Abstract
The metastatic state of most solid cancers traditionally has been regarded as an incurable dissemination of disease, with treatment focused on delaying progression rather than eliminating all tumour burden. In this setting, local therapies including surgery and radiotherapy are directed at quality of life end points and not at improvement in survival. However, improvements in imaging and systemic therapy have highlighted populations of patients with lower burden of metastatic disease, termed “oligometastatic,” who may present an exception. This condition is hypothesized to bridge the gap between incurable metastatic disease and locoregional disease, where miliary spread either has not occurred or remains eradicable. Consequently, elimination of such low-burden residual disease may “cure” some patients or delay further progression. Accordingly, use of local therapies with the intent of improving survival in oligometastatic disease has increased. Technological advances in radiation delivery with stereotactic ablative body radiotherapy (SAbR) in particular have provided a non-invasive and low-morbidity option. While observational studies have provided interesting preliminary data, significant work remains necessary to prove the merits of this treatment paradigm. This review discusses the data for the oligometastatic state and its treatment with SAbR, as well as challenges to its investigation.
INTRODUCTION
Significant strides in the management of localized solid cancers have been made in recent decades. Earlier detection of disease by screening has been complemented by improved treatments, as in the example of breast cancer, where a pairing of mammography and multimodal treatment has enhanced outcomes compared with the Halsted era of radical mastectomy.1–4 Further, extent of disease work-up has benefitted from better imaging and minimally invasive sampling techniques, resulting in stage migration and the ability to refine locoregional treatments. Thus, the most productive advances in deciphering the heterogeneity of cancer in an actionable manner arguably have occurred in the localized setting.
In contrast, metastatic spread of most solid cancers for much of the twentieth century remained an opaque category, nihilistically considered refractory to control and certainly cure. Only modest improvements in survival have been noted in the majority of cancers, when accounting for state migration. Long-term control and “cure” has been rare and confined largely to uniquely chemosensitive diseases such as germ cell neoplasms.5
An “oligometastatic” state
With advances in imaging and systemic therapy, it has been observed that a group of patients with low burden of metastatic disease across almost all major solid tumour subtypes experience comparably favourable long-term outcomes. Local therapies in these patients against such limited burden disease appear to augment these outcomes. Many of the earliest empiric data for this low-burden metastatic state and its favourable prognostic significance stemmed from hepatic and pulmonary metastasis resections in colorectal carcinoma and sarcoma.6–9 Hellman and Weichselbaum first formalized this concept over two decades ago, creating the so-called “oligometastatic” theory, wherein a spectrum of intermediate states between localized and systemic disease was hypothesized, which may remain curable with aggressive local therapy.10
These observations have generated considerable interest in defining the oligometastatic population and assessing the influence of local therapy in producing long-term control with or without systemic therapy. While early experiences primarily focused on surgical resection, or metastectomy, other less or non-invasive techniques for ablation have also arisen. Drawing upon the imaging and radiotherapy (RT) advances at the heart of the success of stereotactic radiosurgery (SRS) for brain metastases, stereotactic ablative radiotherapy (SAbR) has arisen as a particularly attractive non-invasive strategy in multiple disease sites for the management of oligometastases.11
Stereotactic ablative body radiotherapy
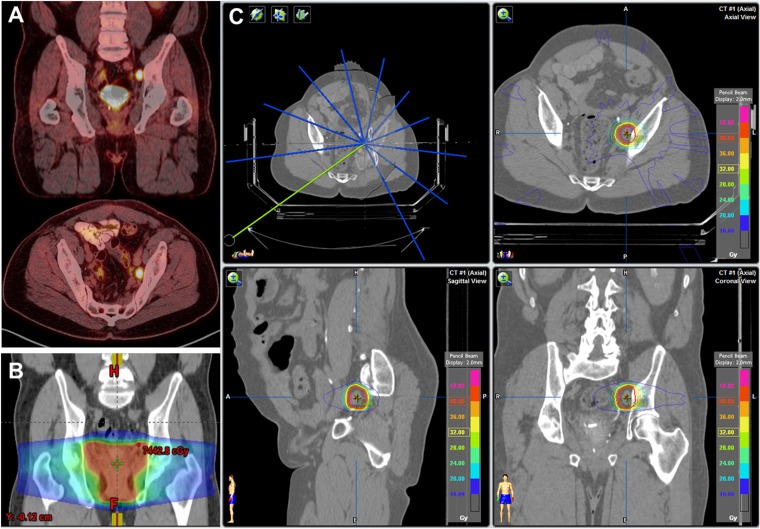
SAbR, also referred to as stereotactic body radiotherapy, enables uniquely focused and high-dose radiation treatments by leveraging rigid patient setup protocols, modern treatment planning and radiation delivery platforms with millimetre tolerances, motion management specific to anatomic sites such as the lung and interfraction and/or intrafraction image guidance. These techniques enable a safe, efficacious treatment across a broad array of anatomic locations, in proximity to critical normal organ structures, and even adjacent to or within prior RT fields, as demonstrated in Figure 1. Dose regimens vary by disease site, proximity of at-risk normal structures such as the spinal cord and therapeutic goal. For instance, dose for curative intent treatment of early stage non-small-cell lung cancer (NSCLC) may range from 50 Gy in five “fractions” of 10 Gy each for tumours near central airway structures to 34 Gy in a single fraction in more favourable locations with these approaches being associated with 2–3-year local control rates of approximately 90%.12,13
Figure 1.
Use of stereotactic ablative body radiotherapy (SAbR) in a 64-year-old male with oligometastatic prostate cancer developing metachronously at a single left pelvic sidewall lymph node just above prior salvage prostate fossa radiotherapy (RT) field for biochemical recurrence after prostatectomy: (a) systemic scans including fluorine-18 fludeoxyglucose positron emission tomography demonstrated a single site of disease. (b) Prior salvage RT was directed to the prostate fossa alone with lymph node oligometastasis occurring immediately above the field. (c) Oligometastasis was treated with SAbR to 40 Gy in 5 fractions with daily CT imaging, use of stereotactic frame and careful constraints placed on adjacent bowel, sciatic nerve and any potential for overlap of incident beams with prior treated areas. Image fusion and composite plan including prior RT facilitated this treatment, which resulted in swift biochemical and radiographic response (currently without evidence of disease) without need for androgen deprivation therapy or grade 2 or higher toxicity.
SAbR rationale
The unifying principle of SAbR regimens is to deliver a high dose of radiation per treatment fraction, typically over five or fewer treatments. In contrast to traditionally fractionated regimens which are guided by normal tissue repair tolerances related to broader fields of radiation, the radiobiologic rationale for SAbR lies in mechanistic data suggesting ablative, linear log kill effects at higher fraction doses, typically at 8 Gy or higher. At these ablative doses, achievable safely only with SAbR techniques associated with steep peritumoral dose fall-off and careful lesion selection, it is hypothesized that the tumour DNA damage repair variances that underlie histology-specific “radioresistance” to conventional fractionation may be overcome. Further, historical observations of the immunomodulatory effect of RT, such as the abscopal effect, are favoured to occur at higher fraction doses seen in SAbR.14,15
At the same time, the definition of the oligometastatic condition, its biologic basis and whether its natural history can be affected by local therapy still lack high-level data in most settings. The rapid adoption of metastectomy, SAbR and other ablative therapies in this context carry financial and ethical considerations that require clarification. Several ongoing trials are attempting to answer these questions. This review will discuss the available data for oligometastatic state, the role of local therapy and specifically SAbR and the challenges in its study.
CHARACTERIZING OLIGOMETASTASIS
No consensus criteria exist for the oligometastatic state. Beyond the number of lesions, other prognostic factors are needed to select ideally those patients exhibiting a “stunted” metastatic state still amenable to local intervention. These factors may be specific to disease site or histology. Broadly, investigators consistently have highlighted the importance of age/performance status, disease site/histology, size/location of lesions, kinetics of disease progression and development of metastasis in relation to diagnosis of a primary. A subgroup of these has been termed the “four aces” by some (young age, patient fitness, low disease burden, slow-growing disease).16 Performance status and/or age are well-recognized prognosticators in virtually every solid tumour type, and their relevance is perhaps even more important in the metastatic setting, by dictating not only tumour aggressiveness but also tolerance of therapy. Examples include suggested prognostic criteria for metastatic renal cell carcinoma, which variously include tumour grade, necrosis and putative biomarker expression, but nearly always incorporate age or performance status.17 Data and rationale for other suggested modifiers of the oligometastatic definition are described below and summarized in Table 1.
Table 1.
Factors affecting selection of oligometastatic states for local therapy intervention based on influence on prognosis
| Factor | Common criteria | Limitations |
|---|---|---|
| Actionable alterations | “Druggable” targets | Limited ability to sample for resistant subclones Intratumoral heterogeneity |
| Age | Young | Cut-off is subjective |
| Disease kinetics | Metachronous vs synchronous Slow progression |
Affected by timing and sensitivity of imaging |
| Histology | Favourable natural history (i.e. breast) | Heterogeneity within histology |
| Location of lesions | Typical route of spread, not visceral | Variable sensitivity of imaging according to anatomic location |
| Performance status | Good | May change during therapy May exclude patients who benefit most from switch from systemic by quality of life metrics |
| Number of lesions | 1–5 | Affected by imaging technique Size of lesions may affect importance |
Histology
Breast cancer is known to exhibit a subpopulation of patients with metastasis achieving long-term survival regardless of therapy. This may relate to a more sustained immune-modulated tumour equilibrium condition in patients of generally better health and younger age. Further, while variations in aggressiveness of metastatic disease may occur in all diseases, the understanding of molecular subtypes is better and a druggable “target” (i.e. oestrogen receptor expression, ERBB2 (erb-b2 receptor tyrosine kinase 2) amplification) more frequently present in breast cancer, helping to account for this. In patients undergoing SAbR for oligometastatic disease, Milano et al presented the largest series demonstrating a comparably better 2-year progression-free survival (PFS) of 36% in breast cancer vs 13% in non-breast cancers, which also translated into a better overall survival of nearly 50% at 6 years.18 In contrast, some have suggested poorer local control of colorectal primary metastases to the lung after SAbR.19–21 However, these series were limited by small size, and another recent series observed only a 7.5% crude rate of local failure, when given adequate dose.22 Thus, there are no clear data separating out the performance of other histologies, with likely heterogeneity in outcome based on characteristics specific to a given disease type.
Actionable genetic alterations
Actionable genetic alterations have helped identify patients more likely to exhibit long-term disease control on targeted agents. The strongest examples of this are in metastatic NSCLC, where activating alterations in epidermal growth factor receptor (EGFR) and a rearrangement giving rise to the ALK (anaplastic large cell tyrosine receptor kinase)-EML4 (echinoderm microtubule-associated protein-like 4) fusion protein sensitize patients to their kinase inhibitors such as erlotinib (Tarceva; Genentech/Astellas) and crizotinib (Xalkori; Pfizer), respectively. While accounting for <10% of metastatic NSCLC, these populations present strong opportunities for examining the potential of oligometastatic disease under the related lens of “oligoprogressive” disease. In this latter theory, clones which have developed resistance to targeted therapy and which threaten to obviate the benefit of kinase inhibition may be amenable to identification and ablation, thereby extending time to targeted therapy and time to functional progression in regard to impact on disease control. Multiple studies have now investigated ablative local therapies in this population under both the oligometastatic and oligoprogressive paradigms and will be discussed.
Influence of lesion number/location
The number of lesions best capturing the oligometastatic population is likely dependent on disease type, imaging use and the other above-mentioned factors. While the earliest descriptions of oligometastatic disease have favoured a threshold of five or less lesions, data do exist for the influence of lesion count below this. In line with the experience of better overall survival with SRS following whole-brain RT for a subgroup of single metastasis in Radiation Therapy Oncology Group 9508,23 Bignardi et al’s experience with SAbR for metastatic disease reported a 42% 2-year PFS for solitary metastasis as compared with 0% for all other patients.24–26 Similarly, both Salama et al and Wersäll et al found an improved overall survival following SAbR for those patients with ≤3 metastatic lesions as compared with those with more.27,28 Further, in addition to the number of lesions, some have postulated that the size of metastatic lesions are influential on outcome, although this may affect a more local control constraining SAbR dose and is not consistently found to be predictive.18,29,30
The location of metastasis is likely to influence the prognosis of the patient with oligometastasis. Several studies argue for worse performance with visceral oligometastatic involvement. Milano et al demonstrated better PFS of patients with single organ system involvement by metastasis of thoracic lymph nodes or bone, as compared with visceral disease of the liver or lung.31 Adrenal lesions were historically thought to be associated with worse outcome on the basis of assumed haematologic spread,32 although this may be related to technical difficulty in local therapy for this site before the development of laparoscopic surgical approaches33 and SAbR.34
Further, there is likely an equal, negative prognostic meaning of an atypical metastasis location for a particular disease, when compared with classical routes of spread. For instance, while prostate cancer is frequently found to be metastatic to bone or pelvic/abdominal nodes, the finding of visceral involvement of the lungs, liver or other sites is often associated with very advanced disease or neuroendocrine dedifferentiation,35 where prognosis is especially poor. This appears true at even earlier time points. In a natural history study of patients suffering failure after local RT for prostate cancer by Zumsteg et al, visceral compartment relapse conferred decidedly worse survival than those with “lymphotropic” or osseous only disease.36 In this scenario, one might postulate that atypical metastasis site for a disease even when oligometastatic may not represent the same favourable state of intermediary disease as similar low-burden involvement of a more common metastasis site.
Influence of disease kinetics
The kinetics of oligometastatic disease development is also thought to affect prognosis in oligometastatic disease. This encompasses the type of presentation of oligometastases, either at diagnosis (synchronous) or following local therapy for the primary tumour (metachronous), and time to development of first and subsequent oligometastases. Metachronous development is felt to demonstrate improved outcomes in comparison with synchronous presentation, from which we may infer an indolent disease progression timeline in the former scenario.37 In further evidence of this concept, two independent groups reported improved disease-free intervals following SAbR for patients with metastases occurring after more than 12 months vs sooner.38,39 Several factors may influence interpretation of the oligometastases kinetics and highlight the need to consider disease histology specific factors. Clearly, the utility and frequency of imaging varies by cancer type. In addition, the use of adjuvant therapies, as in the case of post-operative RT or androgen deprivation therapy for biochemically recurrent prostate cancer, must also be considered.
In sum, it is clear that the definition of an oligometastatic state must factor in patient performance status, disease histology, disease kinetics and relapse pattern in addition to lesion number in order to best define a sufficiently homogeneous group for the critical study of aggressive local therapies, including SAbR.
RATIONALE FOR LOCAL THERAPY IN OLIGOMETASTASIS
Quality of life is the key metric of outcome for palliative local therapy in metastatic solid tumours, and local therapy without expectation of active symptom relief is typically viewed with critical eyes. However, as most metastatic solid cancers are not cured by “standard” systemic therapy, advances are arguably assessed more simply by weighing ability to delay progression and/or achieve response against expected toxicity. In this setting, adjunct modalities that augment response or prolong time until progression are of high interest.
Novel approaches such as small molecule inhibitors and immunotherapy have drawn much publicity in this role. However, outside of a handful of examples, the magnitude of extended survival typically has been on the order of months. Indeed, in metastatic colorectal cancer, a recent analysis indicated that only a minority of survival improvement in the last two decades can be attributed to advances in systemic therapy, whereas a majority was likely due to factors such as improved supportive care.40 The reasons for this slow improvement despite large investments in research and drug development include diminishing performance status issues, incomplete understanding of resistance mechanisms and tumour heterogeneity, and pharmacokinetic limitations in delivering blood-borne therapies to their target.41 Most of these limitations are demonstrated by the consistent failure of systemic therapies to control durably gross visible disease.
Thus, there exists a need for strategies outside reliance on traditional or even modern systemic agents. Why and in what context would aggressive local therapy play such a role?
Impact of patterns of failure
Firstly, local therapy would be beneficial if the pattern of failure of disease following systemic therapy is commonly within initial sites of involvement. Even in good responses, the principles of the Norton–Simon hypothesis argue that macroscopic reservoirs of disease, under selection pressures due to hypoxia/metabolic stress, will be less responsive to complete elimination by cytotoxic therapy alone owing to different growth kinetics, arguing for adjunct local therapy.42 This concept appears to drive the particular benefit of consolidative RT in bulky disease even in haematologic malignancies, such as lymphomas. To evaluate this empirically, Rusthoven et al assessed patterns of progression amongst 64 patients with advanced NSCLC meeting their inclusion criteria for assessment following maximal response to first-line chemotherapy.43 Of these, 34 patients met institutional eligibility criteria for SAbR following maximal response to chemotherapy. In turn, upon treatment failure in the total group, a majority (64%) had first progression at initial sites of disease, suggesting that SAbR to these sites would have been of benefit. Of note, this SAbR-eligible population formed 53% of their patients with metastatic NSCLC.
Further, oligometastatic disease may more likely produce oligometastatic progression upon relapse, which remains amenable to local therapy with delay of systemic therapy initiation or change. In an analysis of patients with oligometastatic prostate cancer (1–3 lesions in bones or nodes) undergoing SAbR who suffered subsequent progression, Decaestecker et al noted that a majority of those relapses occurred within the same compartment (ie nodal or bone) and that 75% of these were limited to 3 or fewer sites.44 After accounting for second courses of SAbR or surgery in these cases, >50% of patients were able to defer initiation of androgen deprivation therapy for 2 years after detection of initial metastasis. Thus, the tropism of limited metastatic disease for initially involved site or involved organ system progression argues for use of SAbR along with systemic therapy.
Impact of therapeutic resistant clones
Second, disease progression may occur heterogeneously as outgrowth of resistant subclones or amidst otherwise good response to systemic therapy. In either case, time to therapy may be disproportionately affected by a minority subpopulation of metastatic lesions, which may be thought of as “oligoprogressive” and which may yet be salvaged with local therapy, without an obligate change in systemic therapy. This has been classically demonstrated by instances of poor central nervous system (CNS) disease control despite good extracranial disease response to systemic therapies, owing to blood–brain barrier penetration issues, especially for certain targeted agents.
One retrospective, multi-institution study demonstrated prolonged survival in patients with ALK-rearranged, crizotinib-treated metastatic NSCLC and brain metastases mostly treated with local therapies including resection, whole-brain RT and/or SRS.45 Notably, 45% of patients experienced CNS progression at the time of death, with repeated RT interventions being common and extracranial systemic control as an important prognosticator of better outcome. By focusing on these best-performing “outliers”, the study demonstrated that control of disease that had escaped otherwise effective systemic therapy, by virtue of spread to CNS sanctuary site, was critical to overall outcome. In the extracranial setting, autopsy studies have demonstrated the ability of cross-seeding by metastases to other metastases, providing mechanistic basis for the ability of resistant or escaped tumour clones to cause treatment failure.46
At a molecular level, various mechanisms of resistance to both targeted and cytotoxic therapies have been uncovered, such as with mutations in EGFR abrogating response to in targeted inhibitors in NSCLC and androgen receptor (AR) variants causing resistance to antiandrogens in prostate cancer. While biopsy confirmation of these alterations is limited in practice, advanced imaging modalities may identify these events, before dissemination of the newly refractory disease. A clear-cut resistance mechanism cannot be identified, and/or a salvage systemic therapy may not exist, in most patients treated with chemotherapy. SAbR to such lesions offers the ability to capitalize on the ability of increasingly sensitive imaging modalities, such as positron emission tomography (PET) using prostate specific membrane antigen (PSMA) targeted tracer in prostate cancer, to detect low burdens of progressive metastatic disease in this manner.
Low-toxicity alternative therapy
Third, chemotherapy beyond the first-line regimen typically experiences a sharp drop-off in efficacy and tolerability. There is a need to develop low-toxicity alternative strategies which may include local therapy. This is demonstrated by the limited improvements in second-line cytotoxic therapy in colorectal cancer over the past 20 years, compared with supportive care and selective hepatic metastectomy,40 and the poor approximately 10% overall response rate for second-line regimens in metastatic bladder cancer, where up to 50% of patients are medically ineligible for first-line platinum. This need has set the regulatory approvals bar low for benefit from second-line therapy, as exemplified by recent approvals of high-cost immunotherapies based on efficacy in small minorities of patients.47
Local therapy in selected extracranial oligometastatic state surpasses these thresholds of response and yet remains tolerable beyond the first line of therapy. Early registry studies from the 1980–90s, for instance, suggested that resection of liver deposits from colorectal cancer produced 5-year survival rates of approximately 30%48 and that resection of pulmonary metastectomy in sarcoma and epithelial malignancies achieved 5-year overall survival of 36%.7 Since then, surgical resection of limited metastatic disease has been associated with favourable outcomes in multiple series of metastectomy of various histologies involving pulmonary,49,50 adrenal,33,51 ovarian52,53 and other organ sites.54,55
Despite these results and advances in minimally invasive surgical techniques, surgery entails operative risks and recovery periods that may interrupt systemic therapy for prolonged intervals. Further, not all patients are suitable for surgery based on disease location, number of sites of disease, prior surgeries/RT and/or performance status. Alternatives to surgery for local therapy include ablative techniques, such as embolization/chemoembolization, radiofrequency ablation (RFA) and RT. Each has its pros and cons based on disease- and patient-related factors. In the case of RT, conventional fractionation has a proven role in palliation of disease-related symptoms across a variety of histologies and sites. However, the doses needed for ablation and resultant protracted course of treatment may make it impractical owing to interruptions in systemic therapy or quality of life for the patient. SAbR offers the potential to deliver ablative doses in rapid schedules to even sensitive areas near critical organs and prior RT fields (Figure 1), while maintaining a non-invasive treatment technique that balances efficacy, logistics and toxicity.
Potential synergy with immunotherapy
Lastly, RT is postulated to exert immunomodulatory effects that may enhance the efficacy of novel immunotherapies or expand the population of patients sensitive to those agents. Many have further argued that ablative doses of SAbR are needed to produce these effects,14,15,56 and this combination is not restricted to the setting of oligometastases. A full discussion of this potential role is beyond the scope of this review, with a growing literature summarized elsewhere.57,58
PERFORMANCE AND SAFETY OF SAbR IN LOCAL CONTROL
A growing volume of retrospective and single-arm prospective trial data describes the efficacy and tolerability of SAbR for oligometastases in various disease histologies and anatomic sites. Analogous to the establishment of surgical resection for oligometastases, much of the earliest data for SAbR for low volume or oligometastatic disease come from the treatment of colorectal and pulmonary metastases, as reviewed previously by several authors.58–60 While these series included heterogeneous sets of patients, the local control rates for treated lesions in the lung and/or liver were generally >70% and up to 90%, with low rates of severe toxicity <5%.39,61,62 More specific SAbR series, delineated by disease site and histology, in recent years are detailed in this section. Select series are tabulated in Table 2.
Table 2.
Outcomes for SAbR Treatment for varied metastatic sites
| Study | Site | Patients (N) | Dose | Local control | Survival | Toxicity |
|---|---|---|---|---|---|---|
| Rusthoven et al64 | Lung | 38 with 63 lesions | 48–60 Gy in 3 Fx | 2 years = 96% | Median = 19 months | Grade 3 = 7.9% |
| Rusthoven et al30 | Liver | 47 with 63 lesions | 36–60 Gy in 3 Fx | 2 years = 92% | Median = 20.5 months | Grade 3 = 1 |
| Zelefsky et al31 | Spine | 45 | 24 Gy in 1 Fx | 3 years = 88% | NR | Grade 4 erythema = 1; 2 fractures |
| Holy et al35 | Adrenal | 18 | 20–40 Gy in 5 Fx | 2 years = 77% | Median = 23 months | Grade 1 nausea = 6 |
Fx, fraction; N, patient number enrolled; NR, not reported.
SAbR for pulmonary and hepatic metastases
A notable pair of prospective Phase I/II multicentre trials evaluated pulmonary and hepatic sites individually,29,63 contributing important information on efficacy, feasibility of prospective trial enrolment and dose constraints/pre-therapy parameters for safe treatment. In the pulmonary setting, patients with 1–3 lung metastases of solid tumour origin cumulatively <7 cm and with adequate pulmonary function (forced expiratory volume in 1 second FEV1 >1.0L, diffusing capacity of lungs for carbon monoxide DLCO >40%) were treated according to dose escalation design from 48 to 60 Gy in 3 fractions, which produced a 2-year local control of 96% and median overall survival 19 months, with 7.9% grade 3 toxicity (no grade 4 or 5). Similarly, patients with 1–3 liver metastases of cumulative diameter <6 cm underwent dose escalation from 36–60 Gy in 3 fractions, while preserving a volume of at least 700 cm3 receiving <15 Gy, producing a 2-year local control of 92%, median overall survival 20.5 months and a single grade 3 toxicity (soft tissue) without any instances of radiation-induced liver dysfunction.
SAbR for spine metastases
SAbR for metastatic disease to the spine has received much study outside the oligometastatic setting, owing often to its symptomatic presentation in terms of pain and/or neurologic compromise. While pain relief is readily achieved with conventionally fractionated RT, local control rates were as poor as 50% or lower.64 Further, the same noted study demonstrated that these local failures in the spine following surgery and conventionally fractionated RT resulted in increasing actuarial rates of neurologic compromise between 1 year and 4 years after treatment. Thus, patients with oligometastasis in whom extended survival is anticipated may have curtailed survival and disease control owing to limited progression in the spine, similar to the previously discussed scenario of those with CNS disease. Retrospective and Phase I/II data at multiple institutions have suggested improved local control of spine metastases with dose escalation, particularly through hypofractionation.30,65–67
SAbR offers the technical ability to deliver ablative regimens upwards of 20 Gy in a single fraction,68 utilizing a variety of techniques and dose constraints on the spinal cord or thecal sac.69–71 High-grade toxicity is relatively rare, with myelopathy incidence <1%72 and compression fractures in approximately 11% of patients.73 The data for SAbR in controlling spine metastases from traditionally “radioresistant” histologies, such as renal cell carcinoma or sarcoma, are particularly relevant to its role in the oligometastatic setting, when comparing SAbR with other potential local therapies. In one series from Memorial Sloan Kettering Cancer Center on patients with metastatic renal cell carcinoma, 59 lesions in the spine were treated with SAbR, with a 3-year cumulative incidence of local recurrence of only 12% in patients treated to 24 Gy in a single fraction, as opposed to decidedly higher recurrence rates in those treated with 3–5-fraction SAbR courses.30 The same group also demonstrated a 1-year cumulative incidence of local recurrence of 12% in single-fraction 24-Gy SAbR for spine lesions in 88 patients with sarcoma, as compared with lower control with 3–6-fraction SAbR courses.67
SAbR for adrenal metastases
Treatment of NSCLC metastases to the adrenal glands with SAbR, while initially felt to represent a poor prognosis metastasis site, has been associated with encouraging 1-year local control of 87–100% in more recent series,34,74–76 when treated with 36–45 Gy in 3–5 fractions. In the Holy et al series of SAbR-treated adrenal oligometastases,34 often cited for its encouraging outcomes, a median overall survival of 23 months was achieved, comparable with adrenalectomy series.
SAbR series to other or mixed extracranial site metastases
In comparison with lung, liver, adrenal or spine metastases, data for SAbR in extracranial oligometastatic disease at other organ sites are predominantly drawn from studies evaluating mixed sites of metastatic involvement. This deviates from the historical standard set by surgical series of reporting organ-specific results. However, most primary tumours in fact spread to multiple organs in their natural history, making single-organ reporting seem forced. At any rate, these critically describe the feasibility issues of dose selection, normal tissue constraints and patient recruitment that must be reconciled in comprehensive SAbR approaches to oligometastases ablation.
In a study of mixed-site oligometastases in 121 patients with up to 5 sites of disease of any histology, Milano et al19 treated all sites of disease with SAbR with a preferred dose of 50 Gy in 10 fractions, achieving 2-year 74% local control and median overall survival of approximately 18 months, even when restricting analysis to non-breast histology.18 With only one patient suffering grade 3 toxicity, this study provided early proof of the safety of multisite “comprehensive” ablation of multiple oligometastatic sites. In a similar broad inclusion University of Chicago prospective dose escalation study, in patients with 1–5 oligometastases of any histology treated with SAbR from 24 to 42 Gy in 3 fractions, a median PFS of 5.1 months and median survival of >2 years was achieved, at the cost of 2 acute and 7 chronic grade 3 toxicities.27 While overall 2-year local control was low (53%) compared with other studies, the subgroup of patients treated at higher dose cohorts neared 2-year local control of 90%. In a Japanese study38 combining SRS and SAbR for brain and extracranial oligometastases, respectively, up to 5 total sites (brain, lungs or adrenals) utilized site-specific dose regimens (single or 4-fraction SRS, 48 Gy in 8 fractions for adrenal gland SAbR, 35–60 Gy in 4–8 fractions for lung lesions). The investigators reported 3-year local control of 80% and median survival of 24 months, remarkably without grade 3 or higher toxicities. Multiple further groups have reported on SAbR for mixed sites of involvement by oligometastases, as previously reviewed.59
Effect of histology on response to SAbR
A number of series have described outcomes for SAbR in oligometastatic disease by specific disease histology. At our institution, in a Phase II study of NSCLC patients with <6 extracranial metastases who had progressed on first-line chemotherapy, patients underwent SAbR to all sites in 1–5 fractions along with the EGFR inhibitor erlotinib.77 The median PFS and overall survival of 14.7 and 20.4 months, respectively, were especially impressive considering there was no pre-screening for activating EGFR mutations or discovery of such mutations on post hoc analysis of available tissues. Relapse patterns favoured new sites of distant failure, as compared with the typically original site progression that occurs after systemic therapy alone. More recently, Gomez et al78 reported a Phase II randomized controlled trial comparing consolidative local therapy with or without maintenance chemotherapy with maintenance therapy (which could include observation) alone in patients with Stage IV NSCLC with three or fewer metastases. Local therapy consolidation, which could include SAbR, surgery and conventional or moderately hypofractionated RT, was associated with a significant PFS benefit vs maintenance therapy/observation alone (11.9 vs 3.9 months). Our institution completed a similarly designed randomized Phase II study (unpublished data, article in submission), which focused exclusively on use of RT as the local therapy. In this study, patients with Stage IV NSCLC with six or fewer extracranial metastases who had partial response or stable disease after induction chemotherapy were randomized to consolidative RT (typically SAbR to metastasis and moderately hypofractionated RT to the primary site) or standard systemic therapy. Those receiving RT again achieved substantial and statistically significant PFS benefit of 9.7 vs 3.5 months, and those not receiving RT had a predominantly local pattern of progression.
In prostate cancer, Muacevic et al demonstrated an in-field 2-year local control of over 95% without grade 3 or higher toxicity with SAbR of 20 Gy in 1 fraction to bone metastases in 40 patients with 1–2 lesions by positron emission tomography-CT choline staging.79 More recently, a multi-institution group evaluated outcomes after SAbR to 163 sites in 119 patients who were treatment naïve.80 At median follow-up of 3 years, the median PFS was 21 months, with a majority (70%) of progression events occurring at three or fewer sites, allowing for second local therapy salvage in 51% of cases.
In uterine cervical cancer, the Korean Radiation Oncology Group 14-11 trial demonstrated a 2-year local control of 82.5%, 5-year overall survival 32.9% and 5.8% chronic grade ≥3 toxicity, despite treatment in >50% sites occurring within a prior RT field.81 While local control was significantly worse in retreatment sites, the outcomes altogether were comparable with treatment with systemic therapy.
Overall, the experience with SAbR in oligometastases has been rapidly expanding through primarily Phase I/II prospective and retrospective observation reports. These have demonstrated promising improvements in local control over what would be anticipated from conventionally fractionated RT, with more convenience, feasible integration with systemic and other therapies, and low grade 3 or higher toxicities. In particular, the multisite, multiorgan experiences with SAbR and the potential to substantially prolong disease control, while minimizing time-off systemic therapy, compares favourably with surgical resection.
TRIAL DESIGN CONCEPTS IN SAbR FOR OLIGOMETASTASIS
To date, no randomized data evaluating the overall survival benefit of SAbR for oligometastasis have been completed and reported. As a function of the need to describe preliminary outcomes and safety, the experience of SAbR has been a patchwork of retrospective and prospective single-arm studies evaluating the modality in different scenarios. These experiences have been complemented by continuing study of other local therapies in the oligometastatic setting and better delineation of outcomes for patients with low-burden, good prognosis metastasis in systemic therapy trials on a disease-by-disease basis. Together, these efforts are helping to drive the design of the first oligometastatic randomized trials for SAbR. Several issues arise in the design of such trials, with the need to address obstacles to accrual in this space as important as ensuring scientific rigour. Table 3 summarizes these.
Table 3.
Practical issues affecting design of trials for assessing SAbR in oligometastatic disease
| Trial design challenges |
|---|
| Defining oligometastatic cohort with relatively similar prognosis for study screening |
| Maintaining safety as a primary focus |
| Prescribing allowable therapies that allow reproducibility in multi-institution setting |
| Picking achievable clinical end points or surrogate measures that assess meaningful clinical benefit |
| Collaborative efforts often needed to synthesize technical approaches and attain sufficient power |
| Setting control cohorts to fairly assess efficacy of intervention and its costs |
Defining population
Firstly, identification of a relatively homogeneous cohort for study by pinning down the definition of oligometastatic criteria for a particular disease or treatment site is necessary. As noted in this review, several prognostic factors have been observed to affect the outcome of patients with low-burden metastasis, including number of lesions, histology, actionable alterations, performance status and time to development of metastatic disease in relation to diagnosis of the primary disease. The execution of the local therapy also impacts selection, as invasive metastectomy becomes less flexible with more sites to address. The same may not be true for less invasive local treatments. Investigators proposing oligometastatic trials are tasked with applying existing data to select patients with distinct but reasonably similar expected natural histories, while still maintaining sufficient flexibility to achieve study accrual. It is likely that many questions regarding SAbR for an oligometastatic indication will require multi-institution collaborations to do so, given the rarity of the state, making successful collaboration crucial.
Defining therapeutic intervention
Similarly, delineation of allowable local therapy modalities and frequency of interventions for oligometastatic disease is important to making a study completion practical and its data interpretable. Where multiple options exist for treatment of oligometastasis, such as resection, RFA or various SAbR fractionation schemes, there exist institution-specific and patient preference-related variations in local therapy execution. In cases where toxicity or efficacy is not demonstrably different, these variations may not affect the principle question regarding the impact of local therapy on the natural history of disease. Treating local therapy in such cases as a “black box” has precedent in various fractionation schemes allowed for multi-institution randomized trials. Further, as demonstrated in the discussed studies,44,80 subsequent relapses after SAbR may yet remain amenable to further local therapy, thereby allowing deferment of systemic therapy and its quality of life impacts. If the goal of local therapy intervention in oligometastasis is to extend survival, extend efficacy of a systemic therapy or to defer systemic therapy altogether, then the intervention does not necessarily need to be a one-time event. Indeed, in studies of novel agents in metastatic disease, time to therapy is often used as a surrogate of efficacy, as well as tolerability.
Picking end points and controls
The selection of appropriate end points and control cohort is important in discerning whether oligometastatic disease classification is simply prognostic (good outcome regardless of intervention) or predictive of an even better outcome with the prescribed local intervention. It further follows that the natural history of such patients be precisely known in order to estimate accrual requirements.82
An example of the control arm's significance is demonstrated by the European Organisation for Research and Treatment of Cancer 40004 Phase II randomized trial of systemic treatment or systemic treatment with RFA for unresectable colorectal liver metastases, where 3-year PFS was more than doubled with RFA (27.6% vs 10.6%; p = 0.025), while an overall survival benefit was not detected. This largely was due to much better than expected 30-month overall survival in both arms (61.7% vs 57.6%; not significant), as compared with the primary predicted objective survival of >38%.83 Whether time will reveal a difference in survival in this now effectively underpowered trial as a result of the PFS benefit is unclear. In this setting, absent a clear benchmark of expected outcome for the oligometastatic group under study, the inclusion of such an observation or standard treatment control arm is important. As this experience demonstrated, it is as important to know the outcomes of untreated/control patients as it is to include a control arm.
Similarly, the studied end point of SAbR intervention in oligometastasis should reflect its goal in the disease context. While the “gold standard” of overall survival or PFS is reasonable where disease control is the imperative, in other cases where deferment of systemic therapy continuation or resistance is the goal, quality of life end points are equally important. For instance, in the setting of castrate-sensitive metastatic prostate cancer, survival end points may require years and large accrual numbers, while the very real quality of life benefits of remaining off androgen deprivation may be observed earlier. Moreover, biomarker surrogates of disease control may also need to be adapted to the disease state, as observed in metastatic prostate cancer treated with radium-223, where the ALSYMPCA trial demonstrated the superiority of alkaline phosphatase and lactate dehydrogenase over prostate-specific antigen.84
CONCLUSION
Improvements in defining the outlier “oligometastatic” state of advanced solid tumours have coincided with the rise of innovative local therapies attempting to capitalize on the potential of improving outcomes and quality of life in such patients. A wealth of primarily retrospective and Phase I/II single-arm prospective experiences have demonstrated excellent tolerability and local control with SAbR.
Its efficacy in comparison with other local modalities including metastectomy and the overall impact of local therapy on survival in metastatic disease remain unclear in the absence of randomized data. However, the addition of this non-invasive and potentially immunogenic alternative local treatment option has potentiated broader application and study of desperately needed new strategies in this space.
Contributor Information
Neil B Desai, Email: Neil.Desai@UTSouthwestern.edu.
Aaron M Laine, Email: aaron.laine@utsouthwestern.edu.
Robert D Timmerman, Email: Robert.Timmerman@UTSouthwestern.edu.
REFERENCES
- 1.Nelson HD, Tyne K, Naik A, Bougatsos C, Chan BK, Humphrey L, et al. ; U.S. Preventive Services Task Force. Screening for breast cancer: an update for the U.S. Preventive Services Task Force. Ann Intern Med 2009; 151: 727–37, W237–42. doi: https://doi.org/10.7326/0003-4819-151-10-200911170-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists' Collaborative Group (EBCTCG); Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011; 378: 1707–16. doi: https://doi.org/10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative Group (EBCTCG); Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet 2012; 379: 432–44. doi: https://doi.org/10.1016/S0140-6736(11)61625-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.EBCTCG (Early Breast Cancer Trialists' Collaborative Group); McGale P, Taylor C, Correa C, Cutter D, Duane F, Ewertz M, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014; 383: 2127–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Einhorn LH. Treatment of testicular cancer: a new and improved model. J Clin Oncol 1990; 8: 1777–81. doi: https://doi.org/10.1200/jco.1990.8.11.1777 [DOI] [PubMed] [Google Scholar]
- 6.Aloia TA, Vauthey JN, Loyer EM, Ribero D, Pawlik TM, Wei SH, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006; 141: 460–6; discussion 6–7. doi: https://doi.org/10.1001/archsurg.141.5.460 [DOI] [PubMed] [Google Scholar]
- 7.Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. ; International Registry of Lung Metastases. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997; 113: 37–49. doi: https://doi.org/10.1016/S0022-5223(97)70397-0 [DOI] [PubMed] [Google Scholar]
- 8.Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev 2013; 2: 35. doi: https://doi.org/10.1186/2046-4053-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei AC, Greig PD, Grant D, Taylor B, Langer B, Gallinger S. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006; 13: 668–76. doi: https://doi.org/10.1245/ASO.2006.05.039 [DOI] [PubMed] [Google Scholar]
- 10.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995; 13: 8–10. doi: https://doi.org/10.1200/jco.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 11.Timmerman RD, Kavanagh BD, Cho LC, Papiez L, Xing L. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol 2007; 25: 947–52. doi: https://doi.org/10.1200/JCO.2006.09.7469 [DOI] [PubMed] [Google Scholar]
- 12.Mehta N, King CR, Agazaryan N, Steinberg M, Hua A, Lee P. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: a pooled analysis of biological equivalent dose and local control. Pract Radiat Oncol 2012; 2: 288–95. doi: https://doi.org/10.1016/j.prro.2011.10.004 [DOI] [PubMed] [Google Scholar]
- 13.Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010; 303: 1070–6. doi: https://doi.org/10.1001/jama.2010.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell 2005; 8: 89–91. doi: https://doi.org/10.1016/j.ccr.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003; 300: 1155–9. [DOI] [PubMed] [Google Scholar]
- 16.Palma DA, Louie AV, Rodrigues GB. New strategies in stereotactic radiotherapy for oligometastases. Clin Cancer Res 2015; 21: 5198–204. doi: https://doi.org/10.1158/1078-0432.CCR-15-0822 [DOI] [PubMed] [Google Scholar]
- 17.Sun M, Shariat SF, Cheng C, Ficarra V, Murai M, Oudard S, et al. Prognostic factors and predictive models in renal cell carcinoma: a contemporary review. Eur Urol 2011; 60: 644–61. doi: https://doi.org/10.1016/j.eururo.2011.06.041 [DOI] [PubMed] [Google Scholar]
- 18.Milano MT, Katz AW, Zhang H, Okunieff P. Oligometastases treated with stereotactic body radiotherapy: long-term follow-up of prospective study. Int J Radiat Oncol Biol Phys 2012; 83: 878–86. doi: https://doi.org/10.1016/j.ijrobp.2011.08.036 [DOI] [PubMed] [Google Scholar]
- 19.Takeda A, Kunieda E, Ohashi T, Aoki Y, Koike N, Takeda T. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011; 101: 255–9. doi: https://doi.org/10.1016/j.radonc.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 20.Yamamoto T, Jingu K, Shirata Y, Koto M, Matsushita H, Sugawara T, et al. Outcomes after stereotactic body radiotherapy for lung tumors, with emphasis on comparison of primary lung cancer and metastatic lung tumors. BMC Cancer 2014; 14: 464. doi: https://doi.org/10.1186/1471-2407-14-464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoki M, Hatayama Y, Kawaguchi H, Hirose K, Sato M, Akimoto H, et al. Clinical outcome of stereotactic body radiotherapy for primary and oligometastatic lung tumors: a single institutional study with almost uniform dose with different five treatment schedules. Radiat Oncol 2016; 11: 5. doi: https://doi.org/10.1186/s13014-016-0581-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippi AR, Badellino S, Ceccarelli M, Guarneri A, Franco P, Monagheddu C, et al. Stereotactic ablative radiation therapy as first local therapy for lung oligometastases from colorectal cancer: a single-institution cohort study. Int J Radiat Oncol Biol Phys 2015; 91: 524–9. doi: https://doi.org/10.1016/j.ijrobp.2014.10.046 [DOI] [PubMed] [Google Scholar]
- 23.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004; 363: 1665–72. doi: https://doi.org/10.1016/S0140-6736(04)16250-8 [DOI] [PubMed] [Google Scholar]
- 24.Bignardi M, Navarria P, Mancosu P, Cozzi L, Fogliata A, Tozzi A, et al. Clinical outcome of hypofractionated stereotactic radiotherapy for abdominal lymph node metastases. Int J Radiat Oncol Biol Phys 2011; 81: 831–8. doi: https://doi.org/10.1016/j.ijrobp.2010.05.032 [DOI] [PubMed] [Google Scholar]
- 25.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol 1991; 9: 1618–26. doi: https://doi.org/10.1200/jco.1991.9.9.1618 [DOI] [PubMed] [Google Scholar]
- 26.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Prognostic factors in patients with advanced non-small cell lung cancer: data from the phase III FLEX study. Lung Cancer 2012; 77: 376–82. doi: https://doi.org/10.1016/j.lungcan.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 27.Salama JK, Hasselle MD, Chmura SJ, Malik R, Mehta N, Yenice KM, et al. Stereotactic body radiotherapy for multisite extracranial oligometastases: final report of a dose escalation trial in patients with 1 to 5 sites of metastatic disease. Cancer 2012; 118: 2962–70. doi: https://doi.org/10.1002/cncr.26611 [DOI] [PubMed] [Google Scholar]
- 28.Wersäll PJ, Blomgren H, Lax I, Kälkner KM, Linder C, Lundell G, et al. Extracranial stereotactic radiotherapy for primary and metastatic renal cell carcinoma. Radiother Oncol 2005; 77: 88–95. [DOI] [PubMed] [Google Scholar]
- 29.Rusthoven KE, Kavanagh BD, Cardenes H, Stieber VW, Burri SH, Feigenberg SJ, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009; 27: 1572–8. doi: https://doi.org/10.1200/jco.2008.19.6329 [DOI] [PubMed] [Google Scholar]
- 30.Zelefsky MJ, Greco C, Motzer R, Magsanoc JM, Pei X, Lovelock M, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys 2012; 82: 1744–8. doi: https://doi.org/10.1016/j.ijrobp.2011.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milano MT, Katz AW, Okunieff P. Patterns of recurrence after curative-intent radiation for oligometastases confined to one organ. Am J Clin Oncol 2010; 33: 157–63. doi: https://doi.org/10.1097/coc.0b013e3181979238 [DOI] [PubMed] [Google Scholar]
- 32.Milano MT, Katz AW, Muhs AG, Philip A, Buchholz DJ, Schell MC, et al. A prospective pilot study of curative-intent stereotactic body radiation therapy in patients with 5 or fewer oligometastatic lesions. Cancer 2008; 112: 650–8. doi: https://doi.org/10.1002/cncr.23209 [DOI] [PubMed] [Google Scholar]
- 33.Strong VE, D'Angelica M, Tang L, Prete F, Gonen M, Coit D, et al. Laparoscopic adrenalectomy for isolated adrenal metastasis. Ann Surg Oncol 2007; 14: 3392–400. doi: https://doi.org/10.1245/s10434-007-9520-7 [DOI] [PubMed] [Google Scholar]
- 34.Holy R, Piroth M, Pinkawa M, Eble MJ. Stereotactic body radiation therapy (SBRT) for treatment of adrenal gland metastases from non-small cell lung cancer. Strahlenther Onkol 2011; 187: 245–51. doi: https://doi.org/10.1007/s00066-011-2192-z [DOI] [PubMed] [Google Scholar]
- 35.Papandreou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, et al. Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol 2002; 20: 3072–80. doi: https://doi.org/10.1200/jco.2002.12.065 [DOI] [PubMed] [Google Scholar]
- 36.Zumsteg ZS, Spratt DE, Romesser PB, Pei X, Zhang Z, Kollmeier M, et al. Anatomical patterns of recurrence following biochemical relapse in the dose escalation era of external beam radiotherapy for prostate cancer. J Urol 2015; 194: 1624–30. doi: https://doi.org/10.1016/j.juro.2015.06.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashworth AB, Senan S, Palma DA, Riquet M, Ahn YC, Ricardi U, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014; 15: 346–55. doi: https://doi.org/10.1016/j.cllc.2014.04.003 [DOI] [PubMed] [Google Scholar]
- 38.Inoue T, Katoh N, Aoyama H, Onimaru R, Taguchi H, Onodera S, et al. Clinical outcomes of stereotactic brain and/or body radiotherapy for patients with oligometastatic lesions. Jpn J Clin Oncol 2010; 40: 788–94. doi: https://doi.org/10.1093/jjco/hyq044 [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Xiao JP, Zhang HZ, Yin WB, Hu YM, Song YX, et al. Stereotactic body radiation therapy favors long-term overall survival in patients with lung metastases: five-year experience of a single-institution. Chin Med J (Engl) 2011; 124: 4132–7. [PubMed] [Google Scholar]
- 40.Jawed I, Wilkerson J, Prasad V, Duffy AG, Fojo T. Colorectal cancer survival gains and novel treatment regimens: a systematic review and analysis. JAMA Oncol 2015; 1: 787–95. doi: https://doi.org/10.1001/jamaoncol.2015.1790 [DOI] [PubMed] [Google Scholar]
- 41.Jain RK. Physiological barriers to delivery of monoclonal antibodies and other macromolecules in tumors. Cancer Res 1990; 50: 814s–9s. [PubMed] [Google Scholar]
- 42.Simon R, Norton L. The Norton-Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Clin Pract Oncol 2006; 3: 406–7. doi: https://doi.org/10.1038/ncponc0560 [DOI] [PubMed] [Google Scholar]
- 43.Rusthoven KE, Hammerman SF, Kavanagh BD, Birtwhistle MJ, Stares M, Camidge DR. Is there a role for consolidative stereotactic body radiation therapy following first-line systemic therapy for metastatic lung cancer? A patterns-of-failure analysis. Acta Oncol 2009; 48: 578–83. doi: https://doi.org/10.1080/02841860802662722 [DOI] [PubMed] [Google Scholar]
- 44.Decaestecker K, De Meerleer G, Lambert B, Delrue L, Fonteyne V, Claeys T, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014; 9: 135. doi: https://doi.org/10.1186/1748-717X-9-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johung KL, Yeh N, Desai NB, Williams TM, Lautenschlaeger T, Arvold ND, et al. Extended survival and prognostic factors for patients with ALK-rearranged non-small-cell lung cancer and brain metastasis. J Clin Oncol 2016; 34: 123–9. doi: https://doi.org/10.1200/JCO.2015.62.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015; 520: 353–7. doi: https://doi.org/10.1038/nature14347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014; 515: 558–62. doi: https://doi.org/10.1038/nature13904 [DOI] [PubMed] [Google Scholar]
- 48.Nordlinger B, Guiguet M, Vaillant JC, Balladur P, Boudjema K, Bachellier P, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer 1996; 77: 1254–62. doi: https://doi.org/10.1002/(sici)1097-0142(19960401)77:7<1254::aid-cncr5>3.0.co;2-i [PubMed] [Google Scholar]
- 49.Fletcher WS, Pommier RF, Lum S, Wilmarth TJ. Surgical treatment of metastatic melanoma. Am J Surg 1998; 175: 413–7. doi: https://doi.org/10.1016/S0002-9610(98)00041-5 [DOI] [PubMed] [Google Scholar]
- 50.Leo F, Cagini L, Rocmans P, Cappello M, Van Geel AN, Maggi G, et al. Lung metastases from melanoma: when is surgical treatment warranted? Br J Cancer 2000; 83: 569–72. doi: https://doi.org/10.1054/bjoc.2000.1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paul CA, Virgo KS, Wade TP, Audisio RA, Johnson FE. Adrenalectomy for isolated adrenal metastases from non-adrenal cancer. Int J Oncol 2000; 17: 181–7. doi: https://doi.org/10.3892/ijo.17.1.181 [PubMed] [Google Scholar]
- 52.Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol 2004; 94: 477–82. doi: https://doi.org/10.1016/j.ygyno.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 53.Jiang R, Tang J, Cheng X, Zang RY. Surgical treatment for patients with different origins of Krukenberg tumors: outcomes and prognostic factors. Eur J Surg Oncol 2009; 35: 92–7. doi: https://doi.org/10.1016/j.ejso.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 54.Kager L, Zoubek A, Potschger U, Kastner U, Flege S, Kempf-Bielack B, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol 2003; 21: 2011–8. doi: https://doi.org/10.1200/JCO.2003.08.132 [DOI] [PubMed] [Google Scholar]
- 55.Selzner M, Morse MA, Vredenburgh JJ, Meyers WC, Clavien PA. Liver metastases from breast cancer: long-term survival after curative resection. Surgery 2000; 127: 383–9. doi: https://doi.org/10.1067/msy.2000.103883 [DOI] [PubMed] [Google Scholar]
- 56.Perez CA, Fu A, Onishko H, Hallahan DE, Geng L. Radiation induces an antitumour immune response to mouse melanoma. Int J Radiat Biol 2009; 85: 1126–36. doi: https://doi.org/10.3109/09553000903242099 [DOI] [PubMed] [Google Scholar]
- 57.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015; 16: e498–509. doi: https://doi.org/10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 58.Timmerman RD, Bizekis CS, Pass HI, Fong Y, Dupuy DE, Dawson LA, et al. Local surgical, ablative, and radiation treatment of metastases. CA Cancer J Clin 2009; 59: 145–70. doi: https://doi.org/10.3322/caac.20013 [DOI] [PubMed] [Google Scholar]
- 59.Tree AC, Khoo VS, Eeles RA, Ahmed M, Dearnaley DP, Hawkins MA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013; 14: e28–37. doi: https://doi.org/10.1016/S1470-2045(12)70510-7 [DOI] [PubMed] [Google Scholar]
- 60.Westover KD, Iyengar P, Sharma AN, Timmerman R. SABR for aggressive local therapy of metastatic cancer: a new paradigm for metastatic non-small cell lung cancer. Lung Cancer 2015; 89: 87–93. doi: https://doi.org/10.1016/j.lungcan.2015.04.009 [DOI] [PubMed] [Google Scholar]
- 61.Dhakal S, Corbin KS, Milano MT, Philip A, Sahasrabudhe D, Jones C, et al. Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys 2012; 82: 940–5. doi: https://doi.org/10.1016/j.ijrobp.2010.11.052 [DOI] [PubMed] [Google Scholar]
- 62.Ricardi U, Badellino S, Filippi AR. Stereotactic body radiotherapy for early stage lung cancer: history and updated role. Lung Cancer 2015; 90: 388–96. doi: https://doi.org/10.1016/j.lungcan.2015.10.016 [DOI] [PubMed] [Google Scholar]
- 63.Rusthoven KE, Kavanagh BD, Burri SH, Chen C, Cardenes H, Chidel MA, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009; 27: 1579–84. doi: https://doi.org/10.1200/jco.2008.19.6386 [DOI] [PubMed] [Google Scholar]
- 64.Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien) 1998; 140: 957–67. doi: https://doi.org/10.1007/s007010050199 [DOI] [PubMed] [Google Scholar]
- 65.Damast S, Wright J, Bilsky M, Hsu M, Zhang Z, Lovelock M, et al. Impact of dose on local failure rates after image-guided reirradiation of recurrent paraspinal metastases. Int J Radiat Oncol Biol Phys 2011; 81: 819–26. doi: https://doi.org/10.1016/j.ijrobp.2010.06.013 [DOI] [PubMed] [Google Scholar]
- 66.Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976) 2009; 34: S78–92. doi: https://doi.org/10.1097/brs.0b013e3181b8b6f5 [DOI] [PubMed] [Google Scholar]
- 67.Folkert MR, Bilsky MH, Tom AK, Oh JH, Alektiar KM, Laufer I, et al. Outcomes and toxicity for hypofractionated and single-fraction image-guided stereotactic radiosurgery for sarcomas metastasizing to the spine. Int J Radiat Oncol Biol Phys 2014; 88: 1085–91. doi: https://doi.org/10.1016/j.ijrobp.2013.12.042 [DOI] [PubMed] [Google Scholar]
- 68.Yamada Y, Bilsky MH, Lovelock DM, Venkatraman ES, Toner S, Johnson J, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 2008; 71: 484–90. doi: https://doi.org/10.1016/j.ijrobp.2007.11.046 [DOI] [PubMed] [Google Scholar]
- 69.Lovelock DM, Hua C, Wang P, Hunt M, Fournier-Bidoz N, Yenice K, et al. Accurate setup of paraspinal patients using a noninvasive patient immobilization cradle and portal imaging. Med Phys 2005; 32: 2606–14. doi: https://doi.org/10.1118/1.1951042 [DOI] [PubMed] [Google Scholar]
- 70.Yamada Y, Lovelock DM, Yenice KM, Bilsky MH, Hunt MA, Zatcky J, et al. Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: a preliminary report. Int J Radiat Oncol Biol Phys 2005; 62: 53–61. doi: https://doi.org/10.1016/j.ijrobp.2004.09.006 [DOI] [PubMed] [Google Scholar]
- 71.Sahgal A, Bilsky M, Chang EL, Ma L, Yamada Y, Rhines LD, et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine 2011; 14: 151–66. doi: https://doi.org/10.3171/2010.9.SPINE091005 [DOI] [PubMed] [Google Scholar]
- 72.Gibbs IC, Patil C, Gerszten PC, Adler JR, Jr, Burton SA. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery 2009; 64(Suppl. 2): A67–72. doi: https://doi.org/10.1227/01.neu.0000341628.98141.b6 [DOI] [PubMed] [Google Scholar]
- 73.Cunha MV, Al-Omair A, Atenafu EG, Masucci GL, Letourneau D, Korol R, et al. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int J Radiat Oncol Biol Phys 2012; 84: e343–9. doi: https://doi.org/10.1016/j.ijrobp.2012.04.034 [DOI] [PubMed] [Google Scholar]
- 74.Milgrom SA, Goodman KA. The role of radiation therapy in the management of adrenal carcinoma and adrenal metastases. J Surg Oncol 2012; 106: 647–50. doi: https://doi.org/10.1002/jso.23096 [DOI] [PubMed] [Google Scholar]
- 75.Barney BM, Olivier KR, Macdonald OK, Fong de Los Santos LE, Miller RC, Haddock MG. Clinical outcomes and dosimetric considerations using stereotactic body radiotherapy for abdominopelvic tumors. Am J Clin Oncol 2012; 35: 537–42. doi: https://doi.org/10.1097/coc.0b013e31821f876a [DOI] [PubMed] [Google Scholar]
- 76.Casamassima F, Livi L, Masciullo S, Menichelli C, Masi L, Meattini I, et al. Stereotactic radiotherapy for adrenal gland metastases: university of Florence experience. Int J Radiat Oncol Biol Phys 2012; 82: 919–23. doi: https://doi.org/10.1016/j.ijrobp.2010.11.060 [DOI] [PubMed] [Google Scholar]
- 77.Iyengar P, Kavanagh BD, Wardak Z, Smith I, Ahn C, Gerber DE, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014; 32: 3824–30. doi: https://doi.org/10.1200/JCO.2014.56.7412 [DOI] [PubMed] [Google Scholar]
- 78.Gomez DR, Blumenschein GR, Jr., Lee JJ, Hernandez M, Ye R, Camidge DR, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016; 17: 1672–82. doi: https://doi.org/10.1016/S1470-2045(16)30532-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Muacevic A, Kufeld M, Rist C, Wowra B, Stief C, Staehler M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol 2013; 31: 455–60. doi: https://doi.org/10.1016/j.urolonc.2011.02.023 [DOI] [PubMed] [Google Scholar]
- 80.Ost P, Jereczek-Fossa BA, As NV, Zilli T, Muacevic A, Olivier K, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol 2016; 69: 9–12. doi: https://doi.org/10.1016/j.eururo.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 81.Park HJ, Chang AR, Seo Y, Cho CK, Jang WI, Kim MS, et al. Stereotactic body radiotherapy for recurrent or oligometastatic uterine cervix cancer: a cooperative study of the korean radiation oncology group (KROG 14-11). Anticancer Res 2015; 35: 5103–10. [PubMed] [Google Scholar]
- 82.Aberg T, Malmberg KA, Nilsson B, Nou E. The effect of metastasectomy: fact or fiction? Ann Thorac Surg 1980; 30: 378–84. [DOI] [PubMed] [Google Scholar]
- 83.Ruers T, Punt C, Van Coevorden F, Pierie JP, Borel-Rinkes I, Ledermann JA, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann oncol 2012; 23: 2619–26. doi: https://doi.org/10.1093/annonc/mds053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoskin P, Sartor O, O'Sullivan JM, Johannessen DC, Helle SI, Logue J, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014; 15: 1397–406. doi: https://doi.org/10.1016/S1470-2045(14)70474-7 [DOI] [PubMed] [Google Scholar]