Abstract
Objective:
To determine the adverse drug reaction (ADR) profile of non-ionic iodinated contrast media in populations with underlying diseases and risk factors and to provide guidance for more safe and rational use of iodinated contrast media (ICMs) in the clinic.
Methods:
Data from 120,822 cases who underwent enhanced CT examination in our hospital from January 2014 to March 2016 were collected. A standardized case report form was used for data collection and analysis.
Results:
The incidence of ADRs was 0.4% and 0.44% in patients with and without underlying diseases, respectively (p = 0.378). Risk factor analysis revealed that patients with asthma had the highest incidence of ADRs, followed by patients with cardiac insufficiency and patients who were aged had the lowest incidence. There was a low incidence of ADRs in patients under metformin (0.36%) and β-adrenaline receptor antagonist (0.20%) medication. The incidence was the highest in patients with previous ADRs to ICMs (7.17%) and the lowest in those with a history of ICM usage but no previous reactions (0.32%). ADRs were more common in patients at high risk at a higher injection dose (≥100 ml; p < 0.01) and speed (≥5 ml s−1; p < 0.01).
Conclusion:
The incidence of ADRs was extremely low in patients regardless of underlying diseases. Some high-risk factors have certain correlations with the occurrence of ADRs. Particular attention should be given to patients at high risk when performing enhanced CT examination.
Advances in knowledge:
The correlation between various risk factors and underlying diseases and ADRs was comprehensively analyzed in a large-scale population.
INTRODUCTION
Non-ionic iodinated contrast media (NICMs) are the preferred option for enhanced CT examination currently worldwide, and many surveillance studies have investigated their safety profiles for clinical use.1–3 Despite being generally considered to be relatively safe, adverse drug reactions (ADRs) still occur in a significant number of patients, ranging from simple cutaneous manifestations to fatal and life-threatening complications.4–6 Some relevant underlying diseases and uncertain risk factors, defined by the Chinese Society of Radiology (CSR), American College of Radiology (ACR) and European Society of Urogenital Radiology, bring confusion to clinicians and radiologists on the screening and assessment of patients.7–9 The risk magnitude of an enhanced CT examination for the special population is still controversial and lacks sufficient clinical evidence. There have been no unified evaluation standards on weighing the risk–benefit for receiving iodinated contrast media (ICMs) in clinical practice yet. Katayama et al2 reported that patients with cardiac diseases were at increased risk for ADRs to ICMs. However, there are no large-scale clinical trials involving various ICMs to support this. Up till now, there have been very few studies on the incidence of ADRs to NICMs in populations with underlying diseases and risk factors. As there are over 75 million ICM-requiring procedures performed annually worldwide, it is urgently needed to identify and separate out the contributing risk factors and underlying diseases associated with ADRs.10 It will help radiologists to better gauge riskiness, make assessment procedures and take more effective precautions, and thus help in improving the safety of patients receiving an enhanced CT examination. Herein, a large-scale clinical observation for up to 26 months from January 2014 was performed aimed at providing further knowledge for clinicians and radiologists.
METHODS AND MATERIALS
Clinical data
From January 2014 to March 2016, a total of 122,293 patients received NICMs in our institution. Among these, 1471 cases were ineligible for analysis and had to be excluded for the following reasons: (1) non-standard form filling and unclear description of underlying diseases; (2) patients who were unconscious, not accompanied and unable to answer detailed medical history enquiry; (3) patients with emergency conditions at night with an unclear medical history. Therefore, 120,822 patients were enrolled in this study. Among these, there were 54,069 females (44.75%) and 66,753 males (55.25%), with a mean age of 57.6 ± 14.6 years (ranging from 0 to 104 years). The ICMs used included iopromide 370 (Bayer Healthcare, Leverkusen, Germany), iodixanol 270 (GE Healthcare, London, UK), iopamidol 350 (Bracco, Milen, Italy), loversol 320 (Jiangsu Hengrui Medicine Co., Ltd, Jiangsu, China), iobitridol 350 (Guerbet, Paris, France) and iohexol 350 (Yangtze River Pharmaceutical Co., Ltd, Jiangsu, China). All the ICMs were intravenously injected by using a high-pressure injector (Ulrich Medical® Inc., Ulm, Germany). The self-designed case report form (Supplementary Table A) included the following information: patient general characteristics, underlying diseases, risk factors, history of ICM usage and previous reactions, other allergic history, special medication (metformin and β-adrenergic receptor antagonists), type, dose and injection speed of ICMs, scanning regions, clinical presentations of ADRs and treatments. This study was approved by the institutional review board of our institution.
Inclusion criteria
Patients who were routinely examined were carefully assessed and screened according to the case report form of ADRs to NICMs before undergoing the enhanced CT examination. The underlying diseases and risk factors were recorded in detail. All the patients were informed about the indications and contradictions for receiving NICMs and signed informed consent before examination. For the cases with risk factors of ADRs that needed to receive examination indeed, clinicians in charge were asked to help weigh the pros and cons and make decisions to whether or not to perform the examination.
Classification criteria of non-ionic iodinated contrast medium-related adverse drug reactions
NICM-related ADRs were classified according to the criteria issued by CSR (v. 2) and ACR (v. 9).7,8 Physiological reactions such as heat sensation and angialgia were excluded.
Statistical analysis
The SPSS® v. 19.0 (IBM Corp., New York, NY; formerly SPSS Inc., Chicago, IL) software was used and Pearson's χ2 analysis was performed for comparisons. A p-value of <0.05 was considered to establish a statistically significant difference.
RESULTS
Incidence of adverse drug reactions
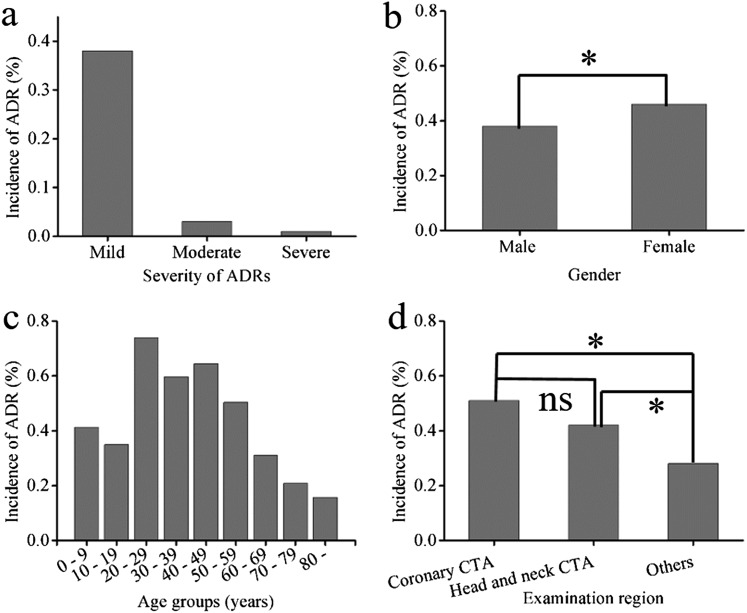
The details of patient demographics and baseline characteristics are shown in Table 1. The overall incidence of ADRs was 0.42% (n = 506). The mild, moderate and severe ADRs accounted for 90.91%, 7.71% and 1.38% incidences, respectively (Figure 1a). Supplementary Table B shows the characteristics of seven cases that developed severe ADRs. The patients who developed ADRs included 256 males (50.59%) and 250 females (49.41%), with a mean age of 52.3 ± 13.7 years (ranging from 4 to 90 years). The incidence of ADRs in males (0.38%) was lower than that in females (0.46%) (p < 0.05) (Figure 1b). The incidence of ADRs was the highest in the age group of 20–29 years, followed by the 30–59-year age group and the lowest in the ≥80-year age group (p < 0.01) (Figure 1c). All the seven severe ADRs occurred in the 46–90-year age group. The incidence of ADRs was 0.51% (120 cases), 0.42% (140 cases) and 0.28% (246 cases) for patients who underwent coronary CT angiography (CTA), head and neck CTA and examination of the body and other regions, respectively. The difference was statistically significant (p < 0.01) (Figure 1d). ADRs occurred more commonly in patients with body mass index >24 than in those with a normal weight (body mass index = 18.5–23.9; p = 0.027).
Table 1.
Patient demographics and clinical characteristics
| Characteristics | Number of patients (%) | ADR (%) |
|---|---|---|
| Total | 120,822 | 506 (0.42) |
| Mean age (years) | 57.6 ± 14.6 | 52.3 ± 13.7 |
| Age range (years) | 0–104 | 4–90 |
| Gender | ||
| Male | 66,753 (55.25) | 256 (0.38) |
| Female | 54,069 (44.75) | 250 (0.46) |
| Examination regions | ||
| Coronary CTA | 23,718 (16.20) | 120 (0.51) |
| Head and neck CTA/CTP | 33,707 (23.00) | 140 (0.42) |
| The body and other regions | 88,970 (60.82) | 246 (0.28) |
| Age groups (years) | ||
| 0–9 | 485 (0.40) | 2 (0.41) |
| 10–19 | 1146 (0.95) | 4 (0.35) |
| 20–29 | 3382 (2.80) | 25 (0.74) |
| 30–39 | 6874 (5.69) | 41 (0.60) |
| 40–49 | 21,751 (18) | 140 (0.64) |
| 50–59 | 27,853 (23.05) | 140 (0.50) |
| 60–69 | 33,263 (27.53) | 103 (0.31) |
| 70–79 | 19,681 (16.29) | 41 (0.21) |
| ≥80 | 6387 (5.29) | 10 (0.16) |
| BMI | ||
| <18.5 | 11,801 (9.77) | 44 (0.37) |
| 18.5–23.9 | 61,050 (50.53) | 236 (0.39) |
| ≥24 | 47,381 (39.22) | 225 (0.47) |
| Unknown | 590 (0.49) | 1 (0.17) |
| Injection dose (ml) | ||
| ≤60 | 51,321 (42.48) | 216 (0.42) |
| 61–99 | 53,650 (44.40) | 197 (0.37) |
| ≥100 | 12,264 (10.15) | 73 (0.60) |
| Unknown | 3587 (2.97) | 20 (0.56) |
| Injection speed (ml s−1) | ||
| <4 | 28,469 (23.56) | 85 (0.30) |
| 4–4.9 | 42,366 (35.06) | 153 (0.36) |
| ≥5 | 42,878 (35.49) | 245 (0.57) |
| Unknown | 7109 (5.88) | 23 (0.32) |
| Types of NICMs | ||
| Iso-osmolar | 18,614 (15.41) | 129 (0.69) |
| Hypo-osmolar | 102208 (84.59) | 377 (0.37) |
ADR, adverse drug reaction; BMI, body mass index; CTA, CT angiography; CTP, CT perfusion imaging; NICM, non-ionic iodinated contrast medium.
Figure 1.
Incidences of adverse drug reactions (ADRs) with different severities (a) and in patients of different sexes (b), age groups (c) and examination regions (d). CTA, CT angiography; ns, not significant.
Incidence of adverse drug reactions in patients with underlying diseases
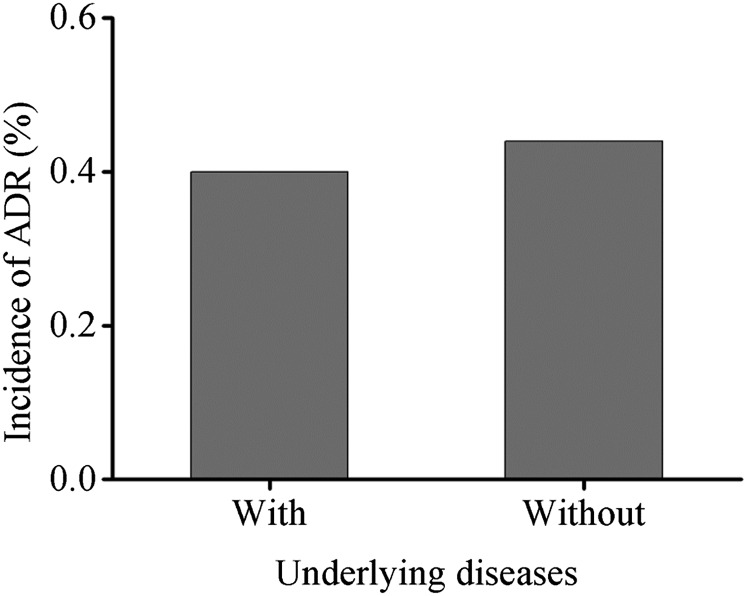
In the present study, there were 58,599 patients (84,982 cases) with different underlying diseases, of which 237 patients (322 cases) developed ADRs, with an incidence of 0.40% (0.38%). In the various underlying diseases (Table 2), ADRs were most common in patients with diseases of the thyroid (hyperthyroidism, hypothyroidism, thyroiditis, thyroid nodule and thyroid carcinoma), followed by the respiratory system (asthma, chronic bronchitis, obstructive pulmonary emphysema, lung cancer, pulmonary tuberculosis, pneumocomosis and nasopharynx cancer), the nervous system (brain tumour and cerebrovascular diseases), hepatic (hepatitis, liver cancer and liver cirrhosis), renal (renal tumour, chronic nephritis and renal insufficiency), cardiac (coronary disease, congenital heart disease, rheumatic heart disease and cardiac insufficiency), diabetes, hypertension, haematological system (leukaemia, multiple myeloma, lymphoma and non-Hodgkin's lymphoma), gastrointestinal (gastrointestinal cancer and ileus), pelvic (cervical, ovarian, bladder and prostatic cancers) and others (breast cancer and multiple injuries). In the various risk factors, the incidence of ADRs was the highest in patients with asthma, followed by those with cardiac insufficiency and the lowest in patients who were aged (Table 3). No ADRs occurred in patients with prior kidney surgery, impaired renal function, hyperthyroidism, nephrotoxic medication and myasthenia gravis. 269 of 61,494 cases without underlying diseases developed ADRs, with an incidence of 0.44%. There was no difference in ADR incidence in patients with and without underlying diseases (p = 0.378) (Figure 2).
Table 2.
Incidence of adverse drug reactions (ADRs) in patients with underlying diseases (n = 84,982)
| Underlying diseases | Total patients | ADR (%) |
|---|---|---|
| Thyroid | 571 | 5 (0.88) |
| Respiratory system | 6205 | 37 (0.60) |
| Nervous system | 4867 | 27 (0.55) |
| Hepatic | 4046 | 20 (0.49) |
| Renal | 1210 | 5 (0.41) |
| Cardiac | 12,709 | 46 (0.36) |
| Hypertension | 32,500 | 117 (0.36) |
| Diabetes | 14,769 | 47 (0.32) |
| Gastrointestinal system | 4839 | 10 (0.21) |
| Pelvic | 1563 | 3 (0.19) |
| Haematological system | 222 | 0 |
| Others | 752 | 4 (0.53) |
| Unknown | 729 | 1 (0.14) |
Each patient may suffer from one or more underlying diseases. There were nine cases of severe ADRs in total, of which one case suffered from cardiac disease and hypertension, and another case suffered from diabetes and hypertension.
Table 3.
Incidence of adverse drug reactions (ADRs) in patients with high-risk factors (n = 68,808)
| Risk factors | Total patients | ADR (%) |
|---|---|---|
| Asthma | 147 | 3 (2.04) |
| Cardiac insufficiency | 91 | 1 (1.10) |
| Gout | 142 | 1 (0.70) |
| Coronary heart disease | 6147 | 34 (0.55) |
| Hypertension | 22,379 | 116 (0.52) |
| Chemoradiotherapy | 3439 | 18 (0.52) |
| Diabetes with metformin medication | 12,732 | 46 (0.36) |
| Age > 70 years | 23,557 | 46 (0.20) |
| Prior kidney surgery | 77 | 0 |
| Impaired renal function | 45 | 0 |
| Hyperthyroidism | 24 | 0 |
| Nephrotoxic medication | 23 | 0 |
| Myasthenia gravis | 5 | 0 |
Each patient may have one or more risk factors.
Figure 2.
Incidence of adverse drug reactions (ADRs) in patients with underlying diseases.
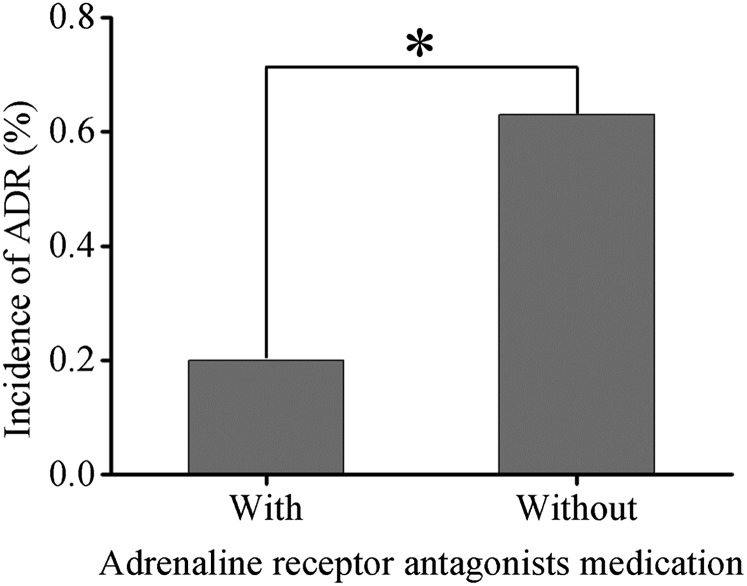
In the present study, there were 12,732 cases that suffered from diabetes with metformin medication, of which 46 (0.36%) cases developed ADRs, and no severe ADRs were found. There were 18,934 cases that underwent coronary CTA, 5128 cases of which received adrenaline receptor antagonist medication. Among these, 10 (0.20%) cases developed ADRs, and 1 severe ADR was found. Patients with adrenaline receptor antagonist medication had a lower incidence of ADRs than those without (0.63%, 87 cases) (p < 0.01) (Figure 3).
Figure 3.
Incidence of adverse drug reactions (ADRs) in patients with adrenaline receptor antagonist medication (n = 18,934).
Incidence of adverse drug reactions in patients with allergic history
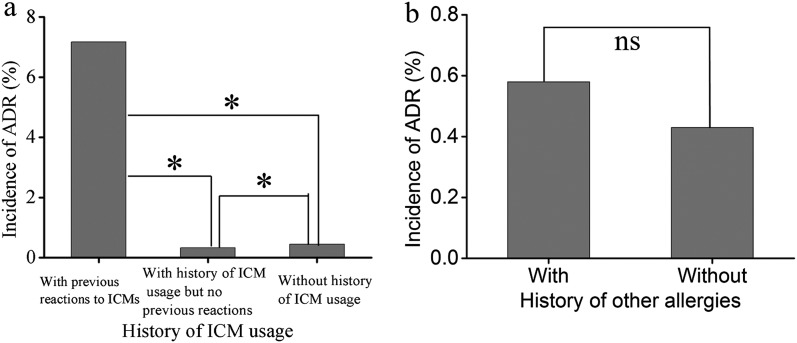
In the present study, 10,592 cases had an allergic history, of which 85 cases had >1 allergic history. The incidence of ADRs in patients with an allergic history (0.82%, 87 cases) was significantly higher than that in those without (0.38%, 419 cases) (p < 0.01). Patients with previous ADRs to ICMs (7.17%) were at a 20-fold increased risk for developing ADRs than those with previous ICM examination but no prior ICM reactions (0.32%) (Figure 4a). There was no significant difference between patients with (0.58%) and without (0.43%) a history of other allergy (p = 0.106) (Figure 4b). Six cases of severe ADRs had no allergic history.
Figure 4.
Incidences of adverse drug reactions (ADRs) in patients with a history of iodinated contrast medium (ICM) usage (a, n = 120,822) and other allergies (b, n = 76,656). ns, not significant.
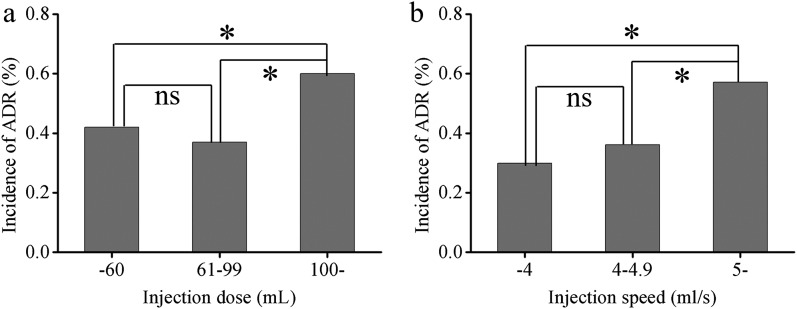
Incidence of adverse drug reactions in patients with different injection doses and velocities
The incidence of ADRs was the lowest at an injection dose of 61–99 ml (0.37%, 197/53,650 cases) and the highest when ≥100 ml (0.60%, 73/12,264 cases) (Figure 5a). There was a significant difference between the ≥100-ml and ≤60-ml groups (0.42%, 216/51,321 cases) (p < 0.05), while there was no difference between the ≤60-ml and 61–99-ml groups (p = 0.165). The incidence of ADRs was the lowest at an injection speed of <4 ml s−1 (0.30%, 85/28,469 cases) and the highest when ≥5 ml s−1 (0.57%, 245/42,878 cases) (Figure 5b). There was a significant difference between the ≥5-ml s−1 and 4–4.9-ml s−1 groups (0.36%, 153/42,366 cases) (p < 0.01), while there was no difference between the <4-ml s−1 and 4–4.9-ml s−1 groups (p = 0.158).
Figure 5.
Incidences of adverse drug reactions (ADRs) in patients with different injection doses and speeds. ns, not significant.
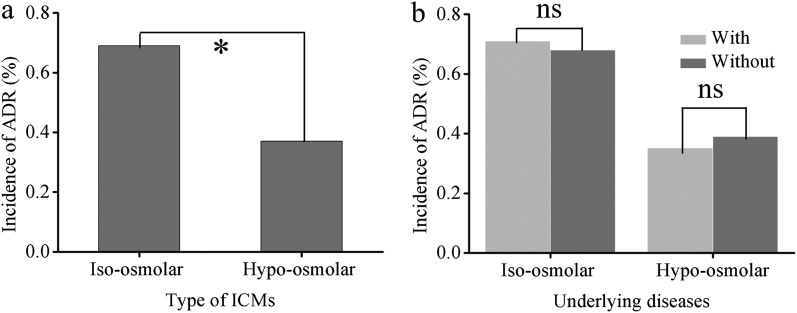
Incidence of adverse drug reactions in patients receiving different types of non-ionic iodinated contrast media
In the present study, 129 (0.69%) of 18,614 cases receiving iso-osmolar ICMs developed ADRs. The incidence of mild, moderate and severe ADRs was 0.63% (117 cases), 0.06% (11 cases) and 0.01% (1 case), respectively. Of these, the incidence of ADRs was 0.71% and 0.68% in patients with and without underlying diseases, respectively (p = 0.858) (Figure 6b). 377 (0.37%) of 102,208 cases receiving hypo-osmolar ICMs developed ADRs. The incidence of mild, moderate and severe ADRs was 0.34% (343 cases), 0.03% (28 cases) and 0.01% (6 cases), respectively. Among these patients, the incidence of ADRs was 0.35% and 0.39% in patients with and without underlying diseases, respectively (p = 0.24) (Figure 6b). The total incidence of ADRs in patients receiving iso-osmolar ICMs was higher than that in those receiving hypo-osmolar ICMs (p < 0.01) (Figure 6a), mainly presenting as delayed skin symptoms. There was no statistical difference in the incidence of severe ADRs between the two groups.
Figure 6.
Incidence of adverse drug reactions (ADRs) in patients with different types of iodinated contrast media (ICMs). ns, not significant.
DISCUSSION
This clinical observation included 120,822 cases which received an i.v. injection of NICMs for enhanced CT examination. On the basis of our previous retrospective study involving 109,255 cases, the present study gives special emphasis to the ADR incidence to NICMs in populations with underlying diseases and risk factors.1 The overall incidence of ADRs was 0.42%, slightly higher than that in a previous retrospective study (0.34%; p < 0.01), mainly presenting as increased mild ADRs and decreased moderate and severe ADRs. The explanation may be the improvement of assessment methods. When encountering patients with uncertain risk factors of ADRs, clinicians in charge were asked to help weigh the risk–benefit and make decisions to whether perform the examination or not. In order to provide more diagnostic information, the assessment criterion was broadened properly for the cases that need examination indeed. During the observation period after examination, special nurses would ask patients about their discomfort profiles, and the transient mild discomfort (expected for heat sensation and angialgia) was included in ADRs. Despite an increased incidence of ADRs, the overall incidence is still lower than those reported in many studies, ranging from 0.47% to 3.13% (Supplementary Table C), with one notable exception of 0.31% in a latest study.11 The incidence of ADRs in different sexes, age groups and examination regions were similar to that in our previous retrospective study.
There are many studies focused on ICM-induced nephropathy, but very few on the correlation between other underlying diseases and ICM-related ADRs.12–15 The risk factors suggested by various guides bring confusion to clinicians and radiologists on patient assessment. Considering these uncertain risk factors, most hospitals often give up examination to avoid the risk of ADRs, which may bring difficulties in diagnosis. The degree of risk has become a focus problem in ICM usage. Although there was no difference in ADR incidence in patients with and without underlying diseases, all the seven severe ADRs occurred in patients with various types and degrees of underlying diseases, including cardiac diseases, diseases of the nervous system, hypertension, diabetes and diseases of the respiratory system. Cardiac diseases had the highest risk, consistent with previous reports.2,8,16 We also found that ADRs had a connection with the severity of diseases. They more likely occurred in patients with cardiac insufficiency (Level III above), hypertension (Level II above), diabetes requiring treatment, severe asthma, hypertensive encephalopathy and >1 risk disease. The risk factors analysis revealed that patients with asthma had the highest incidence of ADRs, consistent with previous reports,2,3,17–19 followed by those with cardiac insufficiency and the lowest in patients who were aged. However, five patients with severe ADRs were older than 60 years, suggesting that severe ADRs were more common in patients who were elderly, in line with a previous study.1 As the guidelines indicate that renal insufficiency, prior kidney surgery, nephrotoxic medication and hyperthyroidism are risk factors of ADRs, the vast majority of patients with severe renal insufficiency had been ruled out when screening. For a minority of patients requiring examination indeed (including 42 cases with dialysis), the examination was completed within 1 h before dialysis treatment. Rational i.v. and oral hydration were performed before examination, and close monitoring of the renal function and urine output changes was carried out after the examination. All these patients had no bad feedback. 21 cases with hyperthyroidism were in recovery, with normal or minimal abnormal T3/T4 values and mild symptoms. No special changes of manifestations and thyroid function occurred after examination. Unfortunately, long-term follow-up was difficult for these patients after discharge and the potential long-term effect remains further observation. Although there was no notable difference in ADR incidence in patients with and without underlying diseases, all the seven severe ADRs occurred in patients at a high risk. The results suggest that stratification of risk assessment is of great importance to provide guidance for a more safe and rational use of ICMs in the clinic. For patients at risk requiring examination indeed, appropriate interventions should be performed, such as preparation well before the examination, observation of time during examination and adopting an appropriate injection dose and speed.
It has been debatable whether diabetics need to stop metformin medication before enhanced CT examination. The most significant adverse reaction during metformin treatment was potential lactic acidosis in patients who were susceptible, and ICMs were not an independent risk factor for patients taking metformin. ICM-induced nephropathy was rare and was even absent in patients with a normal renal function; it needs special attention only in patients with a potential renal dysfunction.8,20 Biguanides medication is recommended to stop at 48 h before receiving ICMs for all the patients by CSR, while for patients with effective glomerular filtration rate between 30 ml min−1 and 40 ml min−1/1.73 m2 by the European Society of Urogenital Radiology, for patients with a renal dysfunction by the Endocrinology Branch of Chinese Medical Association and for patients with effective glomerular filtration rate below 60 ml min−1/1.73 m2 by the Canadian Society of Radiology, respectively. However, ACR commission and the mainstream opinion agree that it is not necessary to stop metformin medication. These unified suggestions make it difficult for radiologists to assess the renal function of patients comprehensively. Following the clinician advice, 12,732 cases suffering from diabetes received examination without stopping metformin medication. 46 (0.36%) cases developed ADRs, all of which were mild ADRs and mainly presented as skin symptoms. There were no special changes of basic renal function indexes at Day 3 and Day 7 after examination compared with those before examination, such as the levels of serum creatinine, urea nitrogen, glomerular clearance rate and urine output. No discomfort and manifestation of lactic acidosis was found in other patients after examination. The 46 cases of ADRs were not considered to be related to metformin medication. The results suggest that by combining careful risk stratification, rational i.v. and oral hydration prior to examination and limited dosage of ICMs for patients with metformin medication, the risk of lactic acidosis will not increase.
According to ACR, β-adrenergic antagonists can decrease the threshold and increase the response intensity of ICM-related ADRs. In this study, 5128 of 18,934 cases that underwent coronary artery CTA received adrenaline receptor antagonist medication to control the heart rate. Among them, 10 (0.20%) cases developed ADRs, including 1 severe ADR. The patient who developed severe ADR had a history of coronary heart disease, with a heart rate of 95 times/min before examination. After taking 25-mg metoprolol tartrate (Betaloc®, AstraZeneca company, Shanghai, China), the heart rate was controlled at 75–76 times/min. After examination for 2 min, chest tightness, dyspnoea, whole-body tingling and pale complexion occurred for this patient. Electrocardiogram monitoring showed a heart rate of 56 times/min, blood pressure 66/45 mmHg, respiratory rate 18 times/min and oxygen saturation (SPO2) 98%. After receiving oxygen inhalation, rehydration and vasopressors for 40 min, the symptoms relieved and the patient was transferred to the ward for further treatment and observation. This patient mainly presented as slow rhythm of heart and hypotension. Considering the low dosage of Betaloc, this severe ADR was more likely to be associated with underlying disease or vasovagal reflex, rather than Betaloc itself. The incidence of ADRs in patients receiving β-adrenergic antagonist medication was lower than that in those without (p < 0.01), inconsistent with the ACR guideline. Thus, once the indications of β-adrenergic antagonists were mastered in clinical practice, the risk of ADRs did not increase.
The correlation between prior allergy and ADRs was also observed in this study (Table 4). Patients with previous ADRs to ICMs are at 3.6–12-fold risk of repeat ADRs to ICMs.3,18,19,21 In our study, the incidence of ADRs in patients with previous ADRs to ICMs was 20 times higher than that in those with previous ICM examination but no prior ICM reactions (Figure 4a), including 20 mild and 1 moderate ADRs. The patients without a history of ICM usage had a moderate incidence, but contributed to all the seven cases of severe ADRs. A possible explanation for this phenomenon was special focus on patients with previous reactions to ICMs. Patients with a history of severe hypersensitivity had been ruled out when screening and subsequent ICMs was avoided. Owing to the first time of receiving ICMs for patients without a history of ICM usage, potential risk factors were lack of self-knowledge and easy underestimation or neglect when screening, resulting in a higher incidence of ADRs. Previous studies demonstrated that patients with prior hypersensitivity were at an increased risk of developing ADRs than those without.3,21–23 In the present study, there was no difference of ADR incidence in patients with and without other history of allergy (Figure 4b). On the whole, ADRs were significantly more common in patients with a history of allergy than that in those without. The incidence was the highest in patients with previous ADRs to ICMs, mainly presenting as mild ADRs, of which most were skin symptoms and rarely fatal and life-threatening. Notably, the reaction of patients to ICMs at different times is uncertain and unpredictable.24 Patients without previous reactions to ICMs may have developed ADRs when receiving ICMs repeatedly. So, the risk–benefit assessment should be seriously considered by radiologists when screening. In this study, six cases of severe ADRs occurred in patients without a history of ICM usage and other allergy, of which three cases were hypertension, two cases were coronary heart disease and one case was cerebral infarction. These severe ADRs may be related to their underlying diseases. Observation, prevention and nursing should be strengthened for patients with not only a history of allergy but also high-risk diseases.
Table 4.
Incidence of adverse drug reactions (ADRs) in patients allergic to different types of allergens (n = 11,195)
| Types of allergy | Total patients | ADR (%) |
|---|---|---|
| ICMs | 293 | 21 (7.17) |
| Pollinosis | 78 | 2 (2.56) |
| Specific allergy | 41 | 1 (2.44) |
| Drugs | 9129 | 61 (0.67) |
| Foods | 860 | 5 (0.58) |
| Others | 263 | 5 (1.90) |
| Unknown | 531 | 0 (0.00) |
ICM, iodinated contrast medium.
Each patient may be allergic to more than one type of allergen.
The correlations between injection dosage, speed and the incidence of ADRs are complicated issues. ICM-induced ADRs are divided into dose-related type (chemotoxic reaction) and non-dose-related type (anaphylactic reaction). This study focuses on the relationship between injection dosage, speed and the incidence of ADRs in patients with underlying diseases. Patients receiving a high injection dose (≥100 ml) and speed (≥5 ml s−1) were at increased risk of developing ADRs. In patients with a high injection dose (≥100 ml) and speed (≥5 ml s−1), the majority of them received coronary CTA and head–neck CTA/CT perfusion imaging examination (Supplementary Table D). Most of these patients suffered from cadiocerebrovascular diseases and they mainly presented as increased mild ADRs. Two of seven cases which developed severe ADRs underwent multiple-region examination with an injection dose of ≥100 ml and four of the seven were at an injection speed of ≥5 ml s−1. It indicated that there is a correlation between injection dosage, speed and the incidence of severe ADRs in patients with cadiocerebrovascular diseases. The i.v. infusion volume and speed were strictly controlled for such patients in the clinic. However, rapid injection of ICMs in a short time may worsen the manifestations of cadiocerebrovascular diseases (e.g. palpitation, dizziness, dyspnoea) or even induce other symptoms. The types and degrees of underlying diseases, injection dose and speed are three important factors for causing severe ADRs. Injection dose and speed should be strictly controlled for patients with underlying high-risk diseases. Low flow rate and dose are recommended for examination under the precondition of normal diagnosis.
In this study, patients receiving iso-osmolar ICMs had a higher overall incidence of ADRs than those receiving hypo-osmolar ICMs (p < 0.01), while there was no difference in severe ADRs. It is not clear what is responsible for this phenomenon. The likely explanation is that patients receiving iso-osmolar ICMs have a high incidence of delayed skin symptoms. It may also be attributable to the chemical structure and pharmacological mechanism of ICMs with different osmolalities. There was no apparent difference in the incidence of ADRs in patients with and without risk factors in each group. Among the seven cases that developed severe ADRs, two cases received iopromide 370, one case iodixanol 270, one case iopamidol 350, one case loversol 320, one case iobitridol 350 and one case iohexol 350. This suggests that severe ADRs may have occurred for all kinds of ICMs.
The major strength of this clinical observation is that it included a very large patient population enrolled in various types of imaging examinations, which ensures the accuracy and reliability of the results. Compared with multicentre studies, it minimizes regional variations between constitutions. Comprehensive and detailed analyses were performed on the incidence of ADRs to NICMs in populations with underlying diseases and risk factors. As with other surveillance studies, this study also has limitations. There was no elaboration on the entities and clinical severity of underlying diseases. For outpatients with ADRs and patients with ADRs who were discharged, no long-term follow-up was conducted and some delayed-type ADRs may be missed, which need further observation and summary.
CONCLUSION
The incidence of ADRs following NICM administration was extremely low in patients regardless of underlying diseases, and no death was found, indicating the well-established good safety profile of NICMs in the clinic. Some risk factors and underlying diseases have correlations with the occurrence of ADRs. ADRs are more common in patients at a high risk at a high injection dose (≥100 ml) and speed (≥5 ml s−1) and present a small relationship with the types of NICMs, metformin and adrenaline receptor antagonist medication. The incidence was the highest in patients with previous ADRs to ICMs and the lowest in those with a history of ICM usage but no prior reactions. Particular attention should be paid to patients with underlying diseases (e.g. cardiac disease, asthma, hypertension, diabetes and cerebrovascular disease) and those at high risk when performing enhanced CT examination.
Acknowledgments
ACKNOWLEDGMENTS
The authors would like to thank all the nurses in our department for data collection.
Contributor Information
Xue Li, Email: lixue928136@163.com.
Heng Liu, Email: 303609940@qq.com.
Li Zhao, Email: 275398302@qq.com.
Junling Liu, Email: 373072057@qq.com.
Li Cai, Email: 849087005@qq.com.
Lei Liu, Email: ttcrystalma@163.com.
Weiguo Zhang, Email: wgzhang01@163.com.
REFERENCES
- 1.Li X, Chen J, Zhang L, Liu H, Wang S, Chen X, et al. Clinical observation of the adverse drug reactions caused by non-ionic iodinated contrast media: results from 109,255 cases who underwent enhanced CT examination in Chongqing, China. Br J Radiol 2015; 88: 20140491. doi: https://doi.org/10.1259/bjr.20140491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katayama H, Yamaguchi K, Kozuka T, Takashima T, Seez P, Matsuura K. Adverse reactions to ionic and nonionic contrast media. A report from the Japanese Committee on the Safety of Contrast Media. Radiology 1990; 175: 621–8. doi: https://doi.org/10.1148/radiology.175.3.2343107 [DOI] [PubMed] [Google Scholar]
- 3.Kopp AF, Mortele KJ, Cho YD, Palkowitsch P, Bettmann MA, Claussen CD. Prevalence of acute reactions to iopromide: postmarketing surveillance study of 74,717 patients. Acta Radiol 2008; 49: 902–11. doi: https://doi.org/10.1080/02841850802282811 [DOI] [PubMed] [Google Scholar]
- 4.Meth MJ, Maibach HI. Current understanding of contrast media reactions and implications for clinical management. Drug Saf 2006; 29: 133–41. doi: https://doi.org/10.2165/00002018-200629020-00003 [DOI] [PubMed] [Google Scholar]
- 5.Namasivayam S, Kalra MK, Torres WE, Small WC. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg Radiol 2006; 12: 210–5. doi: https://doi.org/10.1007/s10140-006-0488-6 [DOI] [PubMed] [Google Scholar]
- 6.Bettmann MA. Frequently asked questions: iodinated contrast agents. Radiographics 2004; 24(Suppl. 1): S3–10. doi: https://doi.org/10.1148/rg.24si045519 [DOI] [PubMed] [Google Scholar]
- 7.Chinese Society of Radiology. Iodine contrast agents application guideline. 2nd edn; 2013. [Google Scholar]
- 8.American College of Radiology. ACR manual on contrast media. v. 9; 2013. [Google Scholar]
- 9.European Society of Urogenital Radiology. ESUR contrast media guidelines v. 8.1; 2015. [Google Scholar]
- 10.Christiansen C. X-ray contrast media—an overview. Toxicology 2005; 209: 185–7. doi: https://doi.org/10.1016/j.tox.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 11.Zhang B, Dong Y, Liang L, Lian Z, Liu J, Luo X, et al. The incidence, classification, and management of acute adverse reactions to the low-osmolar iodinated contrast media isovue and ultravist in contrast-enhanced computed tomography scanning. Medicine (Baltimore) 2016; 95: e3170. doi: https://doi.org/10.1097/md.0000000000003170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katzberg RW. Urography into the 21st century: new contrast media, renal handling, imaging characteristics, and nephrotoxicity. Radiology 1997; 204: 297–312. doi: https://doi.org/10.1148/radiology.204.2.9240511 [DOI] [PubMed] [Google Scholar]
- 13.Abujudeh HH, Gee MS, Kaewlai R. In emergency situations, should serum creatinine be checked in all patients before performing second contrast CT examinations within 24 hours? J Am Coll Radiol 2009; 6: 268–73. doi: https://doi.org/10.1016/j.jacr.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 14.Pahade JK, LeBedis CA, Raptopoulos VD, Avigan DE, Yam CS, Kruskal JB, et al. Incidence of contrast-induced nephropathy in patients with multiple myeloma undergoing contrast-enhanced CT. AJR Am J Roentgenol 2011; 196: 1094–101. doi: https://doi.org/10.2214/ajr.10.5152 [DOI] [PubMed] [Google Scholar]
- 15.Trivedi H, Foley WD. Contrast-induced nephropathy after a second contrast exposure. Ren Fail 2010; 32: 796–801. doi: https://doi.org/10.3109/0886022X.2010.495441 [DOI] [PubMed] [Google Scholar]
- 16.Shehadi WH. Adverse reactions to intravascularly administered contrast media. A comprehensive study based on a prospective survey. Am J Roentgenol Radium Ther Nucl Med 1975; 124: 145–52. doi: https://doi.org/10.2214/ajr.124.1.145 [DOI] [PubMed] [Google Scholar]
- 17.Rao QA, Newhouse JH. Risk of nephropathy after intravenous administration of contrast material: a critical literature analysis. Radiology 2006; 239: 392–7. doi: https://doi.org/10.1148/radiol.2392050413 [DOI] [PubMed] [Google Scholar]
- 18.Vogl TJ, Honold E, Wolf M, Mohajeri H, Hammerstingl R. Safety of iobitridol in the general population and at-risk patients. Eur Radiol 2006; 16: 1288–97. doi: https://doi.org/10.1007/s00330-005-0061-9 [DOI] [PubMed] [Google Scholar]
- 19.Maurer M, Heine O, Wolf M, Freyhardt P, Schnapauff D, Hamm B. Safety and tolerability of iobitridol in general and in patients with risk factors: results in more than 160,000 patients. Eur J Radiol 2011; 80: 357–62. doi: https://doi.org/10.1016/j.ejrad.2010.03.018 [DOI] [PubMed] [Google Scholar]
- 20.Thomsen HS; European Society of Urogenital Radiology. European Society of Urogenital Radiology guidelines on contrast media application. Curr Opin Urol 2007; 17: 70–6. doi: https://doi.org/10.1097/MOU.0b013e328011c96f [DOI] [PubMed] [Google Scholar]
- 21.Zhang BC, Hou L, Lv B, Xu YW. Post-marketing surveillance study with iodixanol in 20 185 Chinese patients from routine clinical practices. Br J Radiol 2014; 87: 20130325. doi: https://doi.org/10.1259/bjr.20130325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coakley FV, Panicek DM. Iodine allergy: an oyster without a pearl? AJR Am J Roentgenol 1997; 169: 951–2. doi: https://doi.org/10.2214/ajr.169.4.9308442 [DOI] [PubMed] [Google Scholar]
- 23.Haussler MD. Safety and patient comfort with iodixanol: a postmarketing surveillance study in 9515 patients undergoing diagnostic CT examinations. Acta Radiol 2010; 51: 924–33. [DOI] [PubMed] [Google Scholar]
- 24.Morcos SK. Acute serious and fatal reactions to contrast media: our current understanding. Br J Radiol 2005; 78: 686–93. doi: https://doi.org/10.1259/bjr/26301414 [DOI] [PubMed] [Google Scholar]