Abstract
Objective:
To perform a systematic review of the methods used for background parenchymal enhancement (BPE) evaluation on breast MRI.
Methods:
Studies dealing with BPE assessment on breast MRI were retrieved from major medical libraries independently by four reviewers up to 6 October 2015. The keywords used for database searching are “background parenchymal enhancement”, “parenchymal enhancement”, “MRI” and “breast”. The studies were included if qualitative and/or quantitative methods for BPE assessment were described.
Results:
Of the 420 studies identified, a total of 52 articles were included in the systematic review. 28 studies performed only a qualitative assessment of BPE, 13 studies performed only a quantitative assessment and 11 studies performed both qualitative and quantitative assessments. A wide heterogeneity was found in the MRI sequences and in the quantitative methods used for BPE assessment.
Conclusion:
A wide variability exists in the quantitative evaluation of BPE on breast MRI. More studies focused on a reliable and comparable method for quantitative BPE assessment are needed.
Advances in knowledge:
More studies focused on a quantitative BPE assessment are needed.
INTRODUCTION
As stated by the research committee of the European Society of Radiology, the future of medicine lies in the so-called “personalized medicine” (PM).1,2 The concept of PM could be reassumed in delivering the right treatment to the right patient at the right time. The concept of PM is strictly linked to “precision medicine”, which has been defined in 2011 by the National Research Council of the National Academies white paper entitled “Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a new Taxonomy of Disease”.3 In light of these new goals of modern medicine, biomedical imaging requires a correct and rational use of quantitative imaging biomarkers (QIBs).4
In addition, implementation of quantitative imaging on a large scale will be critical to meet the demands of PM.4 Indeed, PM presents new challenges to the radiologists with the need for validation and assessment of QIBs for diagnosis and treatment response assessment.1–6 One primary metrology area of interest in the assessment of performance of a QIB is the ability of the QIB to consistently reproduce equivalent results when conditions change, as would be expected in any clinical trial.6 In this perspective, background parenchymal enhancement (BPE), the term used to describe the enhancement of the normal breast tissue, is emerging as an imaging biomarker.7
The “degree” of BPE is linked to the risk of developing breast cancer, may affect the reading of breast MRI, the staging and the risk of cancer and even the long-term outcome, particularly in patients with certain subtypes at immunohistochemistry.8–15 BPE can be visually assessed qualitatively using the Breast Imaging-Reporting and Data System (BI-RADS) scores or quantitatively using software.7,16 However, radiologist agreement for BPE qualitative evaluation is fair17 and, to the best of our knowledge, there is a lack of uniformity on quantitative measurements of BPE on breast MRI. Indeed, an absolute categorizing method based on percentage is not supported by the American College of Radiology (ACR), suggesting the need for further research in this topic.16 It is crucial that in the era of PM, the methods used for the evaluation of BPE, as for other imaging biomarkers, are reliable and comparable among different imaging sites.5 Therefore, the purpose of this study was to perform a systematic review of the methods currently adopted to assess BPE on breast MRI and to drive future research on this QIB.
METHODS AND MATERIALS
We followed the guidelines defined by the Preferred Reporting Items for Systematic Reviews and Meta-analyses.18 The protocol of this study was published on the International Prospective Register of Systematic Reviews (protocol number: CRD42015026904) on 8 October 2015 (http://www.crd.york.ac.uk/PROSPERO/).
Search strategy
We identified all relevant studies that assessed the evaluation of BPE on breast MRI. A literature search using PubMed (http://www.pubmed.org), Embase (http://www.embase.com.proxy.medlib.iupui.edu/search), ISI Web of Science (http://apps.webofknowledge.com), SpringerLink, ScienceDirect and Cochrane library (http://www.thecochranelibrary.com) was performed independently by four reviewers (AT, BB, FV and FR) up to 6 October 2015. A manual revision of the reference lists was also performed to integrate the initial search with additional studies, if necessary. We did not directly contact the authors for additional data.
The search strategy included the following terms related to studies on humans: “background parenchymal enhancement” or “parenchymal enhancement”, in combination with “magnetic resonance imaging”, “evaluation” or “assessment”, “breast”.
The detailed search strategy in PubMed is presented in the Supplementary Material.
Inclusion criteria
Studies were included if they met all the following criteria:
(1) females older than 18 years who underwent breast MRI
(2) BPE assessed on MRI.
(3) The method used for BPE assessment clearly stated: qualitative with BI-RADS, qualitative without BI-RADS, automated quantitative on two-dimensional MRI slices, automated quantitative on three-dimensional MRI volumes, semi-automated quantitative on two-dimensional MRI slices and semi-automated quantitative on three-dimensional MRI volumes.
(4) Only publications in English language were included.
Exclusion criteria: (1) case reports or case series, review articles, letters and comments; (2) duplicate publication; (3) BPE not assessed; (4) MRI examinations below 1.5 T.
No publication date restriction was used.
Study selection
Two authors (AT and BB) independently and manually reviewed article titles and abstracts for study selection, based on the predefined criteria. Then, the same authors independently read the methods in the full text of those studies to confirm fulfilment of the inclusion criteria. Disagreements arising during each phase of the study selection were resolved in consensus. If consensus could not be reached, a clinical expert (MC) was asked to resolve any disagreements.
Data extraction and analysis
Two authors (AT and BB) independently extracted the data from each eligible study. A duplicate data extraction was performed and discrepancies were resolved by consensus. The following data were extracted from each study: first author, journal and publication year, country of the study, study designation (retrospective or prospective), study population, magnetic field of the MRI scanner (1.5 T or 3.0 T), menstrual period of patients undergoing MRI, the type of contrast media used (high-relaxivity contrast media and no high-relaxivity contrast media), the type of BPE assessment (qualitative method, quantitative method, including automated software), the sequences in which BPE was qualitatively and quantitatively assessed and the method used for the quantitative evaluation of BPE. In particular, we recorded studies assessing BPE quantitatively using a region of interest (ROI), fibroglandular tissue segmentation, automatic method or other methods. To assess studies using ROI, we considered studies in which BPE was assessed by using an ROI traced to include a normal fibroglandular tissue, or the most enhanced part of the normal fibroglandular tissue, or the normal tissue extending from the tumour, e.g. excluding breast lesion enhancement. To assess studies using fibroglandular tissue segmentation, we considered studies in which BPE was calculated by enhancements of every pixel/voxel contained within a previously segmented fibroglandular tissue. To assess studies using an automatic method, we considered studies in which the use of fully automatic software that gives the value of BPE without the need for further control by a radiologist was specified. We also recorded studies using other methods, different from the ROI, fibroglandular or automatic ones.
Among the studies assessing BPE qualitatively, we recorded each study with intrareader and interreader agreement assessments for all readings by using the kappa statistics. We recorded kappa values for both ordinal (minimal, mild, moderate or marked BPE) and dichotomized variables (low and high BPE), when assessed. The strength of kappa agreement was defined as follows: 0.00–0.20, slight; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.81, substantial; 0.81–1.00, almost perfect.
We divided articles published in 2015 from those published before 2015 to evaluate the increased interest on this topic in the past year. We performed a narrative synthesis of the qualitative and quantitative methods reported.
Risk of bias
The quality assessments of the eligible study were evaluated independently by two authors (Blind, Blind) using a modified Quality Assessment of Studies of Diagnostic Accuracy Studies (QUADAS-2) checklist, which comprised four domains: patient selection, index test and reference standard, and flow and timing. For the purpose of this study, the domains “index test” and “reference standard” were considered together: in addition to the standard questions of these domains, we included the quality of the description of BPE assessment and the quality of MR images where the BPE assessment was performed, when available. Each domain is assessed in terms of risk of bias and the first three in terms of concerns regarding applicability. The answers “yes” (+), “no” (−) or “unclear” (?) to the standard questions of each domain represent the judgment regarding bias and applicability: low risk of bias, high risk of bias and insufficient data to permit a judgement, respectively. The two authors then discussed the results of their quality assessments. Disagreements were resolved by consensus.
RESULTS
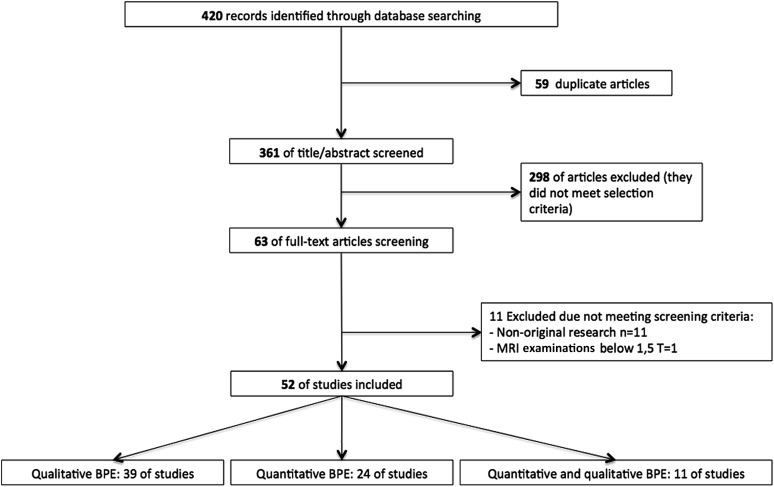
The initial database search identified 420 articles. A total of 63 full-text articles were assessed after removal of duplicates and reading abstracts because they did not meet the selection criteria. From the 63 full-text articles, 11 studies were excluded because they did not meet the screening criteria and a total of 52 articles were included in the systematic review (Figure 1). Tables 1 and 2 show the characteristics of the included studies that assessed BPE with a qualitative and quantitative method, respectively. Among these 52 studies, 28 (54%) studies performed only a qualitative assessment, 13 (25%) studies performed only a quantitative assessment and 11 (21%) studies performed both qualitative and quantitative assessments of BPE and were included in both tables. Among these 52 studies, 20 (38%) studies were published in 2015.
Figure 1.
A flowchart of the selection of studies. BPE, background parenchymal enhancement.
Table 1.
Characteristic of the 39 studies that assess background parenchymal enhancement (BPE) qualitatively included in the systematic review
| Study | Year | Journal | Country | Design | Study population | Magnetic field (T) | Contrast media (commercial names) | Sequences used for qualitative assessment of BPE |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combination of unenhanced and contrast-enhanced fat-suppressed T1 weighted and subtracted images | Combination of the unenhanced, initial contrast-enhanced subtraction and MIP images | MIP | Post-contrast series and/or subtracted images | Not clear | ||||||||
| Albert et al19 | 2015 | Clin Imaging | USA | R | 475 | 3.0 and 1.5 | Magnevist® (Bayer Schering Pharma AG, Berlin, Germany) | xa | ||||
| Amarosa et alb 20 | 2013 | Radiology | USA | R | 58 | 3.0 | Magnevist | x | ||||
| Baek et al21 | 2014 | Eur J Radiol | Korea | R | 322 | 3.0 and 1.5 | Magnevist | xc | ||||
| Cho et alb 22 | 2015 | Eur J Radiol | USA | R | 77 | 3.0 | Magnevist | x | ||||
| Choi and Kim23 | 2015 | Acta Radiol | Korea | R | 98 | 1.5 | Magnevist | xa | ||||
| Cubuk et alb 24 | 2010 | Rad Med | Turkey | R | 26 | 1.5 | Magnevist | x | ||||
| DeMartini et al11 | 2012 | AJR Am J Roentgenol | USA | R | 736 | Not clear | not clear | x | ||||
| DeLeo et al25 | 2015 | AJR Am J Roentgenol | USA | R | 55 | 3.0 and 1.5 | not clear | x | ||||
| Dontchos et al10 | 2015 | Radiology | USA | R | 487 | 1.5 | Omniscan (gadodiamide) | x | ||||
| Grimm et al26 | 2015 | AJR Am J Roentgenol | USA | R | 222 | 3.0 and 1.5 | Magnevist | x | ||||
| Hambly et al12 | 2011 | AJR Am J Roentgenol | USA | R | 250 | 1.5 | Magnevist | x | ||||
| Hansen et al27 | 2014 | J Magn Reson Imaging | Germany | R | 468 | 1.5 | Gadovist® (gadobutrol, Bayer Inc., ON) | xa | ||||
| Iacconi et al28 | 2014 | Eur J Radiol | USA | R | 96 | 3.0 and 1.5 | Magnevist | x | ||||
| Jansen et alb 29 | 2011 | Eur Radiol | USA | R | 229 | 1.5 | Omniscan | x | ||||
| Kajihara et alb 30 | 2013 | Magn Reson Med Sci | Japan | R | 165 | 1.5 | Magnevist | x | ||||
| Kawamura et al31 | 2015 | Nagoya J Med Sci | Japan | R | 160 | 3.0 | Magnevist | xa | ||||
| Kim JY et alb 32 | 2015 | Magn Reson Imaging | Korea | R | 81 | 3.0 | Gadovist | xa | ||||
| Kim MY et al33 | 2015 | Clin Radiol | Korea | R | 178 | 3.0 | Dotarem® | x | ||||
| Kim MY, et alb 34 | 2013 | Acta Radiol | Korea | R | 133 | 1.5 | Gadovist | xa | ||||
| Kim SA et alb 35 | 2014 | Radiology | Korea | R | 215 | 1.5 | MultiHance® (Bracco Imaging, Milan, Italy) | xa | ||||
| Kim YJ et al36 | 2014 | Asian Pac j Cancer Prev | Korea | R | 62 | 3.0 | Gadovist | xa | ||||
| King et al8 | 2012 | Radiology | USA | R | 149 | 3.0 and 1.5 | Magnevist | x | ||||
| King et al37 | 2012 | Breast J | USA | R | 88 | 1.5 | Magnevist | x | ||||
| King et al38 | 2012 | Eur Radiol | USA | R | 330 | 3.0 and 1.5 | Magnevist | x | ||||
| King et al39 | 2011 | Radiology | USA | R | 1275 | 1.5 | Magnevist | x | ||||
| Kohara et al40 | 2015 | Nagoya | Japan | R | 91 | 3.0 | Magnevist | xa | ||||
| Koo et al41 | 2013 | Eur J Radiol | Korea | R | 52 | 1.5 | Gadovist | xa | ||||
| Melsaether et al17 | 2014 | AJR Am J Roentgenol | USA | R | 119 | 3.0 and 1.5 | Magnevist | xa | ||||
| Myers et al42 | 2015 | Clin Breast Cancer | USA | R | 168 | 1.5 | MultiHance | x | ||||
| Park et al43 | 2015 | BrJ Radiol | Korea | R | 314 | 3.0 and 1.5 | Magnevist | x | ||||
| Preibsch et al44 | 2015 | Eur Radiol | Germany | R | 73 | 1.5 | Gadovist | xd | ||||
| Price et al45 | 2014 | Eur Radiol | USA | R | 18 | 1.5 | Magnevist | xa | ||||
| Scaranelo et alb 46 | 2013 | Radiology | Canada | R | 147 | 1.5 | Gadovist | xa | ||||
| Schrading et alb 47 | 2014 | Radiology | Germany | P | 40 | 1.5 | Magnevist | xa | ||||
| Tagliafico et alb 7 | 2015 | Br J Radiol | Italy | R | 48 | 3.0 | MultiHance | xa | ||||
| Uematsu et al15 | 2012 | Breast Cancer | Japan | R | 70 | 1.5 | Magnevist | xa | ||||
| Uematsu et al13 | 2011 | Eur Radiol | Japan | R | 146 | 1.5 | Magnevist | xa | ||||
| Uematsu et al48 | 2012 | Eur J Radiol | Japan | R | 146 | 1.5 | Magnevist | xa | ||||
| Yoon et al49 | 2015 | Eur Radiol | Korea | R | 145 | 3.0 | Magnevist | x | ||||
MIP, maximum intensity projection; P, prospective study; R, retrospective study.
Early post-contrast images were used.
Articles with both qualitative and quantitative assessments of BPE.
The unenhanced images were not used.
Only subtracted images were used.
Table 2.
Characteristic of the 24 studies that assess background parenchymal enhancement (BPE) quantitatively included in the systematic review
| Study | Year | Journal | Country | Design | Study population | Magnetic field (T) | Contrast media | Method used for quantitative assessment of BPE |
Software used | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | Fibroglandular tissue segmentation | Other | |||||||||
| Amarosa et ala 20 | 2013 | Radiology | USA | R | 58 | 3.0 | Magnevist® (Bayer Schering Pharma AG, Berlin, Germany) | x | Interactive Data Language (Exelis, Boulder, CO) | ||
| Chen et al50 | 2015 | Translational Oncology | USA | R | 46 | 1.5 | Omniscan | x | |||
| Chen et al51 | 2013 | Magn Reson Imaging | USA | R | 45 | 1.5 | Omniscan | x | |||
| Cho et ala 22 | 2015 | Eur J Radiol | USA | R | 77 | 3.0 | Magnevist | x | MATLAB®, (MathWorks®, Natick, MA) | ||
| Cubuk et ala 24 | 2010 | Rad Med | Turkey | R | 26 | 1.5 | Magnevist | x | |||
| Hattangadi et al52 | 2008 | Am J Roentgenol | USA | P | 42 | 1.5 | Magnevist | x | |||
| Hegenscheid et al53 | 2012 | Eur Radiol | Germany | P | 651 | 1.5 | Gadovist® (gadobutrol, Bayer Inc., ON) | x | Syngo 2008A MultiModality Workplace (Siemens Medical Solutions, Erlangen, Germany) | ||
| Hegenscheid et al54 | 2013 | Radiology | Germany | P | 651 | 1.5 | Gadovist | x | Syngo 2008A MultiModality Workplace | ||
| Jansen et ala 29 | 2011 | Eur Radiol | USA | R | 101 | 1.5 | Omniscan | x | CADstream research v. 5.0 (Confirma, CA) | ||
| Kajihara et ala 30 | 2013 | Magn Reson Med Sci | Japan | R | 165 | 1.5 | Magnevist | x | Aquarius (TeraRecon Inc., San Mateo, CA) | ||
| Kang et al55 | 2014 | J Magn Reson Imaging | Korea | P | 272 | 3.0 | Magnevist | x | Extended MR Work Space (Philips Medical Systems) | ||
| Kim JY et ala 32 | 2015 | Magn Reson Imaging | Korea | R | 81 | 3.0 | Gadovist | x | |||
| Kim MY et ala 34 | 2013 | Acta Radiol | Korea | R | 133 | 1.5 | Gadovist | x | |||
| Kim SA et ala 35 | 2014 | Radiology | Korea | R | 215 | 1.5 | MultiHance® (Bracco Imaging, Milan, Italy) | x | |||
| Klifa et al56 | 2011 | J Magn Reson Imaging | USA | R | 16 | 1.5 | Magnevist | x | |||
| Mazurowski et al57 | 2014 | Radiology | USA | R | 48 | 1.5 | not clear | xb | |||
| Mousa et al58 | 2012 | Menopause | Canada | P | 14 | 1.5 | Gadovist/Omniscan | x | |||
| Scaranelo et ala 46 | 2013 | Radiology | Canada | R | 147 | 1.5 | Gadovist | x | |||
| Schrading et ala 47 | 2014 | Radiology | Germany | P | 40 | 1.5 | Magnevist | x | View Forum (Philips, Best, Netherlands) | ||
| Schrading and Kuhl59 | 2015 | Radiology | Germany | P | 62 | 1.5 | Magnevist | x | DynaCAD software package, v. 3.0 (Invivo, Philips Healthcare, Best, Netherlands) | ||
| Tagliafico et ala 7 | 2015 | Br J Radiol | Italy | R | 48 | 3.0 | MultiHance | xb | MedDensity© (Genova, Italy) | ||
| Van der Velden et al14 | 2015 | Radiology | Netherlands | R | 531 | 1.5 | ProHance® (gadoteridol, Bracco Diagnostics, Inc., Singen Germany) | x | Insight Segmentation and Registration Toolkit and Visualization Toolkit (Kitware, Clifton Park, NY) and MeVisLab software (MeVis Medical Solutions, Bremen, Germany) | ||
| Wu et al60 | 2015 | Breast Cancer Res | USA | R | 55 | 1.5 | Omniscan | xb | |||
| Yang et al61 | 2015 | Med Phys | China | R | 115c | 1.5 | Magnevist | x | |||
P, prospective study; R, retrospective study; ROI, region of interest.
In the last column, there is the name of the software used, when retrievable.
Articles with both qualitative and quantitative assessments of BPE.
Automatic method.
MR images.
Qualitative background parenchymal enhancement assessment
Among the 39 (28+11) studies that assessed BPE qualitatively,7,8,10–13,15,17,19–49 38% (15/39) studies were published in 2015 (January–October 2015) and 62% (24/39) studies were published during 2010–2014. Most of the studies were performed in the USA (17/39 studies), the Korea (10/39 studies) and Japan (6/39 studies). Only one study47 had a prospective study design. The patient population of the included studies ranged from 18 to 1275 patients. 20 studies performed breast MRI using a 1.5-T scanner, 9 studies performed breast MRI using a 3.0-T scanner and 9 studies used both 1.5-T and 3.0-T scanners. In one study,11 the MRI scanner was not clearly stated but it was above 1.5 T. Most of the studies (59%; 23/39 studies) used gadopentetate dimeglumine as contrast agent. Only 3/39 (8%) studies used a high-relaxivity contrast agent.7,35,42 All the studies graded BPE on a four-point scale as minimal, mild, moderate or marked in accordance with the BI-RADS categories.16 Iacconi et al28 classified BPE according to the BI-RADS lexicon but for statistical purpose, clumped the studies into two groups (low and high BPE). 16 studies qualitatively assessed BPE using a combination of unenhanced and contrast-enhanced fat-suppressed T1 weighted and subtracted images, and 5 studies added maximum intensity projection images also; 14 studies qualitatively assessed BPE using a combination of post-contrast fat-suppressed T1 weighted and/or subtraction images; 1 study33 used only maximum intensity projection images; in 3 studies, the sequences used for qualitative BPE assessment were not clearly stated (Table 1).
A total of nine studies evaluated the intrareader and/or interreader agreement of qualitative evaluation of BPE.7,8,17,25,39,44–46,49 In particular, four studies7,8,17,49 evaluated both intrareader and interreader agreements and the other five studies evaluated only interreader agreement. Kappa values for intrareader agreement were moderate to almost perfect, while more variability was found for kappa values for interreader agreement, which was demonstrated to be fair to almost perfect (Table 3).
Table 3.
Intrareader and interreader agreement for all readings for qualitative background parenchymal enhancement (BPE) evaluation among the nine studies that assessed agreement by using kappa statistics
| Study | Year | Journal | Number of readers | Agreement |
|
|---|---|---|---|---|---|
| Intrareader (for dichotomized variables) | Interreader (for dichotomized variables) | ||||
| DeLeo et al25 | 2015 | AJR Am J Roentgenol | 2 | n.a. | 0.49 |
| King et al39 | 2012 | Eur Radiol | 2 | n.a. | 0.95 |
| King et al8 | 2011 | Radiology | 2 | 0.62(0.69) | 0.47(0.57) |
| Melsaether et ala 17 | 2014 | AJR Am J Roentgenol | 4 | 0.79(0.80) | 0.45(0.47) |
| Preibsch et alb 44 | 2015 | Eur Radiol | 2 | n.a. | Right breast:0.73 Left breast:0.77 |
| Price et al45 | 2014 | Eur Radiol | 3 | n.a. | 0.3–0.6 |
| Scaranelo et al46 | 2013 | Radiology | 2 | n.a. | 0.37 |
| Tagliafico et al7 | 2015 | Br J Radiol | 2 | 0.69 | 0.70 |
| Yoon et al49 | 2015 | Eur Radiol | 2 | 0.82 | 0.85 |
In the majority of studies (seven of nine studies), the agreement was assessed for ordinal variables. In the studies by King et al8 and Melsaether et al,17 the authors assessed intrareader and interreader agreements for both ordinal and dichotomized variables, but the strength of kappa agreement was not changed.
Quantitative background parenchymal enhancement assessment
Among the 24 (13 + 11) studies that assessed BPE quantitatively,7,14,20,22,24,29,30,32,34,35,46,47,50–61 33% (8/24) studies were published in 2015 (January–October 2015) and 67% (16/24) studies were published during 2008–2014. Most of the studies were performed in the USA (9/24 studies), the Korea (4/24 studies) and Germany (4/24 studies). A total of 7 studies were prospective, and 17 studies were retrospective. The patient population of the included studies ranged from 16 to 651 patients. 18 studies performed breast MRI using a 1.5-T scanner and 5 studies performed breast MRI using a 3.0-T scanner. Most of the studies (42%; 10/24 studies) used gadopentetate dimeglumine (Magnevist®; Bayer Schering Pharma AG, Berlin, Germany) as contrast agent. Only 2/24 (8%) studies7,35 used a high-relaxivity contrast agent (gadobenate dimeglumine, MultiHance®; Bracco Imaging, Milan, Italy). 15 (62%) studies performed a quantitative evaluation of parenchymal enhancement from an ROI. Among these studies, BPE was described as a signal enhancement ratio in four studies.29,34,35,52 The signal enhancement ratio was based on the comparison of signal intensity in an early contrast-enhanced image with signal intensity in a delayed contrast-enhanced image relative to a pre-contrast image.
BPE was described as percentage enhancement rates or a relative percentage enhancement in 11 studies,22,24,30,32,46,47,53–55,58,59 with the use of both pre- and post-contrast images. There was a wide heterogeneity in the time selection of images obtained after contrast agent injection for relative percentage enhancement or percentage enhancement rate calculation.
Three studies performed a quantitative evaluation of BPE using an automatic method.7,57,60 Tagliafico et al7 assessed BPE using fully automated software that performed an objective and reproducible voxel-by-voxel analysis. This software used an algorithm based on the maximum entropy method and a threshold value.7 Mazurowski et al57 used computer vision algorithms that extracted all the features automatically, including a dynamic feature of the background parenchyma.57 Wu et al60 used a validated fully automated method that allowed segmentation and quantitative measure of fibroglandular tissues and BPE.60
Qualitative and quantitative background parenchymal enhancement assessment
Among the 11 studies that assessed BPE in both qualitative and quantitative methods,7,20,22,24,29,30,32,34,35,46,47 27% (3/11) studies were published in 2015 (January–October 2015) and 73% (8/11) studies were published during 2010–2014. Most of the studies were performed in the USA (3/11 studies) and the Korea (3/11 studies). The majority of the studies (10/11 studies) were prospective. The patient population of the included studies ranged from 26 to 229 patients. Seven studies performed breast MRI using a 1.5-T scanner and four studies performed breast MRI using a 3.0-T scanner. Most of the studies (45%; 5/11 studies) used gadopentetate dimeglumine (Magnevist) as contrast agent. Among these 11 studies that assessed BPE in both qualitative and quantitative methods, only the study of Kim et al34 found a statistical difference between qualitative and quantitative data.
Considering the menstrual period of patients who were pre-menopausal who underwent MRI, in the majority of studies (30 of 52 studies), the patient menstrual cycle was unknown or not available.8,10,12–15,21,23,26,28,32–36,38,40–43,48–54,57,59,61 In five studies,11,17,19,29,44 the authors acknowledged that owing to the retrospective nature of the study, it was not possible to analyze the point of menstrual cycle, although, following institutional protocol, screening breast MRI of patients who were pre-menopausal was performed during the second week of the menstrual cycle. In a total of 14 studies, the authors stated the menstrual period.7,20,22,24,25,27,30,31,39,45,46,55,56,60 In 8 of these 14 studies, breast MRI were performed ideally in the second week of the menstrual cycle.7,22,24,25,27,45,56,60 In three studies,37,47,58 the patients were post-menopausal females.
Risk of bias
Assessment of the methodological quality of the included studies by the modified QUADAS-2 tool is depicted in Tables 4 and 5.
Table 4.
Risk of bias table demonstrating the overall risk of bias for each of the domains of patient selection, index test and reference standard, and flow and timing
| Study | Patient selection | Index test and reference standard | Flow and timing |
|---|---|---|---|
| Albert et al19 | + | + | + |
| Amarosa et ala 20 | + | + | ? |
| Baek et al21 | + | + | + |
| Cho et ala 22 | + | + | + |
| Choi and Kim23 | ? | + | ? |
| Cubuk R et ala 24 | + | ? | ? |
| DeMartini et al11 | ? | + | + |
| DeLeo et al25 | + | + | + |
| Dontchos et al10 | + | + | + |
| Grimm et al26 | + | − | ? |
| Hambly et al12 | + | + | − |
| Hansen et al27 | + | + | + |
| Iacconi et al28 | + | + | ? |
| Jansen et ala 29 | ? | + | ? |
| Kajihara et ala 30 | ? | + | ? |
| Kawamura et al31 | + | + | + |
| Kim JY et ala 32 | ? | + | + |
| Kim MY et al33 | + | ? | + |
| Kim MYa 34 | + | + | + |
| Kim SA et ala 35 | + | + | + |
| Kim YJ et al36 | + | ? | − |
| King et al8 | + | + | + |
| King et al37 | + | + | + |
| King et al38 | + | + | + |
| King et al39 | + | + | + |
| Kohara et al40 | + | + | ? |
| Koo et al41 | + | + | + |
| Melsaether et al17 | + | + | + |
| Myers et al42 | + | − | ? |
| Park et al43 | ? | + | ? |
| Preibsch et al44 | + | + | ? |
| Price et al45 | + | + | + |
| Scaranelo et ala 46 | + | + | + |
| Schrading et ala 47 | + | + | + |
| Tagliafico et ala 7 | + | + | + |
| Uematsu et al15 | + | + | ? |
| Uematsu et al13 | + | + | ? |
| Uematsu et al48 | + | + | ? |
| Yoon et al49 | + | + | ? |
+, low risk of bias; −, high risk of bias; ?, unclear.
Studies that assessed background parenchymal enhancement with both qualitative and quantitative methods.
Table 5.
Risk of bias table demonstrating the overall risk of bias for each of the domains of patient selection, index test and reference standard, and flow and timing
| Study | Patient selection | Index test and reference standard | Flow and timing |
|---|---|---|---|
| Amarosa et ala 20 | + | + | ? |
| Chen et al50 | + | − | ? |
| Chen et al51 | + | + | ? |
| Cho et ala 22 | + | + | + |
| Cubuk R et ala 24 | + | ? | − |
| Hattangadi et al52 | + | ? | ? |
| Hegenscheid et al53 | + | + | + |
| Hegenscheid et al54 | + | + | + |
| Jansen et ala 29 | + | + | ? |
| Kajihara et ala 30 | ? | + | ? |
| Kang et al55 | ? | + | + |
| Kim JY et ala 32 | ? | + | + |
| Kim MY et ala 34 | + | ? | ? |
| Kim SA et ala 35 | + | + | + |
| Klifa et al56 | + | + | + |
| Mazurowski et al57 | + | ? | ? |
| Mousa et al58 | + | + | − |
| Scaranelo et ala 46 | + | + | + |
| Schrading et ala 47 | + | + | + |
| Schrading and Kuhl59 | + | ? | + |
| Tagliafico et ala 7 | + | + | + |
| Van der Velden et al14 | + | + | + |
| Wu et al60 | + | + | + |
| Yang et al61 | + | + | ? |
+, low risk of bias; −, high risk of bias; ?, unclear.
Studies that assessed background parenchymal enhancement with both qualitative and quantitative methods.
The domain of “patient selection” for the qualitative and quantitative BPE evaluation was unclear in the studies of DeMartini et al,11 Choi and Kim,23 Jansen et al,29 Kajihara et al,30 Kang et al,55 Kim JY et al32 and Park et al.43 The domain “index test and reference standard” was described in detail in most of the studies that assessed BPE qualitatively and quantitatively. A risk of bias and concerns regarding applicability were judged in the study of Chen et al50 and in the studies of Grimm et al26 and Myers et al,42 specifically for the low quality of MRI examinations where the BPE assessment was performed and a low detail of the qualitative assessment of BPE, respectively. The domain of “flow and timing” was the only domain to potentially contribute a high risk of bias in the studies evaluated. However, we believe that this domain could be less relevant because we focused only on the methods of assessment of BPE which, in most instances, are performed with a retrospective review of a data set of breast MRI.
DISCUSSION
We performed a systematic review of the literature currently available on qualitative and quantitative assessments of BPE in breast MRI. We divided the 52 articles included in the systematic review into those that performed a qualitative evaluation of BPE and those that performed a quantitative evaluation of BPE. Most of the studies found (28/52 studies) performed only a qualitative evaluation of BPE, 13 studies performed only a quantitative evaluation and 11 studies performed both qualitative and quantitative evaluations of BPE. Therefore, a total of 24 studies performed a quantitative assessment of BPE. Among these 24 studies, one of the most difficult issues was the analysis of the quantitative method used, owing to the lack of standardization of the BPE quantitative assessment. Indeed, the studies used different methods and software to evaluate BPE, although the majority of these studies performed a quantitative evaluation of parenchymal enhancement from an ROI. However, the use of ROI usually needs radiologist involvement, and this issue should be faced in the perspective of a standardized quantitative imaging evaluation of BPE. In addition, only three studies used an automatic method, and in all these studies, different software were used. We can state that in the “era” of PM and emerging QIBs, BPE quantitative assessment is still far from standardized. The ACR distances itself from prescribing an absolute quantification method for BPE assessment16 and this is probably the source of heterogeneity that we found in our study. Indeed, our study found extensive heterogeneity in the methods used for BPE quantitative assessments and encourages further studies assessing comparable methods for quantitative BPE evaluation.
Among the 11 studies that performed a BPE assessment with both qualitative and quantitative methods, only 1 study34 reported a statistical difference between the qualitative and quantitative methods used. Noteworthy, the study by Kim et al32 was able to associate high values of BPE around the tumours on pre-operative MRI with an increased risk of ipsilateral breast tumour recurrence. Without the use a quantitative approach, this information would have been missed. Indeed, with a study design similar to that of Kim et al,34 a huge number of breast MRI examinations were necessary to obtain the same information.
Our systematic review found that the majority of studies published had a retrospective design, and only few studies were prospective. A retrospective study design reduces the possibility of associating BPE with other factors relevant to tumour biology. In addition, in the majority of the studies, the menstrual period of pre-menopausal females who underwent MRI was unknown or not available.
Regarding the contrast media used, we found that only few studies used high-relaxivity contrast media. The use of high-relaxivity contrast media such as gadobenate dimeglumine is reported to offer advantages of lesion conspicuity, detection rate and sensitivity for malignant breast lesions.62 Besides, a higher enhancement of benign lesions and breast parenchyma is possible with high-relaxivity contrast media;62 therefore, we cannot confirm that the amount of BPE assessed with the same method, but different contrast media, is comparable.
Regarding the quality assessment, we used a modified QUADAS-2 checklist, since our systematic review did not focus on diagnostic accuracy studies; indeed, we merged the domains “index test” and “reference standard”. In addition to the standard questions of these domains,63 we also considered the quality of the description of BPE assessment and the quality of MR images in which the BPE assessment was performed. In spite of the modified method of quality assessment, the domain of “flow and timing” was the only domain to potentially contribute a high risk of bias in the included studies. However, this review focused on the methods used on BPE evaluation, and the majority of the studies performed the assessment with a retrospective review of the breast MRI data set; therefore, we believe that this domain could be less relevant and the overall risk of bias in these studies could be considered low.
Considering qualitative evaluation, BPE was always graded on a four-point scale by the BI-RADS categories representing the main standardized area in BPE assessment, as recommended by the ACR BI-RADS fifth edition itself.16 However, a huge variability in the MRI sequences adopted to assess BPE was noted, although the main principle was to find the sequences in which the amount of BPE was most evident. It is clear that there is no consensus on what MRI sequences the BPE should be assessed even with the relatively simple suggested BI-RADS grading system. In addition, a wide variability was found among kappa values for the interreader agreement, from fair to almost perfect agreement. Considering intrareader agreement, kappa values were moderate to almost perfect. However, only 9 of 39 studies assessed intrareader and/or interreader agreement for the qualitative evaluation of BPE, and further studies could be useful on this topic.
Considering quantitative evaluation, we acknowledge that our study did not include a detailed description of the methods used for the quantitative assessment of BPE. However, we performed the division of these studies among four main different methods (ROI, fibroglandular tissue segmentation, automatic methods or other methods) to allow a more uniform analysis. Further systematic reviews that focus on this topic could be useful to provide future directions for a standardization of quantitative methods used to assess BPE.
Finally, the first study on BPE assessment was published in 200852 and 38% (20/52) of all the studies included were published in 2015, reflecting the growing interest in this topic. The relatively recent interest in BPE assessment could be another possible explanation for the wide variability found in the sequences used for the qualitative assessment and in the methods used for the quantitative assessment.
In conclusion, since BPE is considered an emerging imaging biomarker, new methods to assess BPE quantitatively are being developed. However, a wide variability exists in the methods used to perform a quantitative evaluation of BPE on breast MRI. In addition, no consensus exists on the sequences to be used to visually assess BPE. Therefore, more studies on quantitative BPE assessment are needed.
FUNDING
This study was partially funded by the University of Genoa, and the Associazione Italiana per la Ricerca sul Cancro (AIRC) provided grants to Alberto Tagliafico.
Contributor Information
Bianca Bignotti, Email: bignottibianca@gmail.com.
Alessio Signori, Email: alessio.signori@medicina.unige.it.
Francesca Valdora, Email: valdorafrancesca@gmail.com.
Federica Rossi, Email: federossi0590@gmail.com.
Massimo Calabrese, Email: massimo.calabrese@hsanmartino.it.
Manuela Durando, Email: mdurando@cittadellasalute.to.it.
Giovanna Mariscotto, Email: giovanna.mariscotti@libero.it.
Alberto Tagliafico, Email: albertotagliafico@gmail.com.
REFERENCES
- 1.European Society of Radiology (ESR). Medical imaging in personalised medicine: a white paper of the research committee of the European Society of Radiology (ESR). Insights Imaging 2015; 6: 141–55. doi: https://doi.org/10.1007/s13244-015-0394-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Society of Radiology (ESR). Medical imaging in personalised medicine: a white paper of the research committee of the European Society of Radiology (ESR). Insights Imaging 2011; 2: 621–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Research Council (US) Committee on A Framework for Developing a New Taxonomy of Disease. Toward precision medicine: building a knowledge network for biomedical research and a new taxonomy of disease. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]
- 4.Herold CJ, Lewin JS, Wibmer AG, Thrall JH, Krestin GP, Dixon AK, et al. Imaging in the age of precision medicine: summary of the proceedings of the 10th biannual symposium of the international society for strategic studies in radiology. Radiology 2015; 279: 226–38. [DOI] [PubMed] [Google Scholar]
- 5.Sullivan DC, Obuchowski NA, Kessler LG, Raunig DL, Gatsonis C, Huang EP, et al. Metrology standards for quantitative imaging biomarkers. Radiology 2015; 277: 813–25. doi: https://doi.org/10.1148/radiol.2015142202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raunig DL, McShane LM, Pennello G, Gatsonis C, Carson PL, Voyvodic JT, et al. Quantitative imaging biomarkers: a review of statistical methods for technical performance assessment. Stat Methods Med Res 2015; 24: 27–67. doi: https://doi.org/10.1177/0962280214537344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tagliafico A, Bignotti B, Tagliafico G, Tosto S, Signori A, Calabrese M. Quantitative evaluation of background parenchymal enhancement (BPE) on breast MRI. A feasibility study with a semi-automatic and automatic software compared to observer-based scores. Br J Radiol 2015; 88: 20150417. doi: https://doi.org/10.1259/bjr.20150417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology 2011; 260: 50–60. doi: https://doi.org/10.1148/radiol.11102156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pike MC, Pearce CL. Mammographic density, MRI background parenchymal enhancement and breast cancer risk. Ann Oncol 2013; 24(Suppl. 8): viii37–41. doi: https://doi.org/10.1093/annonc/mdt310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dontchos BN, Rahbar H, Partridge SC, Korde LA, Lam DL, Scheel JR. Are qualitative assessments of background parenchymal enhancement, amount of fibroglandular tissue on MR images, and mammographic density associated with breast cancer risk? Radiology 2015; 276: 371–80. doi: https://doi.org/10.1148/radiol.2015142304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol 2012; 198: W373–80. doi: https://doi.org/10.2214/AJR.10.6272 [DOI] [PubMed] [Google Scholar]
- 12.Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA. Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol 2011; 196: 218–24. doi: https://doi.org/10.2214/AJR.10.4550 [DOI] [PubMed] [Google Scholar]
- 13.Uematsu T, Kasami M, Watanabe J. Does the degree of background enhancement in breast MRI affect the detection and staging of breast cancer? Eur Radiol 2011; 21: 2261–7. doi: https://doi.org/10.1007/s00330-011-2175-6 [DOI] [PubMed] [Google Scholar]
- 14.van der Velden BH, Dmitriev I, Loo CE, Pijnappel RM, Gilhuijs KG. Association between parenchymal enhancement of the contralateral breast in dynamic contrast-enhanced MR imaging and outcome of patients with unilateral invasive breast cancer. Radiology 2015; 276: 675–85. doi: https://doi.org/10.1148/radiol.15142192 [DOI] [PubMed] [Google Scholar]
- 15.Uematsu T, Kasami M, Watanabe J. Should breast MRI be performed with adjustment for the phase in patients' menstrual cycle? Correlation between mammographic density, age, and background enhancement on breast MRI without adjusting for the phase in patients' menstrual cycle. Eur J Radiol 2012; 81: 1539–42. doi: https://doi.org/10.1016/j.ejrad.2011.04.059 [DOI] [PubMed] [Google Scholar]
- 16.Morris EA, Comstock CE, Lee CH, et al. ACR BI-RADS® magnetic resonance imaging. In: ACR BI-RADS® Atlas, Breast imaging reporting and data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 17.Melsaether A, McDermott M, Gupta D, Pysarenko K, Shaylor SD, Moy L. Inter- and intrareader agreement for categorization of background parenchymal enhancement at baseline and after training. AJR Am J Roentgenol 2014; 203: 209–15. doi: https://doi.org/10.2214/ajr.13.10952 [DOI] [PubMed] [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. doi: https://doi.org/10.1371/journal.pmed.1000100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albert M, Schnabel F, Chun J, Schwartz S, Lee J, Klautau Leite AP, et al. The relationship of breast density in mammography and magnetic resonance imaging in high-risk women and women with breast cancer. Clin Imaging 2015; 39: 987–92. doi: https://doi.org/10.1016/j.clinimag.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amarosa AR, McKellop J, Klautau Leite AP, Moccaldi M, Clendenen TV, Babb JS, et al. Evaluation of the kinetic properties of background parenchymal enhancement throughout the phases of the menstrual cycle. Radiology 2013; 268: 356–65. doi: https://doi.org/10.1148/radiol.13121101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baek JE, Kim SH, Lee AW. Background parenchymal enhancement in breast MRIs of breast cancer patients: impact on tumor size estimation. Eur J Radiol 2014; 83: 1356–62. doi: https://doi.org/10.1016/j.ejrad.2014.05.007 [DOI] [PubMed] [Google Scholar]
- 22.Cho GY, Moy L, Kim SG, Klautau Leite AP, Baete SH, Babb JS, et al. Comparison of contrast enhancement and diffusion-weighted magnetic resonance imaging in healthy and cancerous breast tissue. Eur J Radiol 2015; 84: 1888–93. doi: https://doi.org/10.1016/j.ejrad.2015.06.023 [DOI] [PubMed] [Google Scholar]
- 23.Choi BB, Kim SH. Effective factors to raise diagnostic performance of breast MRI for diagnosing pathologic complete response in breast cancer patients after neoadjuvant chemotherapy. Acta Radiol 2015; 56: 790–7. doi: https://doi.org/10.1177/0284185114538622 [DOI] [PubMed] [Google Scholar]
- 24.Cubuk R, Tasali N, Narin B, Keskiner F, Celik L, Guney S. Correlation between breast density in mammography and background enhancement in MR mammography. Radiol Med 2010; 115: 434–41. doi: https://doi.org/10.1007/s11547-010-0513-4 [DOI] [PubMed] [Google Scholar]
- 25.DeLeo MJ, 3rd, Domchek SM, Kontos D, Conant E, Chen J, Weinstein S. Breast MRI fibroglandular volume and parenchymal enhancement in BRCA1 and BRCA2 mutation carriers before and immediately after risk-reducing salpingo-oophorectomy. AJR Am J Roentgenol 2015; 204: 669–73. doi: https://doi.org/10.2214/ajr.13.12146 [DOI] [PubMed] [Google Scholar]
- 26.Grimm LJ, Anderson AL, Baker JA, Johnson KS, Walsh R, Yoon SC, et al. Interobserver variability between breast imagers using the fifth edition of the BI-RADS MRI lexicon. AJR Am J Roentgenol 2015; 204: 1120–4. doi: https://doi.org/10.2214/ajr.14.13047 [DOI] [PubMed] [Google Scholar]
- 27.Hansen NL, Kuhl CK, Barabasch A, Strobel K, Schrading S. Does MRI breast “density” (degree of background enhancement) correlate with mammographic breast density? J Magn Reson Imaging 2014; 40: 483–9. doi: https://doi.org/10.1002/jmri.24495 [DOI] [PubMed] [Google Scholar]
- 28.Iacconi C, Thakur SB, Dershaw DD, Brooks J, Fry CW, Morris EA. Impact of fibroglandular tissue and background parenchymal enhancement on diffusion weighted imaging of breast lesions. Eur J Radiol 2014; 83: 2137–43. doi: https://doi.org/10.1016/j.ejrad.2014.09.004 [DOI] [PubMed] [Google Scholar]
- 29.Jansen SA, Lin VC, Giger ML, Li H, Karczmar GS, Newstead GM. Normal parenchymal enhancement patterns in women undergoing MR screening of the breast. Eur Radiol 2011; 21: 1374–82. doi: https://doi.org/10.1007/s00330-011-2080-z [DOI] [PubMed] [Google Scholar]
- 30.Kajihara M, Goto M, Hirayama Y, Okunishi S, Kaoku S, Konishi E, et al. Effect of the menstrual cycle on background parenchymal enhancement in breast MR imaging. Magn Reson Med Sci 2013; 12: 39–45. doi: https://doi.org/10.2463/mrms.2012-0022 [DOI] [PubMed] [Google Scholar]
- 31.Kawamura A, Satake H, Ishigaki S, Ikeda M, Kimura R, Shimamoto K, et al. Prediction of background parenchymal enhancement on breast MRI using mammography, ultrasonography, and diffusion-weighted imaging. Nagoya J Med Sci 2015; 77: 425–37. [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Kim SH, Kim YJ, Kang BJ, An YY, Lee AW, et al. Enhancement parameters on dynamic contrast enhanced breast MRI: do they correlate with prognostic factors and subtypes of breast cancers? Magn Reson Imaging 2015; 33: 72–80. doi: https://doi.org/10.1016/j.mri.2014.08.034 [DOI] [PubMed] [Google Scholar]
- 33.Kim MY, Choi N, Yang JH, Yoo YB, Park KS. Background parenchymal enhancement on breast MRI and mammographic breast density: correlation with tumour characteristics. Clin Radiol 2015; 70: 706–10. doi: https://doi.org/10.1016/j.crad.2015.02.017 [DOI] [PubMed] [Google Scholar]
- 34.Kim MY, Cho N, Koo HR, Yun BL, Bae MS, Chie EK, et al. Predicting local recurrence following breast-conserving treatment: parenchymal signal enhancement ratio (SER) around the tumor on preoperative MRI. Acta Radiol 2013; 54: 731–8. doi: https://doi.org/10.1177/0284185113483676 [DOI] [PubMed] [Google Scholar]
- 35.Kim SA, Cho N, Ryu EB, Seo M, Bae MS, Chang JM, et al. Background parenchymal signal enhancement ratio at preoperative MR imaging: association with subsequent local recurrence in patients with ductal carcinoma in situ after breast conservation surgery. Radiology 2014; 270: 699–707. doi: https://doi.org/10.1148/radiol.13130459 [DOI] [PubMed] [Google Scholar]
- 36.Kim YJ, Kim SH, Choi BG, Kang BJ, Kim HS, Cha ES, et al. Impact of radiotherapy on background parenchymal enhancement in breast magnetic resonance imaging. Asian Pac J Cancer Prev 2014; 15: 2939–43. doi: https://doi.org/10.7314/APJCP.2014.15.7.2939 [DOI] [PubMed] [Google Scholar]
- 37.King V, Goldfarb SB, Brooks JD, Sung JS, Nulsen BF, Jozefara JE. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology 2012; 264: 670–8. doi: https://doi.org/10.1148/radiol.12112669 [DOI] [PubMed] [Google Scholar]
- 38.King V, Kaplan J, Pike MC, Liberman L, David Dershaw D, Lee CH, et al. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J 2012; 18: 527–34. doi: https://doi.org/10.1111/tbj.12002 [DOI] [PubMed] [Google Scholar]
- 39.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol 2012; 22: 2641–7. doi: https://doi.org/10.1007/s00330-012-2553-8 [DOI] [PubMed] [Google Scholar]
- 40.Kohara S, Ishigaki S, Satake H, Kawamura A, Kawai H, Kikumori T, et al. Background parenchymal enhancement in preoperative breast MRI. Nagoya J Med Sci 2015; 77: 373–82. [PMC free article] [PubMed] [Google Scholar]
- 41.Koo HR, Moon WK, Chun IK, Eo JS, Jeyanth JX, Chang JM, et al. Background 18F-FDG uptake in positron emission mammography (PEM): correlation with mammographic density and background parenchymal enhancement in breast MRI. Eur J Radiol 2013; 82: 1738–42. doi: https://doi.org/10.1016/j.ejrad.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 42.Myers KS, Kamel IR, Macura KJ. MRI-guided breast biopsy: outcomes and effect on patient management. Clin Breast Cancer 2015; 15: 143–52. doi: https://doi.org/10.1016/j.clbc.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park SY, Kang DK, Kim TH. Does background parenchymal enhancement on MRI affect the rate of positive resection margin in breast cancer patients? Br J Radiol 2015; 88: 20140638. doi: https://doi.org/10.1259/bjr.20140638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Preibsch H, Wanner L, Bahrs SD, Wietek BM, Siegmann-Luz KC, Oberlecher E, et al. Background parenchymal enhancement in breast MRI before and after neoadjuvant chemotherapy: correlation with tumour response. Eur Radiol 2016; 26: 1590–6. doi: https://doi.org/10.1007/s00330-015-4011-x [DOI] [PubMed] [Google Scholar]
- 45.Price ER, Brooks JD, Watson EJ, Brennan SB, Comen EA, Morris EA. The impact of bilateral salpingo-oophorectomy on breast MRI background parenchymal enhancement and fibroglandular tissue. Eur Radiol 2014; 24: 162–8. doi: https://doi.org/10.1007/s00330-013-2993-9 [DOI] [PubMed] [Google Scholar]
- 46.Scaranelo AM, Carrillo MC, Fleming R, Jacks LM, Kulkarni SR, Crystal P. Pilot study of quantitative analysis of background enhancement on breast MR images: association with menstrual cycle and mammographic breast density. Radiology 2013; 267: 692–700. doi: https://doi.org/10.1148/radiol.13120121 [DOI] [PubMed] [Google Scholar]
- 47.Schrading S, Schild H, Kühr M, Kuhl C. Effects of tamoxifen and aromatase inhibitors on breast tissue enhancement in dynamic contrast-enhanced breast MR imaging: a longitudinal intraindividual cohort study. Radiology 2014; 271: 45–55. doi: https://doi.org/10.1148/radiol.13131198 [DOI] [PubMed] [Google Scholar]
- 48.Uematsu T, Kasami M, Watanabe J. Background enhancement of mammary glandular tissue on breast dynamic MRI: imaging features and effect on assessment of breast cancer extent. Breast Cancer 2012; 19: 259–65. doi: https://doi.org/10.1007/s12282-011-0279-0 [DOI] [PubMed] [Google Scholar]
- 49.Yoon HJ, Kim Y, Lee JE, Kim BS. Background 99mTc-methoxyisobutylisonitrile uptake of breast-specific gamma imaging in relation to background parenchymal enhancement in magnetic resonance imaging. Eur Radiol 2015; 25: 32–40. doi: https://doi.org/10.1007/s00330-014-3400-x [DOI] [PubMed] [Google Scholar]
- 50.Chen JH, Yu HJ, Hsu C, Mehta RS, Carpenter PM, Su MY. Background parenchymal enhancement of the contralateral normal breast: association with tumor response in breast cancer patients receiving neoadjuvant chemotherapy. Transl Oncol 2015; 8: 204–9. doi: https://doi.org/10.1016/j.tranon.2015.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen JH, Yu H, Lin M, Mehta RS, Su MY. Background parenchymal enhancement in the contralateral normal breast of patients undergoing neoadjuvant chemotherapy measured by DCE-MRI. Magn Reson Imaging 2013; 31: 1465–71. doi: https://doi.org/10.1016/j.mri.2013.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hattangadi J, Park C, Rembert J, Klifa C, Hwang J, Gibbs J, et al. Breast stromal enhancement on MRI is associated with response to neoadjuvant chemotherapy. AJR Am J Roentgenol 2008; 190: 1630–6. doi: https://doi.org/10.2214/AJR.07.2533 [DOI] [PubMed] [Google Scholar]
- 53.Hegenscheid K, Schmidt CO, Seipel R, Laqua R, Ohlinger R, Hosten N, et al. Contrast enhancement kinetics of normal breast parenchyma in dynamic MR mammography: effects of menopausal status, oral contraceptives, and postmenopausal hormone therapy. Eur Radiol 2012; 22: 2633–40. doi: https://doi.org/10.1007/s00330-012-2544-9 [DOI] [PubMed] [Google Scholar]
- 54.Hegenscheid K, Schmidt CO, Seipel R, Laqua R, Ohlinger R, Kühn JP, et al. Normal breast parenchyma: contrast enhancement kinetics at dynamic MR mammography—influence of anthropometric measures and menopausal status. Radiology 2013; 266: 72–80. doi: https://doi.org/10.1148/radiol.12112590 [DOI] [PubMed] [Google Scholar]
- 55.Kang SS, Ko EY, Han BK, Shin JH, Hahn SY, Ko ES. Background parenchymal enhancement on breast MRI: influence of menstrual cycle and breast composition. J Magn Reson Imaging 2014; 39: 526–34. doi: https://doi.org/10.1002/jmri.24185 [DOI] [PubMed] [Google Scholar]
- 56.Klifa C, Suzuki S, Aliu S, Singer L, Wilmes L, Newitt D, et al. Quantification of background enhancement in breast magnetic resonance imaging. J Magn Reson Imaging 2011; 33: 1229–34. doi: https://doi.org/10.1002/jmri.22545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazurowski MA, Zhang J, Grimm LJ, Yoon SC, Silber JI. Radiogenomic analysis of breast cancer: luminal B molecular subtype is associated with enhancement dynamics at MR imaging. Radiology 2014; 273: 365–72. doi: https://doi.org/10.1148/radiol.14132641 [DOI] [PubMed] [Google Scholar]
- 58.Mousa NA, Eiada R, Crystal P, Nayot D, Casper RF. The effect of acute aromatase inhibition on breast parenchymal enhancement in magnetic resonance imaging: a prospective pilot clinical trial. Menopause 2012; 19: 420–25. doi: https://doi.org/10.1097/gme.0b013e31823772a8 [DOI] [PubMed] [Google Scholar]
- 59.Schrading S, Kuhl CK. Breast cancer: influence of taxanes on response assessment with dynamic contrast-enhanced MR imaging. Radiology 2015; 277: 687–96. doi: https://doi.org/10.1148/radiol.2015150006 [DOI] [PubMed] [Google Scholar]
- 60.Wu S, Weinstein SP, DeLeo MJ, 3rd, Conant EF, Chen J, Domchek SM, et al. Quantitative assessment of background parenchymal enhancement in breast MRI predicts response to risk-reducing salpingo-oophorectomy: preliminary evaluation in a cohort of BRCA1/2 mutation carriers. Breast Cancer Res 2015; 17: 67. doi: https://doi.org/10.1186/s13058-015-0577-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Q, Li L, Zhang J, Shao G, Zheng B. A new quantitative image analysis method for improving breast cancer diagnosis using DCE-MRI examinations. Med Phys 2015; 42: 103–9. doi: https://doi.org/10.1118/1.4903280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Carbonaro LA, Pediconi F, Verardi N, Trimboli RM, Calabrese M, Sardanelli F. Breast MRI using a high-relaxivity contrast agent: an overview. AJR Am J Roentgenol 2011; 196: 942–55. doi: https://doi.org/10.2214/AJR.10.4974 [DOI] [PubMed] [Google Scholar]
- 63.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011; 155: 529–36. doi: https://doi.org/10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]