Abstract
Objective:
To survey the technology and practice of image-guided radiotherapy (IGRT) for prostate cancer in the UK.
Methods:
A pre-tested semi-structured online questionnaire was sent to National Health Service (NHS) and private radiotherapy providers in the UK between March and April 2014. The survey was carried out on the Opinio© online platform.
Results:
There was a high survey response rate of 83%. There is widespread use of intensity-modulated radiotherapy and advanced verification imaging modalities. Cone-beam CT (CBCT) is the main verification imaging modality in radical prostate radiotherapy, used in 66% of UK centres. Fiducial markers in combination with imaging were used in 30% of centres. Over half the centres used a daily imaging schedule, with a Day 1-3 frequency followed by weekly frequency used less commonly. 26% of centres used daily CBCT.
Conclusion:
There is widespread use of volumetric verification imaging with CBCT for prostate radiotherapy in the UK. There is no consensus on the optimal verification imaging schedule.
Advances in knowledge:
This survey provides an insight into contemporary UK practice of IGRT for prostate cancer, both in the NHS and private sector. It demonstrates the widespread use of CBCT imaging and highlights the need for further research to optimize the practice.
INTRODUCTION
Image-guided radiotherapy (IGRT) has become the standard of care for delivery of radiation treatment for prostate cancer. In its broadest definition, it encompasses a wide range of techniques ranging from simple visual checks to advanced techniques incorporating specialist imaging. The clinical benefit of using advanced IGRT in prostate cancer has been demonstrated in retrospective series which used fiducial marker placement. These have demonstrated improvements in biochemical control and reduction in urinary and gastrointestinal toxicity.1
In the UK, the need for advanced verification techniques was affirmed by the National Radiotherapy Action Group in 2007.2 They recommended that all new and replacement radiotherapy machines should have image-guided adaptive radiotherapy capability. The 2008 Royal College of Radiologists (RCR) report “On target: ensuring geometric accuracy in radiotherapy” provided guidelines on verification for tumour sites including the prostate.3 Further guidance on the use of IGRT was provided by the National Radiotherapy Implementation Group in 2012,4 which also provided dedicated clinical support for IGRT implementation.
A previous survey on the use of advanced radiotherapy technology in the UK was performed in 2008.5 It demonstrated limited availability of IGRT equipment, and this was the main reason cited by centres not carrying out advanced IGRT. Online megavoltage (MV) imaging was the main mode of prostate IGRT, with 50% of centres using this verification technique. The UK still lags behind many western European countries in the availability of IGRT equipment, according to the comprehensive European survey data published by the ESTRO-HERO project. This has shown that an average of 35% of MV machines used in the UK in 2010–11 were equipped for IGRT, with a wide variation between England, Scotland, Wales and Northern Ireland.6
The purpose of this survey was to evaluate the current status of prostate IGRT practice in the UK.
METHODS AND MATERIALS
59 National Health Service (NHS) radiotherapy centres were identified from the 2012 RCR Clinical Oncology UK Workforce Report.7 Five private radiotherapy provider networks were also contacted. Centres were invited to the survey individually by phone, and respondents were identified by discussion with radiotherapy service managers. The survey was circulated using Opinio software (ObjectPlanet Inc., Oslo, Norway), with online input of data. The survey was carried out from 1 March 2014 to 30 April 2014. An amendment to clarify one survey question was sent to all centres. Two reminders were sent to all centres which had not completed the survey.
The pre-tested semi-structured survey questionnaire tool had 23 questions on radical prostate radiotherapy using external beam treatment. Post-prostatectomy radiotherapy was not evaluated in this survey. Brachytherapy, either alone or in combination with external beam radiotherapy, was not evaluated in this survey. Survey questions covered details of patient preparation, use of fiducial markers, treatment planning system, radiotherapy dose/fractionation, type of verification imaging and correction strategies. Free text fields were also provided to capture additional data on variations in protocols. The survey tool is included in the Supplementary Material.
The following definitions were used in assessing verification strategies, in line with the 2008 RCR IGRT guideline:3
– Online treatment verification compared reference images with images taken in the treatment delivery room, immediately prior to the treatment being delivered, with any corrections applied before treatment delivery.
– Offline verification analyzed the setup verification at a time after the treatment had been given, with setup data acted upon at the next treatment.
– A gross error is an unacceptably large setup error that could underdose part of the clinical target volume or overdose an organ at risk.
– The systematic component of any error is a deviation that occurs in the same direction and is of a similar magnitude for each fraction throughout the treatment course.
– The random component of any error is a deviation that can vary in direction and magnitude for each delivered treatment fraction.
– Tolerance is the permitted range of setup error from the reference point.
– An action level sets minimum conditions, beyond which performance is deemed unacceptable.
RESULTS
50 NHS centres and 3 private radiotherapy providers responded, giving an overall response rate of 83%. The survey was completed by physicists, radiotherapy dosimetrists, radiotherapy superintendents and specialist radiographers.
Patient preparation
There was a wide variation in bowel preparation protocols for prostate radiotherapy between centres. Daily microenemas (44%) and dietary information (35%) were the most common strategies used. Some centres reported using microenemas daily for the first 9–15 fractions followed by laxatives only if required. 5 (9%) centres reported no fixed bowel preparation protocol.
The majority of centres had a bladder preparation protocol with the patient drinking a specified volume of water prior to treatment. The volume given ranged from 300 ml to 500 ml, followed by a 20–60-min interval before radiotherapy treatment. Two centres used an empty bladder protocol, with one of these treating patients in the prone position.
Fiducial markers
31 (58%) centres did not use fiducial markers for any patients. 11 (21%) centres used fiducial markers for all patients undergoing prostate radiotherapy and 11 (21%) centres used markers for patients who were selected. The availability of marker insertion slots was cited as a limiting factor. Fiducial markers were usually inserted by a consultant urologist, but clinical oncologists, specialist nurses or specialist radiographers performed the procedure in a number of centres (Table 1). The majority of centres used three markers, but one centre reported using two markers per patient. One centre also reported the use of prostate–rectum spacers in selected cases, to reduce dose to the rectum.
Table 1.
Prostate fiducial marker insertion operator
| Operator inserting fiducial markers | Number of centres |
|---|---|
| Urologist | 11 |
| Specialist nurse/radiographer | 7 |
| Oncologist | 6 |
| Radiologist | 4 |
Radiotherapy planning and delivery
There is widespread use of advanced planning and delivery techniques for prostate radiotherapy. 23 (43%) centres used intensity-modulated radiotherapy (IMRT) for all patients undergoing radical prostate radiotherapy, and a further 16 (30%) centres used IMRT for at least 50% of their patients with prostate cancer. A variety of advanced delivery systems were used (Table 2), with some centres using different modalities for specific patient groups having prostate cancer.
Table 2.
Radical prostate cancer radiotherapy—external beam treatment delivery systems
| Radiotherapy delivery system | Number of centres (%) |
|---|---|
| Volumetric modulated arc radiotherapy | 34 (64%) |
| Static beam IMRT | 31 (58%) |
| 3–4-field conformal radiotherapy | 29 (55%) |
| Tomotherapy IMRT | 5 (9%) |
| Cyberknife | 1 (2%) |
IMRT, intensity-modulated radiotherapy.
Dose fractionation regimens
The most commonly used external beam dose fractionation regimen was 74 Gy/37 fractions (Table 3). Dose escalation above an EQD2 of 74 Gy was carried out in only 6% of centres in this survey. Hypofractionated regimes were used in 21% of centres. Case selection criteria for dose fractionation regimes were not assessed in this survey.
Table 3.
Dose fractionation regimens for external beam treatment in radical prostate radiotherapy
| Dose fractionation regime | Number of centres (%) |
|---|---|
| 78 Gy/39 fractions | 2 (4%) |
| 74 Gy/37 fractions | 49 (92%) |
| 60 Gy/20 fractions | 8 (15%) |
| 57 Gy/19 fractions | 3 (6%) |
| 64 Gy/32 fractions | 2 (4%) |
Verification imaging
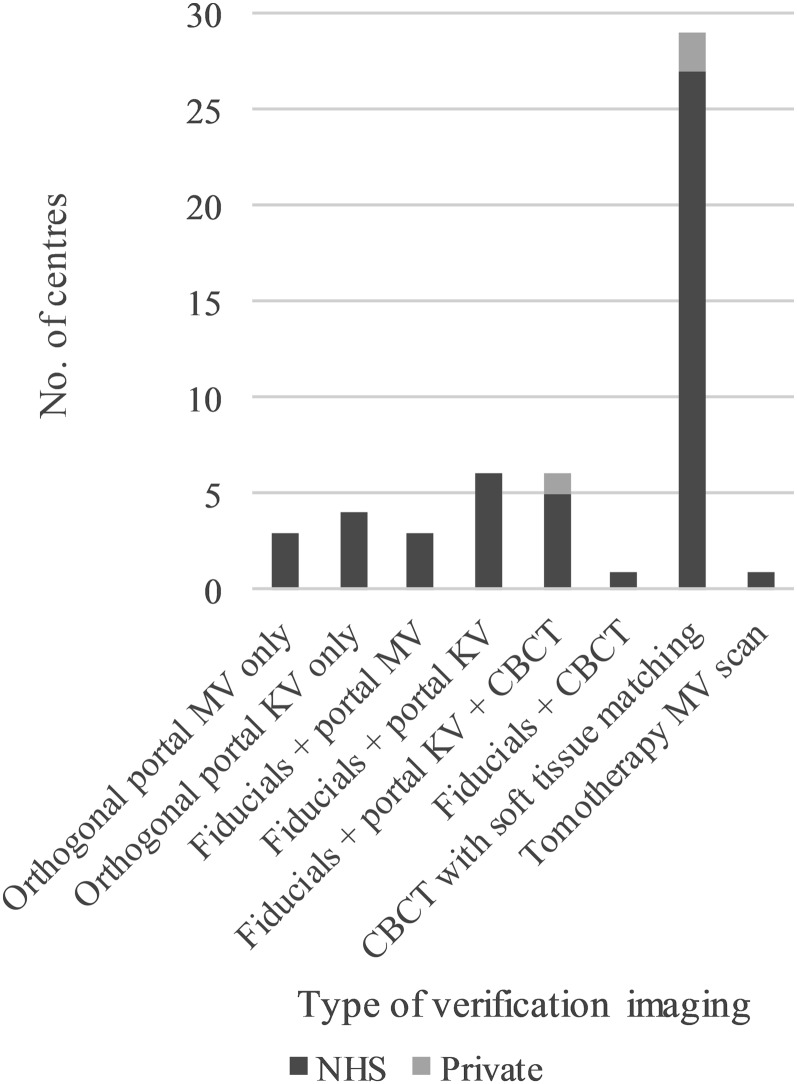
The main verification imaging type used in radical prostate radiotherapy was cone-beam CT (CBCT) with soft-tissue matching (Figure 1). In total, CBCT imaging was used in 35 (66%) centres. 16 (30%) centres used fiducial markers in combination with imaging. This was usually in conjunction with kilovoltage (kV) imaging. Seven centres used fiducial markers and CBCT. 7 (13%) centres used planar imaging only (4 portal kV and 3 portal MV). Only one centre specified tomotherapy MV scan as their main technique for patients with prostate cancer. None of the centres reported using ultrasound for routine verification imaging.
Figure 1.
The main type of verification imaging in radical prostate radiotherapy in the UK. CBCT, cone-beam CT; kV, kilovoltage; MV, megavoltage; NHS, National Health Service.
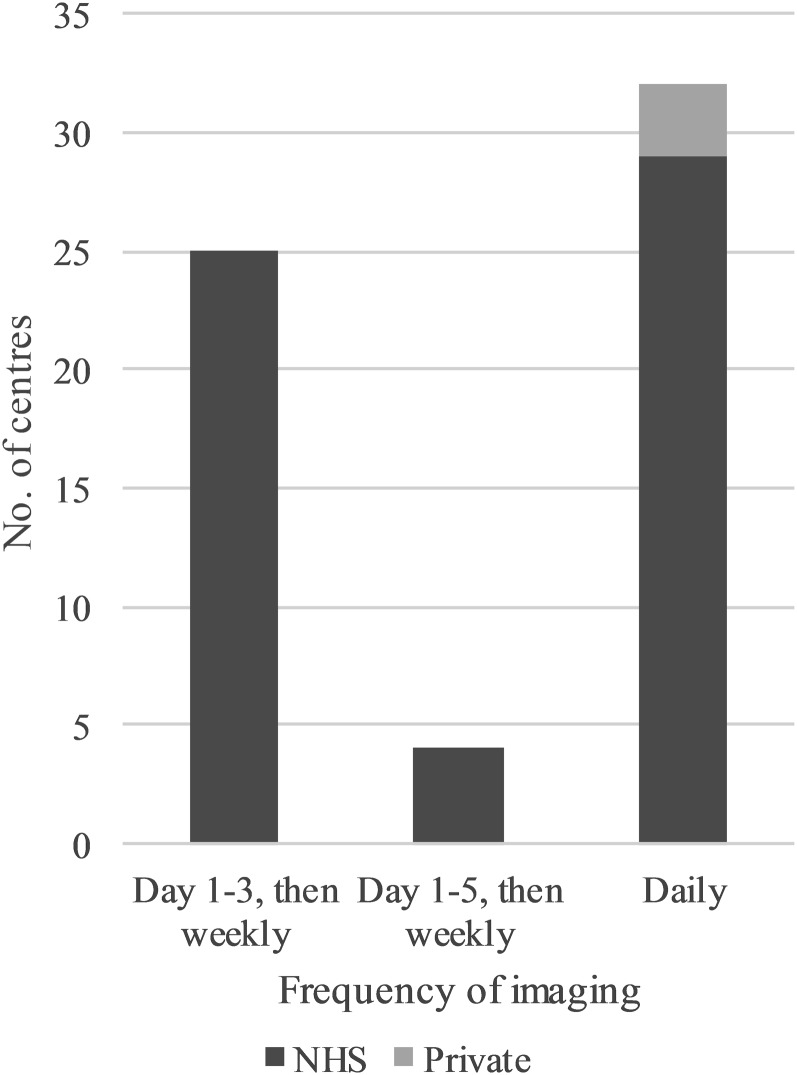
The most common verification imaging frequency was daily, used by 32 (60%) centres (Figure 2). 25 (47%) centres used a Day 1-3 schedule followed by once weekly schedule, and 4 (8%) centres used a Day 1-5 schedule followed by once weekly schedule. A variable verification strategy was used in some centres where daily imaging was used if there was concern about patient setup. 60% of CBCT centres repeated imaging prior to treatment, in situations such as excessive rectal distension.
Figure 2.
The frequency of verification imaging in radical prostate radiotherapy in the UK. NHS, National Health Service.
Verification strategy
A variety of verification strategies were described by the various departments. 41 (77%) centres stated they used online treatment verification. 21 of these centres used a zero tolerance protocol. However, 20 centres had an action level for online correction. The action level varied from 1 mm to 5 mm. Two centres reported using a combined online–offline protocol. 10 centres used an offline verification protocol (2 centres with online imaging for part of the protocol). 34 centres reported using a systematic correction, with a median threshold of 3 mm (range 1–6 mm). The median threshold for gross error setup correction was 10 mm (range 2–10 mm).
Private sector image-guided radiotherapy practice
At the three private sector provider networks who responded, all radical prostate radiotherapy was delivered with IMRT. For IGRT, two providers used daily online CBCT soft-tissue matching. The other provider used CBCT on Days 1–3 and weekly, with daily online matching with fiducials and kV portal imaging on other days. Fiducial marker insertion was consultant-led in all cases. Other than the universal use of IMRT with daily imaging, there were no other major differences seen between private and NHS prostate radiotherapy practices.
DISCUSSION
This survey captures data on contemporary prostate IGRT practice in the UK. The high response rate of 85% amongst NHS centres minimized non-response bias. There was a 60% response rate amongst private provider networks.
There were a number of limitations with this survey. We did not evaluate the margins used during generation of the planning target volume. While the clinical target volume–planning target volume margin should be based on individual institution setup errors, it may be influenced by the type of IGRT available. Each responding institution specified the total number of new patients with prostate cancer treated. However, in centres which used multiple imaging protocols, we did not ascertain the number of patients verified with each modality. This survey did not go into detail on the image guidance utilized in the TomoTherapy® and CyberKnife® (Accuray Inc., Sunnydale, CA) platforms, which were used by only a small proportion of centres. This study also did not specifically evaluate pelvic lymph node irradiation, which is associated with additional challenges in relation to image guidance during treatment.
There has been a clear improvement in utilization of advanced radiotherapy techniques in the UK, with a higher use of IMRT and advanced IGRT compared with previous UK surveys. The high uptake of volumetric imaging for prostate IGRT shows a trend similar to that identified in a survey in the USA in 2009.8 The high proportion of 66% of UK centres using CBCT verification in this survey indicates an improvement in IGRT equipment compared with the 2010 data from the ESTRO-HERO project.6 There remain some inequities in provision of image guidance technology, with seven NHS centres using planar imaging only. However, two of these centres specified that they were planning to introduce more advanced image-guided treatment within the near future.
The majority of centres use a dose fractionation regimen of 74 Gy in 37 fractions. Guidance from the UK National Institute for Health and Care Excellence from January 2014 recommends that radiotherapy doses below 74 Gy or equivalent should not be used. A few centres have started to use dose escalation above an EQD2 of 74 Gy, in line with clinical trial results which show a benefit in progression-free survival.9,10 There has also been early adoption of the hypofractionated course of 60 Gy in 20 fractions, which has been shown to be non-inferior to conventional fractionation in a more recent randomized trial.11 Two centres reported the use of 64 Gy in 32 fractions. This regimen has been shown to have lower toxicity, but lower progression-free survival.12 With the widespread availability of advanced IGRT techniques, the use of dose escalation is likely to be better tolerated, and this lower dose regimen would not be considered the standard of care in current practice.
In this survey, the use of fiducial markers for radical prostate radiotherapy appears limited. This appears to be related to resource limitations for fiducial insertion and the more widespread availability of non-invasive on-board imaging techniques. There is limited research directly comparing CBCT with fiducials, but analysis of shift data suggests correlation between techniques.13 One advantage of CBCT is that it also provides visualization of soft-tissue structures.
There are two main CBCT imaging frequency protocols currently in use in the UK. Over half the centres were using daily imaging, but many centres used a Day 1-3 schedule followed by once weekly schedule. Daily online imaging has the best potential to correct for target position variation through the course of treatment. However, a concomitant pelvic dose of 3.7–4.3 mSv per CBCT exposure14 should be taken into consideration when daily imaging is used. Further research is required to determine the optimal schedule for CBCT verification.
There is continuing progress in IGRT, with the development of prototype hybrid MRI linear accelerators.15 This is a promising technology, with the advantages of excellent image quality and potential for real-time treatment adaptation, without any concomitant radiation dose to the patient. However, it is still a research technology due to be introduced in the UK in 2017. In the meantime, further research is required on existing IGRT techniques to guide their optimum use. It would also be useful to incorporate IGRT research into future prostate radiotherapy trials in order to consolidate the evidence base for its use.
CONCLUSION
Volumetric imaging with CBCT is the main verification modality used for prostate IGRT in the UK. There are a variety of imaging schedules used for verification, with daily imaging used by the majority of centres.
Contributor Information
Hemal Ariyaratne, Email: hemal.ariyaratne@nhs.net.
Hayley Chesham, Email: hayleymkerr@gmail.com.
Roberto Alonzi, Email: ralonzi@nhs.net.
REFERENCES
- 1.Zelefsky MJ, Kollmeier M, Cox B, Fidaleo A, Sperling D, Pei X, et al. Improved clinical outcomes with high-dose image guided radiotherapy compared with non-IGRT for the treatment of clinically localized prostate cancer. Int J Radiat Oncol Biol Phys 2012; 84: 125–9. doi: https://doi.org/10.1016/j.ijrobp.2011.11.047 [DOI] [PubMed] [Google Scholar]
- 2. Radiotherapy: developing a world class service for England. Report to Ministers from National Radiotherapy Advisory Group; 2007. Cited 16 January 2016. Available from: http://webarchive.nationalarchives.gov.uk/20080726235726/, http://dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_074575.
- 3.Royal College of Radiologists; Society and College of Radiographers; Institute of Physics and Engineering in Medicine. On target: ensuring geometric accuracy in radiotherapy. London: Royal College of Radiologists; 2008. [Google Scholar]
- 4. Image Guided Radiotherapy (IGRT): Guidance for implementation and use; 2012. Cited 16 January 2016. Available from: http://www.sor.org/sites/default/files/document-versions/National%20Radiotherapy%20Implementation%20Group%20Report%20IGRT%20Final.pdf.
- 5.Mayles WP; Radiotherapy Development Board. Survey of the availability and use of advanced radiotherapy technology in the UK. Clin Oncol (R Coll Radiol) 2010; 22: 636–42. doi: https://doi.org/10.1016/j.clon.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 6.Grau C, Defourny N, Malicki J, Dunscombe P, Borras JM, Coffey M, et al. Radiotherapy equipment and departments in the European countries: final results from the ESTRO-HERO survey. Radiother Oncol 2014; 112: 155–64. doi: https://doi.org/10.1016/j.radonc.2014.08.029 [DOI] [PubMed] [Google Scholar]
- 7.The Royal College of Radiologists. Clinical Oncology UK Workforce Report 2012. London: Royal College of Radiologists; 2013. [Google Scholar]
- 8.Simpson DR, Lawson JD, Nath SK, Rose BS, Mundt AJ, Mell LK. A survey of image-guided radiation therapy use in the United States. Cancer 2010; 116: 3953–60. doi: https://doi.org/10.1002/cncr.25129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EH, Cheung MR, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. Int J Radiat Oncol Biol Phys 2008; 70: 67–74. doi: https://doi.org/10.1016/j.ijrobp.2007.06.054 [DOI] [PubMed] [Google Scholar]
- 10.Al-Mamgani A, van Putten WL, Heemsbergen WD, van Leenders GJ, Slot A, Dielwart MF, et al. Update of Dutch multicenter dose-escalation trial of radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 2008; 72: 980–8. doi: https://doi.org/10.1016/j.ijrobp.2008.02.073 [DOI] [PubMed] [Google Scholar]
- 11.Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016; 17: 1047–60. doi: https://doi.org/10.1016/S1470-2045(16)30102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dearnaley DP, Jovic G, Syndikus I, Khoo V, Cowan RA, Graham JD, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol 2014; 15: 464–73. doi: https://doi.org/10.1016/S1470-2045(14)70040-3 [DOI] [PubMed] [Google Scholar]
- 13.Moseley DJ, White EA, Wiltshire KL, Rosewall T, Sharpe MB, Siewerdsen JH, et al. Comparison of localization performance with implanted fiducial markers and cone-beam computed tomography for on-line image-guided radiotherapy of the prostate. Int J Radiat Oncol Biol Phys 2007; 67: 942–53. doi: https://doi.org/10.1016/j.ijrobp.2006.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyer DE, Serago CF, Kim S, Li JG, Hintenlang DE. An organ and effective dose study of XVI and OBI cone-beam CT systems. J Appl Clin Med Phys 2010; 11: 3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagendijk JJ, Raaymakers BW, Raaijmakers AJ, Overweg J, Brown KJ, Kerkhof EM, et al. MRI/linac integration. Radiother Oncol 2008; 86: 25–9. doi: https://doi.org/10.1016/j.radonc.2007.10.034 [DOI] [PubMed] [Google Scholar]