 4-mm margin to account for heartbeat.17 To assess the quality of the plans, the skin region was also considered. This was defined as 5 mm of the tissue from the target to the patient outline in the region encompassing the PTV.
Planning technique
VMAT plans were optimized for the RapidArc technique in the Eclipse™ treatment planning system (Varian Medical Systems, Palo Alto, CA), using the Photon Optimizer algorithm (v. 13.6) and calculated with Acuros XB. Arc arrangement used two partial arcs, with the gantry running from about 295° (range 285–307°) to about 173° (range 160–180°), as shown in Figure 1. Plans were optimized for a TrueBeam™ linear accelerator (Varian Medical Systems, Palo Alto, CA) equipped with a Millennium 120 multileaf collimator (5-mm leaf width at the isocentre for the 20-cm central beam). An additional third arc was added for those challenging patients whose dosimetric objectives were not met with only two arcs. The collimator angles were set in a range of 10–22°, with complementary angles for the second arc; in few cases, the collimator was set to 90°, especially for the third arc when needed. The field width (the leaf motion direction) was kept within 15 cm to maximize the modulation power inside the field.
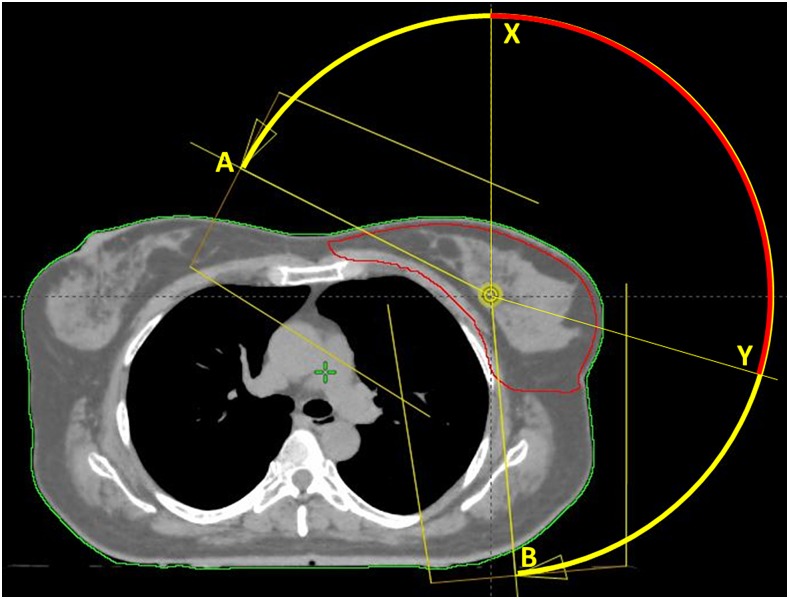
Figure 1.
Arc span in the A–B range; the avoidance sectors are set in the X–Y range of the A–B arc.
Two plans using the same arc arrangement were generated for each patient:
– RA_full: the optimizer used the entire partial arc trajectory
– RA_avoid: avoidance sectors (AvS) were set on all the arcs. In RapidArc, the AvS are a connected subarc fragment, with the length and position defined by the user, where the dose rate is forced to drop to zero. The AvS shall be longer than 15° and the arc lengths before and after it shall be longer than 15°. The start and stop gantry angles of the AvS were manually set from approximately 0° (range 345–20°) to approximately 105° (range 90–110°). Those angles were identified on a patient-by-patient basis according to their anatomy as seen in the beam's eye view: the start angle at approximately 0° was chosen as the position where, during the clockwise rotation, the heart volume in the beam's eye view increased; the stop angle at approximately 105° was the position where the right breast and left lung appeared to be aligned in their anterior surface.
Figure 1 illustrates the concept of the RA_full vs the RA_avoid plans.
Dose prescription and dosimetric objectives
All plans were optimized for prescribing a dose of 40.05 Gy as the mean PTV dose.
The common dosimetric objectives for all the plans were (defining VX as the volume receiving at least X dose, and DY as the dose received by Y of the structure volume):
– PTV: 97% of the PTV should receive at least 95% of the dose (V95% > 97%); the volume receiving >107% of the prescription should not exceed 3 cm3 (V107% < 3 cm3)
– Ilung: mean <8 Gy; minimize V20 Gy, V10 Gy, V5 Gy
– Clung: mean <2 Gy; minimize V5 Gy, V2 Gy
– Heart: mean <5 Gy; minimize V30 Gy, V25 Gy, V2 Gy
– LAD: mean <8 Gy; < 20 Gy
– Cbreast: mean <3 Gy.
To improve the dose distributions and the dose conformality to the target, the body volume outside the PTV receiving 90% of the dose prescription was minimized. The rationale for this resides in the correlation between the V90% and the possible increase of skin toxicity and breast pain.18 This was easier to achieve with RA_full. There was also an attempt to further reduce the mean doses to the contralateral structures (Clung and Cbreast), with the rationale of decreasing the potential risk of second cancer induction from low doses. This was easily achievable with RA_avoid.
Plan quality assurance
To evaluate the accuracy of the delivery in the two planning techniques, especially for RA_avoid, where the dose rate is forced to drop to zero in an arc sector, the institutional standard pre-treatment quality assurance procedure was followed for all patients and plans. The Epiqa™ software (Epidos sro, Bratislava, Slovakia) was used to convert portal images into dose at the depth of the maximum in water and for comparison with Eclipse/Acuros XB calculations. Results were analyzed according to the gamma evaluation using 3% as dose difference and 3 mm as distance to agreement. Data were reported as percentage of points passing the gamma criteria.
Data analysis
Quantitative analysis was performed by means of dose–volume histogram-based metrics. High dose spread outside the target was measured through the normal tissue high dose (NTHD) defined as the ratio between the V90% of the non-target tissue (patient outline subtracting the PTV) and the PTV. This was in analogy with the healthy tissue overdose volume factor defined in Feuvret et al.19 This parameter estimates the unwanted treated volume.
The homogeneity index was defined by the difference between the percentage of D2% and D98%.
Treatment efficiency was evaluated by the monitor units (MU) per each plan.
The dosimetric data were compared for the two sets of plans with the paired Student's t-test.
RESULTS
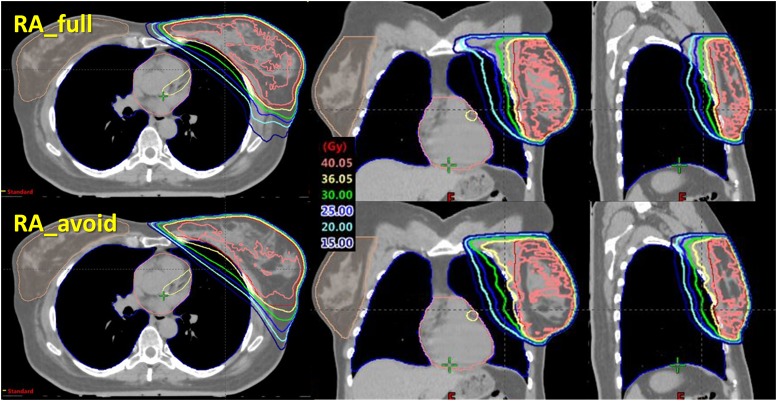
In Table 1, the dosimetric results are reported as mean value and standard deviation of each parameter over all the patients. The mean dose–volume histogram is presented in Figure 2. An example of dose distributions is presented in Figure 3 for the two techniques.
Table 1.
Dosimetric results
| Organ | Parameter | RA_full | RA_avoid | Difference between RA_avoid and RA_full |
|---|---|---|---|---|
| PTV | D2% (%) | 103.7 ± 0.5 | 104.5 ± 0.4 | 0.8 ± 0.5 |
| D98% (%) | 95.3 ± 0.6 | 94.8 ± 0.5 | −0.5 ± 0.6 | |
| SD (Gy) | 0.75 ± 0.08 | 0.90 ± 0.09 | 0.15 ± 0.10 | |
| Body-PTV | V5 Gy (cm3) | 3′753 ± 659 | 2′765 ± 579 | −989 ± 294 |
| V20 Gy (cm3) | 931 ± 206 | 1′173 ± 322 | +243 ± 162 | |
| V30 Gy (cm3) | 434 ± 90 | 649 ± 185 | +215 ± 116 | |
| V90% (cm3) | 165 ± 31 | 299 ± 96 | +134 ± 76 | |
| NTHD | 0.184 ± 0.075 | 0.309 ± 0.079 | 0.125 ± 0.048 | |
| Heart | Mean (Gy) | 3.9 ± 0.6 | 1.9 ± 1.0 | −1.9 ± 0.7 |
| 8.1 ± 1.3 | 4.5 ± 2.2 | −3.5 ± 1.7 | ||
| 6.3 ± 1.1 | 3.4 ± 1.7 | −2.9 ± 1.3 | ||
| D2% (Gy) | 11.0 ± 1.7 | 6.5 ± 2.7 | −4.5 ± 2.2 | |
| V5 Gy (%) | 24.8 ± 7.4 | 7.4 ± 10.0 | −17.4 ± 7.1 | |
| LAD | Mean (Gy) | 6.2 ± 0.7 | 4.0 ± 1.5 | −2.2 ± 1.3 |
| D2% (Gy) | 14.4 ± 2.8 | 10.3 ± 3.8 | −4.1 ± 3.5 | |
| LungI | Mean (Gy) | 7.4 ± 0.5 | 6.5 ± 0.9 | −0.9 ± 0.8 |
| V5 Gy (%) | 46.7 ± 5.0 | 36.5 ± 6.0 | −10.2 ± 5.8 | |
| V20 Gy (%) | 8.5 ± 1.2 | 9.1 ± 1.9 | 0.6 ± 1.8 | |
| V30 Gy (%) | 2.2 ± 0.6 | 3.5 ± 1.2 | 1.4 ± 1.2 | |
| V40 Gy (%) | 0.0 ± 0.0 | 0.04 ± 0.15 | 0.04 ± 0.15 | |
| 29.1 ± 2.2 | 31.7 ± 1.6 | 2.5 ± 1.9 | ||
| 24.7 ± 3.0 | 26.9 ± 2.6 | 2.3 ± 2.3 | ||
| LungC | Mean (Gy) | 1.6 ± 0.2 | 0.3 ± 0.1 | −1.3 ± 0.2 |
| V2 Gy (%) | 25.7 ± 6.9 | 0.6 ± 0.5 | −25.1 ± 6.9 | |
| V5 Gy (%) | 2.7 ± 1.6 | 0.01 ± 0.01 | −2.7 ± 1.6 | |
| 5.2 ± 0.9 | 1.2 ± 0.3 | −4.0 ± 1.0 | ||
| 4.3 ± 0.8 | 0.9 ± 0.2 | −3.3 ± 0.9 | ||
| BreastC | Mean (Gy) | 2.2 ± 0.2 | 0.6 ± 0.2 | −1.6 ± 0.3 |
| V2 Gy (%) | 40.2 ± 9.5 | 5.3 ± 3.1 | −34.8 ± 9.1 | |
| V5 Gy (%) | 5.7 ± 1.7 | 1.4 ± 1.2 | −4.3 ± 1.9 | |
| 4.9 ± 1.2 | 1.8 ± 0.8 | −3.1 ± 1.2 | ||
| 3.7 ± 0.9 | 1.1 ± 0.4 | −2.7 ± 0.8 | ||
| Skin | Mean (Gy) | 30.9 ± 0.4 | 31.7 ± 0.4 | 0.7 ± 0.3 |
| V35 Gy (%) | 37.4 ± 3.7 | 44.1 ± 3.2 | 6.7 ± 2.9 | |
| 35.9 ± 0.5 | 36.6 ± 0.6 | 0.7 ± 0.3 | ||
| 31.1 ± 3.2 | 32.0 ± 3.4 | 0.8 ± 0.3 | ||
| MU | 691 ± 78 | 620 ± 60 | −71 ± 78 |
LAD, left anterior descending artery; MU, monitor units; NTHD, normal tissue high dose; PTV, planning target volume; SD, standard deviation.
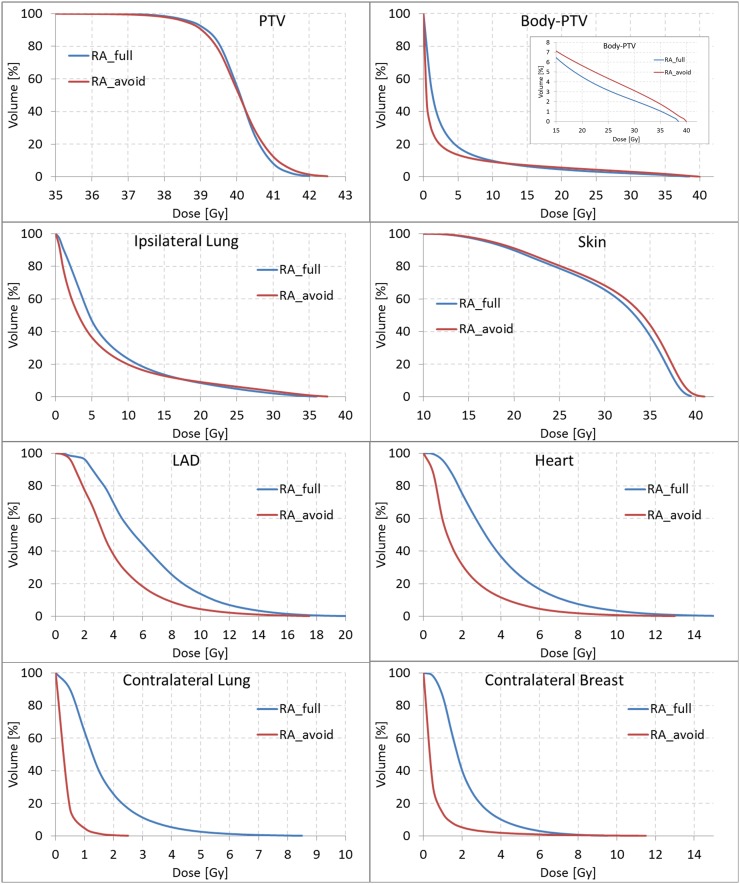
Figure 2.
Mean dose–volume histograms. LAD, left anterior descending artery; PTV, planning target volume.
Figure 3.
An example of isodose distributions.
Target coverage, as D98%, was significantly better for RA_full, as well as for D2%, despite the rather small difference value owing to the dose homogeneity requested. From those values, the homogeneity index was 8.4 ± 1.0% and 9.7 ± 0.9% for RA_full and RA_avoid, respectively (p < 0.0001).
The normal tissue high dose was 18% and 31% for RA_full and RA_avoid. The volume of the healthy tissue receiving 90% of the prescription dose was 165 cm3 in an average for RA_full; the same value was almost doubled (310 cm3 in an average) for RA_avoid.
In addition, the mean dose to the skin was systematically increased by an average of 0.7 ± 0.3 Gy for RA_avoid. This effect was indeed expected, as the irradiation was, for those plans, more tangential.
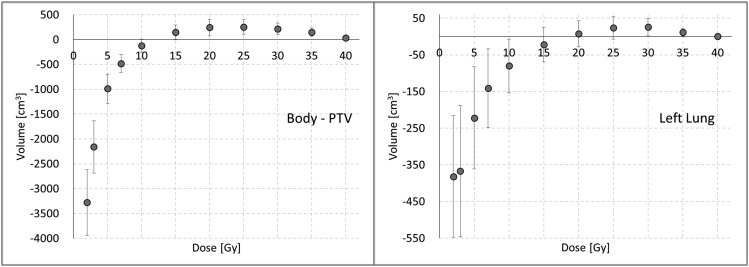
Regarding OAR sparing, the RA_avoid plans significantly lowered the volume receiving low doses for the ipsilateral structures, as well as for the contralateral organs. The numerical findings are summarized in Table 1. All the dosimetric parameters were worse for RA_full, except for the Ilung volume receiving medium–high doses. This effect was due to the effective arc of the RA_avoid plans, limited to gantry regions similar to the conformal tangential entrances, including only the lung sector close to the chestwall. The same trend was observed for the healthy tissue also. To illustrate this feature, Figure 4 shows the average difference of the volumes in cubic centimetres between RA_avoid and RA_full plans as a function of the dose received. For doses lower than 20 (15) Gy to the Ilung (healthy tissue), the RA_avoid presented better results, while the result is the opposite for doses higher than those levels.
Figure 4.
The volume difference between RA_avoid and RA_full as a function of delivered dose for the body planning target volume (PTV) and ipsilateral lung.
For RA_avoid, the contralateral structure doses were very low and likely compatible with the unavoidable scattering and transmission; the average mean doses were 0.7% and 1.5% respectively. For C_Lung and C_Breast, the V2 Gy value dropped from 40% and 26%, respectively, in the case of RA_full to 5% and <1% for RA_avoid.
In terms of delivery efficiency, the RA_full required 11% more MU than RA_avoid (p < 0.001) for the same number of arcs. However, since the RA_avoid arcs have a large sector with no MU delivery, the active arc fragments shall deliver more MU per degree, leading the gantry to slow down in those sectors. For that reason, the time required to deliver the plans is about 20% shorter for RA_full compared with RA_avoid (99 ± 3 vs 117 ± 4 s for RA_full and RA_avoid, respectively).
Pre-treatment quality assurance analysis results showed a gamma agreement of 99.6 ± 0.5% (range 97.6–100.0%) for the RA_full cases and 99.8 ± 0.3% (range 98.9–100.0%) for the RA_avoid arcs.
DISCUSSION
Besides some generally accepted consensus on the dose–volume objectives in the medium–high dose range for the OARs involved in the irradiation of the breast, there is no strong agreement on the radiobiology risk at medium–low dose levels. The toxicity end points and the related tolerance dose levels are quite vague and this fact impacts on the choice of the trade-offs between conflicting aims. Other than radiobiology, there are also other risk factors for late toxicity to be accounted for; these include the role of chemotherapy, for example. It is clear that the optimal trade-off between the low and high dose levels, or between the target dose homogeneity and the surrounding healthy structure irradiation, is a complex multifactorial problem. A safe strategy, in the absence of consensus data, is to apply pragmatically the ALARA principle (as low as reasonably achievable). In the present study, by comparing two similar VMAT techniques, we aimed to emphasize that the planning process should be analyzed carefully, considering which are the trade-offs that have to be balanced. On one side, dose conformality could be preferred at the price of the increased low-dose bath, with a possible attempt at decreasing acute and early late toxicity. On the other hand, the smallest achievable low-dose bath could be preferred at the price of a lower dose conformality, possibly attempting to reduce long-term toxicity and the risk of second cancer induction.
Concerning the heart, the required level of sparing is debated.9,10 The data reported by Darby et al9 correlated the increased rate of major coronary events with the mean dose to the heart, showing a 7.4% increased rate per Gray of mean heart dose. On the other side, the late toxicity is known to be related to the heart volume receiving doses >30 Gy.20 In the present study, to reduce the high dose levels to the heart, patients were planned in DIBH mode, and both the mean heart dose and the high dose volume were minimized.
In the case of lung, there is a clear match between the level of irradiation and tissue damage. Nevertheless, in the case of breast irradiation, there are no definitive data to clarify whether the focus should be set on mean lung dose or on the volume of the parenchyma receiving high–medium dose levels. In the treatment of breast with VMAT, the dose distribution to the lungs is normally grossly different from what is achieved with the conformal tangential beams. In a recent report, Petruzzelli et al21 evaluated the number of B-lines in lung ultrasound examination (Ilung and Clung) on patients treated for breast cancer. Authors found a significantly larger amount of B-lines in the Ilung; in particular, the pattern was mainly in the anterior rather than the posterior chest, i.e. in the region receiving a high dose. These data were not directly correlated to incidence of toxicity, but it might be interesting to further investigate the problem aiming to understand the potential negative impact of dose level vs the presence of this signature.
The minimization of the irradiation of the Cbreast is highly prioritized. The rationale for this resides in the fact that patients with breast cancer are considered long-term survivors, and the risk of radiation-induced carcinogenesis should be minimized. However, the quantitative relationship between low–medium dose levels and the induction of secondary cancer is not clinically proven. Risk models exist,22–24 but the clinical validation is vague. Again, the ALARA principle should be applied.
The recent investigations at planning level aimed to explore the role of IMRT and VMAT and to establish the trade-offs using different optimization options. In this line, in the present study, we compare two VMAT solutions differing in beam geometry (without or with the AvS). The number of arcs was kept low (generally two), as this led to faster treatment, an interesting argument when DIBH is used.
A Dutch group (Jeulink et al25) compared two partial arcs (X jaw setting <18 cm) and six small arcs, doubling the entrances from the tangential direction with respect to the central part of the arcs (the increased number of arcs allowed a smaller X jaw setting). They planned with a simultaneous boost to 40.05 and 50.25 Gy in 15 fractions with RapidArc, no nodal regions and no DIBH. A Finnish group (Virén et al26) compared two continuous partial arcs and two pairs of tangential arcs, hypothesizing a decrease of heart and lung radiation dose without increasing the low dose volume in the Cbreast. They used Elekta VMAT; the dose prescribed to the breast was 50 Gy in 25 fractions, no nodal regions, no simultaneous boost and no DIBH. Finally, a German–Austrian group (Pasler et al27) compared one partial arc with two small tangential arcs. Nodal regions and internal mammary chain were included, simultaneous boost with 50.4 and 60.2 Gy doses in 28 fractions were planned with the Pinnacle planning system for an Elekta linear accelerator and no DIBH. In Table 2, the ratio of the mean doses between tangential and complete partial arc techniques, planned for the heart, Ilung, Clung, Cbreast and healthy tissues, from the above studies are compared with the present work. With this ratio, the difference in the dose prescription is cancelled, and only the variations due to the different geometry/strategies remain relevant. Values <1 present the more tangential solution (RA_avoid in our study) delivering less dose than the complete partial arc(s) solution (RA_full in our study). From this summary, the present study is the only one where the mean doses of all OARs were lowered using the AvS. Pasler et al27 improved with tangential setting all the structures except the Ilung; Jeulink et al25 did not improve the contralateral structures; Virén et al26 were deteriorating the ipsilateral structures, especially the heart. This variability in dose distribution for similar concepts for planning is proof that it is difficult to find the best trade-offs for OAR sparing. It is not sufficient to have the same dosimetric rules to obtain the same results with the same technique. On one side, the optimizer could play a role; on the other side, the planner and the beam geometry could be crucial in finding a solution. In our study, the same initial dose objectives were used in the two geometries, and they were fulfilled in all cases, while the results in the dose distribution were substantially different.
Table 2.
Ratio between mean doses of tangential-like volumetric modulated arc therapy (VMAT) and continuous VMAT for different organs at risks
| Organ at risk | Virén et al26 | Pasler et al27 | Jeulink et al25 | Present work |
|---|---|---|---|---|
| Heart | 1.68 | 0.75 | 0.78 | 0.49 |
| Ilung | 1.10 | 1.01 | 0.72 | 0.88 |
| Clung | 0.56 | 0.37 | 1.06 | 0.19 |
| Cbreast | 0.46 | 0.60 | 1.00 | 0.27 |
| Normal tissue (body—PTV) | 0.87 | 0.93 | 0.83 |
Cbreast, contralateral breast; Clung, contralateral lung; Ilung, ipsilateral lung; PTV, planning target volume.
In the present study, we forced a high level of target dose homogeneity (V97% > 95% and V107% < 3 cm3); on the contrary, none of the three studies mentioned previously25,26,27 applied so stringent rules to the target dose homogeneity as part of the trade-off balance.
From the data reported, it would be incorrect, in general, to identify a certain delivery technique (e.g. VMAT) per se as responsible for, e.g., an excess risk of second cancer induction or a severe risk of toxicity for a critical structure and end point. There are cases where the patient anatomy is convenient for the simple two tangential beam arrangement (chest wall not too concave, heart distant from the chest, limited healthy tissue in the fields). However, in other cases, some dosimetric advantages are present if an intensity-modulated treatment is applied. As shown in the present study, the beam arrangement, even with fine adjustment, like the AvS use (and not simply the use of a technique or the optimizer), could be fundamental for adjusting the trade-off choice. In the decision-making process, e.g. between RA_full and RA_avoid, the attention could be differently focused, or on the long-term toxicity and second cancer risk induction (maybe preferring RA_avoid), or on the acute or “early late” toxicity (maybe with RA_full).
An important improvement in the inverse planning workflow would be to automate the identification of regions where sophisticated tools should be applied. In the present case, the AvS should be ideally automatically set by the optimization engines when their use could remarkably spare regions identified as critical. As per today, this is not yet the case and efforts should be put in this direction.
As a final remark, it is important to notice that our study focused on the case of a whole breast, with no simultaneous boost and without nodal inclusion. This is possibly an important limitation; the rationale for this choice was simplicity. The study was conceived to focus on the maximization of OAR sparing in the absence of confounding factors. Of course, the generalization of the present results to more complex cases shall be carried out with care.
CONCLUSION
An effective reduction of the OAR doses, especially for the contralateral structures, was demonstrated when sectors of the VMAT arcs were required to have zero dose rate delivery; the dose homogeneity in the target is maintained, while a lower degree of conformity is achieved. A deeper investigation of the trade-offs for application to the different OARs should be explored, as well as a validation of the planning objectives to be used based on clinical signatures, and is part of our future project.
CONFLICTS OF INTEREST
L. Cozzi acts as Scientific Advisor to Varian Medical Systems.
Contributor Information
Antonella Fogliata, Email: Antonella.Fogliata@humanitas.it.
Jan Seppälä, Email: jan.seppala@kuh.fi.
Giacomo Reggiori, Email: giacomo.reggiori@humanitas.it.
Francesca Lobefalo, Email: francesca.lobefalo@humanitas.it.
Valentina Palumbo, Email: valentina.palumbo@humanitas.it.
Fiorenza De Rose, Email: fiorenza.de_rose@humanitas.it.
Davide Franceschini, Email: davide.franceschini@humanitas.it.
Marta Scorsetti, Email: marta.scorsetti@humanitas.it.
Luca Cozzi, Email: luca.cozzi@humanitas.it.
REFERENCES
- 1.START Trialists' Group; Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, et al. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet 2008; 371: 1098–107. doi: https://doi.org/10.1016/S0140-6736(08)60348-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fogliata A, Bolsi A, Cozzi L. Critical appraisal of treatment techniques based on conventional photon beams, intensity modulated photon beams and proton beams for therapy of intact breast. Radiother Oncol 2002; 62: 137–45. doi: https://doi.org/10.1016/s0167-8140(01)00476-5 [DOI] [PubMed] [Google Scholar]
- 3.Bartelink H, Horiot JC, Poortmans PM, Struikmans H, Van den Bogaert W, Fourquet A, et al. Impact of a higher radiation dose on local control and survival in breast-conserving therapy of early breast cancer: 10-year results of the randomized boost versus no boost EORTC 22881-10882 trial. J Clin Oncol 2007; 25: 3259–65. doi: https://doi.org/10.1200/JCO.2007.11.4991 [DOI] [PubMed] [Google Scholar]
- 4.Bartelink H, Maingon P, Poortmans P, Weltens C, Fourquet A, Jager J, et al. ; European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015; 16: 47–56. doi: https://doi.org/10.1016/S1470-2045(14)71156-8 [DOI] [PubMed] [Google Scholar]
- 5.Fogliata A, Nicolini G, Alber M, Asell M, Dobler B, El-Haddad M, et al. IMRT for breast. A planning study. Radiother Oncol 2005; 76: 300–10. doi: https://doi.org/10.1016/j.radonc.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 6.Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys 2010; 76: 287–95. doi: https://doi.org/10.1016/j.ijrobp.2009.05.038 [DOI] [PubMed] [Google Scholar]
- 7.Johansen S, Cozzi L, Olsen DR. A planning comparison of dose patterns in organs at risk and predicted risk for radiation induced malignancy in the contralateral breast following radiation therapy of primary breast using conventional, IMRT and volumetric modulated arc treatment techniques. Acta Oncol 2009; 48: 495–503. doi: https://doi.org/10.1080/02841860802657227 [DOI] [PubMed] [Google Scholar]
- 8.Qiu JJ, Chang Z, Wu QJ, Yoo S, Horton J, Yin FF. Impact of volumetric modulated arc therapy technique on treatment with partial breast irradiation. Int J Radiat Oncol Biol Phys 2010; 78: 288–96. doi: https://doi.org/10.1016/j.ijrobp.2009.10.036 [DOI] [PubMed] [Google Scholar]
- 9.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013; 368: 987–98. doi: https://doi.org/10.1056/NEJMoa1209825 [DOI] [PubMed] [Google Scholar]
- 10.Boekel NB, Schaapveld M, Gietema JA, Russell NS, Poortmans P, Theuws JC, et al. Cardiovascular disease risk in a large, population-based cohort of breast cancer survivors. Int J Radiat Oncol Biol Phys 2016; 94: 1061–72. doi: https://doi.org/10.1016/j.ijrobp.2015.11.040 [DOI] [PubMed] [Google Scholar]
- 11.Korreman SS, Pedersen AN, Nøttrup TJ, Specht L, Nyström H. Breathing adapted radiotherapy for breast cancer: comparison of free breathing gating with the breath-hold technique. Radiother Oncol 2005; 76: 311–18. doi: https://doi.org/10.1016/j.radonc.2005.07.009 [DOI] [PubMed] [Google Scholar]
- 12.Korreman SS, Pedersen AN, Aarup LR, Nøttrup TJ, Specht L, Nyström H. Reduction of cardiac and pulmonary complication probabilities after breathing adapted radiotherapy for breast cancer. Int J Radiat Oncol Biol Phys 2006; 65: 1375–80. doi: https://doi.org/10.1016/j.ijrobp.2006.03.046 [DOI] [PubMed] [Google Scholar]
- 13.Aznar MC, Maraldo MV, Schut DA, Lundemann M, Brodin NP, Vogelius IR, et al. Minimizing late effects for patients with mediastinal Hodgkin lymphoma: deep inspiration breath-hold, IMRT, or both? Int J Radiat Oncol Biol Phys 2015; 92: 169–74. doi: https://doi.org/10.1016/j.ijrobp.2015.01.013 [DOI] [PubMed] [Google Scholar]
- 14.Scorsetti M, Alongi F, Fogliata A, Pentimalli S, Navarria P, Lobefalo F. et al. Phase I-II study of hypofractionated simultaneous integrated boost using volumetric modulated arc therapy for adjuvant radiation therapy in breast cancer patients: a report of feasibility and early toxicity results in the first 50 treatments. Radiat Oncol 2012; 7: 145. doi: https://doi.org/10.1186/1748-717X-7-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. RTOG.org. Available from: http://www.rtog.org/ Last access: July 2016.
- 16.Feng M, Moran JM, Koelling T, Chughtai A, Chan JL, Freedman L, et al. Development and validation of a heart atlas to study cardiac exposure to radiation following treatment for breast cancer. Int J Radiat Oncol Biol Phys 2011; 79: 10–8. doi: https://doi.org/10.1016/j.ijrobp.2009.10.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Vidan E, Bergman GW. Cardiac motion of coronary arteries: variability in the rest period and implications for coronary MR angiography. Radiology 1999; 213: 751–8. doi: https://doi.org/10.1148/radiology.213.3.r99dc41751 [DOI] [PubMed] [Google Scholar]
- 18.De Rose F, Fogliata A, Franceschini D, Navarria P, Villa E, Iftode C. et al. Phase II trial of hypofractionated VMAT-based treatment for early stage breast cancer: 2-year toxicity and clinical results. Radiat Oncol 2016; 11: 120. doi: https://doi.org/10.1186/s13014-016-0701-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feuvret L, Noël G, Mazeron JJ, Bey P. Conformity index: a review. Int J Radiat Oncol Biol Phys 2006; 64: 333–42. doi: https://doi.org/10.1016/j.ijrobp.2005.09.028 [DOI] [PubMed] [Google Scholar]
- 20.Darby SC, Cutter DJ, Boerma M, Constine LS, Fajardo LF, Kodama K, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys 2010; 76: 656–65. doi: https://doi.org/10.1016/j.ijrobp.2009.09.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petruzzelli MF, Vasti MP, Tramacere F, D'Errico MP, Gianicolo EA, Andreassi MG, et al. The potential role of lung ultrasound B-lines for detection of lung radio-induced toxicity in breast cancer patients after radiation therapy. Echocardiography 2016; 33: 1374–80. DOI: https://doi.org/10.1111/echo.13249 [DOI] [PubMed] [Google Scholar]
- 22.Schneider U. Mechanistic model of radiation-induced cancer after fractionated radiotherapy using the linear-quadratic formula. Med Phys 2009; 36: 1138–43. doi: https://doi.org/10.1118/1.3089792 [DOI] [PubMed] [Google Scholar]
- 23.Schneider U, Sumila M, Robotka J, Gruber G, Mack A, Besserer J. Dose-response relationship for breast cancer induction at radiotherapy dose. Radiat Oncol 2011; 6: 67. doi: https://doi.org/10.1186/1748-717X-6-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berrington de Gonzalez A, Gilbert E, Curtis R, Inskip P, Kleinerman R, Morton L, et al. Second solid cancers after radiation therapy: a systematic review of the epidemiologic studies of the radiation dose-response relationship. Int J Radiat Oncol Biol Phys 2013; 86: 224–33. doi: https://doi.org/10.1016/j.ijrobp.2012.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jeulink M, Dahele M, Meijnen P, Slotman BJ, Verbakel WF. Is there a preferred IMRT technique for left-breast irradiation? J Appl Clin Med Phys 2015; 16: 5266. doi: https://doi.org/10.1120/jacmp.v16i3.5266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virén T, Heikkilä J, Myllyoja K, Koskela K, Lahtinen T, Seppälä J. Tangential volumetric modulated arc therapy technique for left-sided breast cancer radiotherapy. Radiat Oncol 2015; 10: 79. doi: https://doi.org/10.1186/s13014-015-0392-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pasler M, Lutterbach J, Björnsgard M, Reichmann U, Bartelt S, Georg D. VMAT techniques for lymph node-positive left sided breast cancer. Z Med Phys 2015; 25: 104–11. doi: https://doi.org/10.1016/j.zemedi.2014.03.008 [DOI] [PubMed] [Google Scholar]