Abstract
Objective:
We aimed to evaluate backscatter dose variations in different cranial bone implant materials in an experimental model designed to simulate post-operative radiotherapy.
Methods:
We assessed the radiation backscatter doses associated with sheet- and mesh-type titanium plates and hydroxyapatite (HAP) samples (porosity: 35%, 50% and 85%). The samples were irradiated with 6- and 10-MV photon beams from a linear accelerator. Measurements were obtained using an ionization chamber and radiochromic films cut from the same batch.
Results:
At 6 MV, the titanium sheet showed the highest peak for backscattered radiation, followed by (in decreasing order) HAP30%, HAP50%, titanium mesh and HAP85%. At 10 MV, HAP30% showed the highest peak, followed by HAP50%, titanium sheet, titanium mesh and HAP85%. The peaks were at different depths in the titanium and HAP samples. The thickness of the human scalp is approximately 7 mm; therefore, measurements were obtained 0–7 mm above the implants to assess the likely dose on the scalp. A comparison of the maximum dose on the scalp showed the titanium sheet had the highest dose at both 6 and 10 MV.
Conclusion:
The backscatter dose differed with the density of the material and the backscatter depth was different for each material.
Advances in knowledge:
Ulcer formation due to radiotherapy after brain tumour depends on not only radiation but also the implant material. Therefore, the density and type of implant material should be considered when planning radiotherapy and selecting bone reconstruction materials.
INTRODUCTION
Advanced brain tumours are often treated using a multimodal approach that combines surgery and post-operative radiotherapy. Furthermore, owing to their location, some brain tumours necessitate bone resection and subsequent reconstruction procedures.
The use of alloplastic materials such as titanium plates and hydroxyapatite (HAP) for calvarial reconstruction has recently become more popular.1,2 A few publications have addressed the effects of alloplastic implants on intracranial distributions during linear accelerator-based radiation therapy.3–5
Post-operative radiation can cause ulcer formation, leading to the denudation of skin over the alloplastic material.6 Some reports also describe the influence of osseointegration failure, particularly the failure of titanium screw implants after maxillary and mandibular reconstruction due to oropharyngeal or oral cancer.6–8 Therefore, there is a need to assess the influence of backscatter radiation from cranial implants on the extracranial skin. To our knowledge, no previous study has investigated this issue. In this article, we report the results of backscatter dose variations in artificial bone implants, by employing different photon energies and incidences commonly used in brain radiotherapy practice.
METHODS AND MATERIALS
Materials
The most common alloplastic materials used for cranial reconstruction are titanium plates and HAP.1,2 We prepared two types of titanium plates (sheet and mesh) (Skull-fit; Bear Medic Co. Ltd, Tokyo, Japan), each of which had a thickness of 0.6 mm. The thickness of the HAP was 5 mm with pore diameters between 100 and 500 μmol l−1. We prepared three different porosity types: 35%, 50% and 85% (Apaceram; Hoya Co., Ltd, Tokyo, Japan). The size of each sample was 5 × 5 cm2 (Figure 1).
Figure 1.
The experimental materials: (a) titanium sheet, (b) titanium mesh and (c) hydroxyapatite.
All samples (5 × 5 cm2), which were cut from the same batch, were irradiated with a 6- and 10-MV photon beam from a linear accelerator (Clinac® iX; Varian Medical Systems, Palo Alto, CA). Measurements were performed with an ionization chamber (Model 30013; PTW-Freiburg, Freiburg, Germany) and radiochromic film size 5 × 10 cm2 (Gafchromic EBT2; ISP Corp., Wayne, NJ).
Methods
The implants were set inside a water phantom tank (MP3; PTW-Freiburg). Two water equivalent phantoms (WD-4050; Kyoto Kagaku, Kyoto, Japan) with a size of 30 × 30 × 5 cm3 were placed at the bottom of the water phantom tank. The implants and the films were aligned on the water equivalent phantoms. The implants were placed perpendicular to the beam axis, whereas the films were aligned along the axis in the axial plane (Figure 2). The distances from the beam source to the water surface and to the surface of the implants were set at 90 and 100 cm, respectively. The implants were irradiated with 6- and 10-MV photon beams with a 30 × 30-cm field at 600 MU min−1. The average of the measured doses was obtained three times for each sample. Only the relative dose distributions were used from the film measurement. They were normalized by the dose measured by the ionization chamber to obtain the absolute dose.
Figure 2.
Setup for the measurement of backscatter.
The films were scanned 24 h post irradiation with a flatbed scanner Epson Expression 10000XL (Epson America Inc., Long Beach, CA) using the scanning protocol recommended by Devic et al.9 The scanner settings produced images with a resolution of 72 dots per inch in 48-bit red–green–blue tagged image file format. The films were analysed with a generally used method that averages pixel values inside a region of interest of the scanned film image and converts it to the dose with a dose–response curve. The ImageJ software (National Institutes of Health, Bethesda, MD) was used to analyse the images of the scanned films. The same dose conversion table was used for the 6- and 10-MV beams because the energy dependence of the dose response of the film is negligible.10
The thickness of the human scalp is approximately 7 mm;11 therefore, a depth of 93–100 mm (i.e. 0–7 mm above the implants) represented the scalp. The area under the curve (AUC) between 93 and 100 mm was measured. Each ratio was calculated, based on the AUC of water for 6 MV. Statistical analysis was performed by the one-sample t-test. Statistical significance was set at p < 0.05.
RESULTS
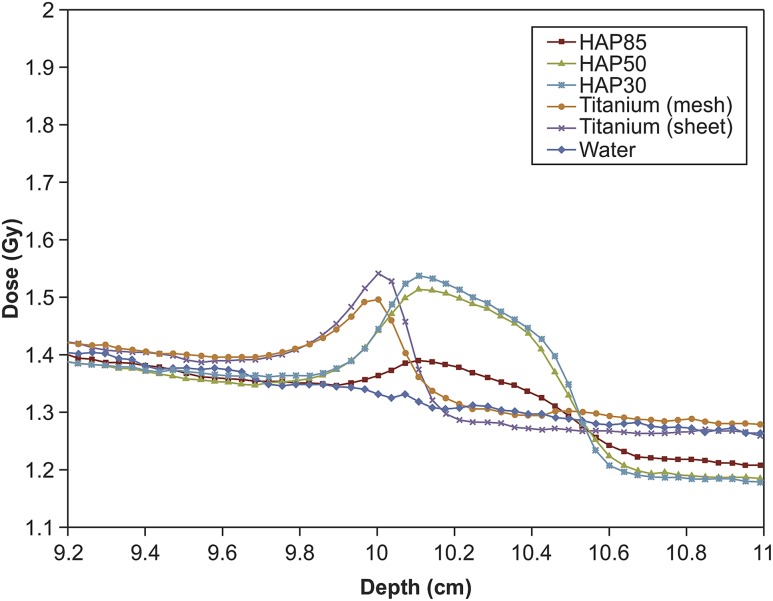
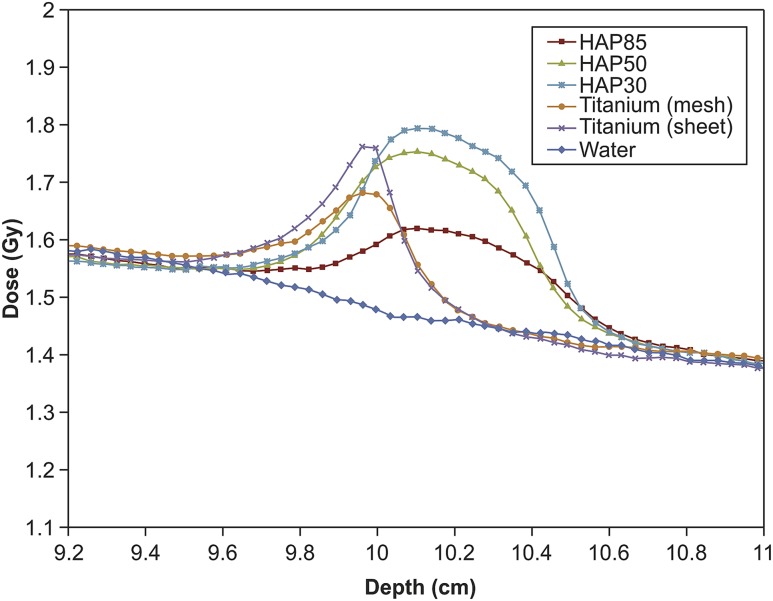
The dose distributions of each implant for the 6- and 10-MV photon beams are presented in Figures 3 and 4, respectively. The dose distributions of the water were measured to serve as a control. The results obtained by the films were normalized at the depth of 8.5 cm. The normalization point was chosen with regard to the dose stability in electron equilibrium and with a sufficient distance from the surface of the implants to avoid obtaining a dose from back-scattered electrons or photons. The peak dose for HAP is effectively within the HAP layer, except when the HAP–alloplast edge is within the field border, in which case, tissue at the edge will be receiving the peak dose due to lateral scatter. The measurement error of the film was estimated as 3.0% at all measurement points. Table 1 shows the maximum doses, maximum doses at the depth of 9.3–10 cm and AUC ratio.
Figure 3.
Dose distribution along the beam axis in the 6-MV photon beam. HAP, hydroxyapatite.
Figure 4.
Dose distribution along the beam axis in the 10-MV photon beam. HAP, hydroxyapatite.
Table 1.
The maximum doses, the depth at maximum dose and the area under the curve (AUC) ratio of each material
| Energy | Material | Max. dose (Gy) | Depth at max. dose (cm) | Max. dose (Gy) at 9.3–10.0 cm | AUC ratio (based on water) |
|---|---|---|---|---|---|
| 6 MV | Titanium sheet | 1.533 ± 0.018 | 9.941 ± 0.041 | 1.533 ± 0.018 | 1.038a |
| Titanium mesh | 1.467 ± 0.014 | 9.941 ± 0.020 | 1.467 ± 0.014 | 1.021a | |
| HAP30% | 1.510 ± 0.001 | 10.118 ± 0.020 | 1.418 ± 0.026 | 0.998 | |
| HAP50% | 1.485 ± 0.018 | 10.106 ± 0.035 | 1.409 ± 0.023 | 0.998 | |
| HAP85% | 1.389 ± 0.029 | 10.059 ± 0.020 | 1.381 ± 0.032 | 0.981 | |
| 10 MV | Titanium sheet | 1.644 ± 0.023 | 9.953 ± 0.020 | 1.644 ± 0.023 | 1.121a |
| Titanium mesh | 1.595 ± 0.018 | 9.952 ± 0.054 | 1.595 ± 0.018 | 1.113a | |
| HAP30% | 1.702 ± 0.015 | 10.094 ± 0.041 | 1.643 ± 0.011 | 1.111a | |
| HAP50% | 1.674 ± 0.012 | 10.118 ± 0.020 | 1.627 ± 0.026 | 1.110a | |
| HAP85% | 1.516 ± 0.003 | 10.106 ± 0.035 | 1.625 ± 0.041 | 1.070a |
HAP, hydroxyapatite; max., maximum.
p < 0.05.
At 6 MV, the maximum value for backscattered radiation (1.533 ± 0.018 Gy) was observed for the titanium sheet, followed by (in decreasing order) HAP30%, HAP50%, titanium mesh and HAP85%. At 10 MV, the maximum peak (1.702 ± 0.015 Gy) was observed for HAP30%, followed by (in decreasing order) HAP50%, titanium sheet, titanium mesh and HAP85%. Each material showed a dose of radioactivity that was higher at 10 mV than at 6 mV.
In addition, the peak dose occurred at the 9.9-mm depth, which was directly on top of the titanium mesh and sheet. This depth lies in the region of the scalp. On the other hand, the peak dose for HAP occurred at a depth of approximately 10.1 cm.
A comparison of the maximum dose on the scalp (mean depth of 9.3–10.0 cm) showed that the titanium sheet had the highest value (1.533 ± 0.018 Gy) at 6 MV, followed by the titanium mesh. Each HAP sample emitted approximately the same dose of backscattered radiation. At 10 MV, the maximum dose occurred in the titanium sheet (1.644 ± 0.023 Gy), followed by (in decreasing order) HAP30%, HAP50%, HAP85% and titanium mesh.
With respect to the AUC ratio, HAP at 6 MV did not show a significant difference between the different porosities. However, all other materials showed significant differences, which suggested that these materials amplified the dose at 6 MV compared with water.
DISCUSSION
Radiotherapy damages small vessels by progressively thickening the subendothelial components of the vessel wall, which leads to fibrosis of the vessels. In addition, radiation induces changes in the skin and subcutaneous tissues that lead to skin atrophy and friability.12,13 As a result, radiotherapy sometimes leads to skin ulcers. To minimize the risk of skin ulcers, the dose of radiation to the skin should be as low as possible.
The cross-section of interactions between photons and materials depends on the atomic number of the material and its density. It has long been known that when metal inhomogeneities with high atomic numbers are in the path of photons or electron beams, the dose distribution is altered and regions of overdosage or underdosage are produced.14–18 Molecules with similar atomic numbers have nearly the same dose distribution. Accordingly, titanium (which is atomic number 22) and HAP, which has calcium (atomic number 20) as a primary component of its structure, have nearly the same dose distribution.
For the same materials, the backscatter dose increased with density. The dose was higher in the sheet type of titanium than in the mesh type of titanium, and the dose was higher in HAP samples with a lower porosity.
The depth of the maximum backscatter dose from each material was interesting. The maximum backscatter dose for titanium occurred at its surface, and the backscatter dose for HAP occurred inside the HAP sample. Although each material is similar in atomic number and has nearly the same dose distribution, the dose to the “skin” was greatly different.
We believe that this difference is caused by the internal structure of the materials. The mesh part in titanium does not reflect photons; therefore, there is little dispersion. Meanwhile, the HAP surface is not flat but irregular because of pores; therefore, the dispersion is high. A portion of the dispersion will be backscatter, and the remaining portion will continue being scattered into the HAP material and enhances the dose, then the effect declines with distance. Therefore, the dose is the highest at the outer layer part of the HAP material.
These findings suggested that the radiation energy should be kept low to prevent radiation ulcers after skull alloplast reconstruction, provided treatment efficacy is not compromised. If the radiation energy can be lowered, the HAP implants should be chosen. After brain tumour resection, HAP should be chosen to prevent radiation ulcers. With regard to the porosity of HAP, the backscatter dose at each porosity level did not change when the energy was low. Therefore, we can conclude that HAP at approximately 30% porosity is much better from the viewpoint of cranial protection.
In conclusion, we investigated the characteristics of backscatter radiation to the scalp in various cranial reconstructions. As a result, we obtained two new findings: (1) the backscatter dose differed, based on the density of the materials and (2) the depth of the backscatter was different for each material.
CONCLUSION
These new findings may be useful for planning radiotherapy and for selecting the reconstruction materials to prevent the risk of skin ulcers.
Contributor Information
Yoshiaki Sakamoto, Email: ysakamoto@z8.keio.jp.
Naoyoshi Koike, Email: koike@rad.med.keio.ac.jp.
Hideyuki Takei, Email: htakei@rad.med.keio.ac.jp.
Mari Ohno, Email: mari.ono@adst.keio.ac.jp.
Tomoru Miwa, Email: tenmiwa@gmail.com.
Kazunari Yoshida, Email: kazrmky@z3.keio.jp.
Naoyuki Shigematsu, Email: shige@rad.med.keio.ac.jp.
Kazuo Kishi, Email: kkishi@a7.keio.jp.
REFERENCES
- 1.Blake GB, MacFarlane MR, Hinton JW. Titanium in reconstruction of the skull and face. Br J Plast Surg 1990; 43: 528–35. doi: https://doi.org/10.1016/0007-1226(90)90115-G [DOI] [PubMed] [Google Scholar]
- 2.Ducic Y. Titanium mesh and hydroxyapatite cement cranioplasty: a report of 20 cases. J Oral Maxillofac Surg 2002; 60: 272–6. doi: https://doi.org/10.1053/joms.2002.30575 [DOI] [PubMed] [Google Scholar]
- 3.Patone H, Barker J, Roberge D. Effects of neurosurgical titanium mesh on radiation dose. Med Dosim 2006; 31: 298–301. doi: https://doi.org/10.1016/j.meddos.2006.05.001 [DOI] [PubMed] [Google Scholar]
- 4.Rakowski JT, Chin K, Mittal S. Effects of titanium mesh implant on dosimetry during gamma knife radiosurgery. J Appl Clin Med Phys 2012; 13: 3833. doi: https://doi.org/10.1120/jacmp.v13i5.3833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimozato T, Yasui K, Kawanami R, Habara K, Aoyama Y, Tabushi K, et al. Dose distribution near thin titanium plate for skull fixation irradiated by a 4-MV photon beam. J Med Phys 2010; 35: 81–7. doi: https://doi.org/10.4103/0971-6203.62199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuttenberger JJ, Hardt N. Long-term results following reconstruction of craniofacial defects with titanium micro-mesh systems. J Craniomaxillofac Surg 2001; 29: 75–81. doi: https://doi.org/10.1054/jcms.2001.0197 [DOI] [PubMed] [Google Scholar]
- 7.Goh BT, Lee S, Stoelinga PJ. Mandibular reconstruction in adults: a review. Int J Oral Maxillofac Surg 2008; 37: 597–605. doi: https://doi.org/10.1016/j.ijom.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 8.Boyd TG, Huber KM, Verbist DE, Bumpous JM, Wilhelmi BJ. Removal of exposed titanium reconstruction plate after mandibular reconstruction with a free fibula osteocutaneous flap with large surgical pin cutters: a case report and literature review. Eplasty 2012; 12: e42. [PMC free article] [PubMed] [Google Scholar]
- 9.Devic S, Seuntjens J, Sham E, Podgorsak EB, Schmidtlein CR, Kirov AS, et al. Precise radiochromic film dosimetry using a flat-bed document scanner. Med Phys 2005; 32: 2245–53. doi: https://doi.org/10.1118/1.1929253 [DOI] [PubMed] [Google Scholar]
- 10.Butson MJ, Cheung T, Yu PK. Weak energy dependence of EBT Gafchromic film dose response in the 50 kVp–10 MVp X-ray range. Appl Radiat Isot 2006; 64: 60–2. doi: https://doi.org/10.1016/j.apradiso.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 11.Parsons FG. The thickness of the living scalp. J Anat 1929; 63(Pt 4): 427–9. [PMC free article] [PubMed] [Google Scholar]
- 12.Guelinckx PJ, Boeckx WD, Fossion E, Gruwez JA. Scanning electron microscopy of irradiated recipient blood vessels in head and neck free flaps. Plast Reconstr Surg 1984; 74: 217–26. doi: https://doi.org/10.1097/00006534-198408000-00008 [DOI] [PubMed] [Google Scholar]
- 13.Olascoage A, Vilar-Compte D, Poitevin-Charcon A, Contreras-Ruiz J. Wound healing in radiated skin: pathophysiology and treatment options. Int Wound J 2008; 5: 246–57. doi: https://doi.org/10.1111/j.1742-481X.2008.00436.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dutreix J, Bernard M. Dosimetry at interfaces for high energy X and gamma rays. Br J Radiol 1966; 39: 205–10. doi: https://doi.org/10.1259/0007-1285-39-459-205 [DOI] [PubMed] [Google Scholar]
- 15.Gibbs FA, Palos B, Goffinet DR. The metal/tissue interface effect in irradiation of the oral cavity. Radiology 1976; 119: 705–7. doi: https://doi.org/10.1148/119.3.705 [DOI] [PubMed] [Google Scholar]
- 16.Gagnon WF, Cundiff JH. Dose enhancement from back-scattered radiation at tissue-metal interfaces irradiated with high energy electrons. Br J Radiol 1980; 53: 466–70. doi: https://doi.org/10.1259/0007-1285-53-629-466 [DOI] [PubMed] [Google Scholar]
- 17.Das IJ, Kahn FM. Backscatter dose perturbation at high atomic number interfaces in megavoltage photon beams. Med Phys 1989; 16: 367–75. doi: https://doi.org/10.1118/1.596345 [DOI] [PubMed] [Google Scholar]
- 18.Das IJ, Kase KR, Meigooni AS, Khan FM, Werner BL. Validity of transition-zone dosimetry at high atomic number interfaces in megavoltage photon beams. Med Phys 1990; 17: 10–6. doi: https://doi.org/10.1118/1.596553 [DOI] [PubMed] [Google Scholar]