Abstract
Pulmonary hypertension (PH) is a rare disease with a significant morbidity and mortality if untreated. The disease has a multifactorial aetiology and is often associated with insidious onset of signs and symptoms. Multimodality imaging is often required for establishing the diagnosis, evaluating the underlying haemodynamic compromise and follow-up after institution of therapy. The range of potential complications associated with PH vary widely. We aimed to summarize the imaging findings of complications that the radiologist should be familiar with.
INTRODUCTION
The classification of pulmonary hypertension (PH) has been updated in 2013 (Table 1).1 The haemodynamic definition of PH is a mean pulmonary arterial pressure ≥25 mmHg at rest or 30 mmHg during exercise during right heart catheterization. There are five main subgroups of PH. Group 1 is pulmonary arterial hypertension (PAH) related to conditions like the connective tissue disease, congenital heart disease, portopulmonary hypertension, secondary to drugs as well as the idiopathic group. By definition, patients with PAH should have a pulmonary artery wedge pressure of ≤15 mmHg and a pulmonary vascular resistance of >3 WU (a Woods unit is defined as 1 WU = 1 mmHg min l−1 = 80 dyne s cm−5). Group 2 is attributed to left heart disease and Group 3 to chronic lung disease. Group 4 is secondary to pulmonary artery obstructions and amongst these, chronic thromboembolic disease is potentially the only surgically treatable condition. Group 5 is PH due to an unclear mechanism or multiple aetiologies. Among these subgroups, Group 2 due to left heart disease is believed to be the commonest. PH is rare with a prevalence of 97 per million in the UK, and the European Society of Cardiology and European Respiratory Society have recommended that expert centres should be responsible for receiving new referrals, assessment, management and research.2
Table 1.
Updated classification of pulmonary hypertension (PH)
| Updated complications of PH |
|---|
| PAH |
| Idiopathic PAH |
| Heritable PAH |
| BMPR2 |
| ALK-1, ENG, SMAD9, CAV1, KCNK3 |
| Unknown |
| Drug and toxin induced |
| Associated with |
| Connective tissue disease |
| HIV infection |
| Portal hypertension |
| Congenital heart disease |
| Schistosomiasis |
| Pulmonary veno-occlusive disease and/or pulmonary capillary haemangiomatosis |
| PPHN |
| PH due to left heart disease |
| Left ventricular systolic dysfunction |
| Left ventricular diastolic dysfunction |
| Valvular disease |
| Congenital/acquired left heart inflow/outflow tract obstruction and congenital cardiomyopathies |
| PH due to lung diseases and/or hypoxia |
| Chronic obstructive pulmonary disease |
| Interstitial lung disease |
| Other pulmonary diseases with mixed restrictive and obstructive pattern |
| Sleep-disordered breathing |
| Alveolar hypoventilation disorders |
| Chronic exposure to high altitude |
| Developmental lung diseases |
| CTEPH |
| PH with unclear multifactorial mechanisms |
| Haematologic disorders: chronic haemolytic anaemia, myeloproliferative disorders, splenectomy |
| Systemic disorders: sarcoidosis, pulmonary histiocytosis, lymphangioleiomyomatosis |
| Metabolic disorders: glycogen storage disease, Gaucher disease, thyroid disorders |
| Others: tumoral obstruction, fibrosing mediastinitis, chronic renal failure, segmental PH |
ALK-1, activin-like receptor kinase 1; BMPR2, bone morphogenic protein receptor type 2; CAV1, caveolin 1; CTEPH, chronic thromboembolic pulmonary hypertension; ENG, endoglin; HIV, human immunodeficiency virus; KCNK3, a gene-encoding potassium channel super family K member 3; PAH, pulmonary arterial hypertension; PPHN, persistent pulmonary hypertension of the newborn; SMAD9, decapentaplegic 9.
The Fifth World Symposium on Pulmonary Hypertension, 2013, Nice, France.
DISCUSSION
Vascular complications
Massive pulmonary dilatation
The range of potential complications associated with PH vary widely (Table 2). The pulmonary artery may increase in size owing to increasing wall sheer stress and volume, e.g. in a left to right shunt. It is considered dilated when the ratio of the main pulmonary artery to the ascending aorta exceeds 0.9. In patients without interstitial lung disease, the upper limit of the pulmonary artery diameter for males is 29 mm and 27 mm for females.3 In patients with interstitial lung disease, the pulmonary artery may be dilated without the corresponding increase in pressure.4 Dilated pulmonary arteries can cause extrinsic compression on the left main stem coronary artery (Figure 1). The left main stem is typically effaced and displaced caudally. Patients may experience angina, myocardial infarction or even sudden death. A potential treatment for left main stem compression includes percutaneous stenting or open bypass, although the optimal treatment is controversial. The former may be more suitable for patients at a high risk. If the cause of PH is congenital heart disease, e.g. atrial septal defect, ventricular septal defect and patent ductus arteriosus, treatment of the left to right shunt may reverse the pulmonary artery dilatation and left main stem compression.5 Pulmonary vasodilators have also been quoted as a successful treatment.6
Table 2.
Complications of pulmonary hypertension
| Complications of pulmonary hypertension |
|---|
| Vascular |
| Massive pulmonary dilatation |
| Pulmonary artery dissection |
| Alveolar haemorrhage |
| In situ thrombosis |
| Balloon angioplasty complications |
| Cardiac complications |
| Right heart failure |
| Pericardial effusion and tamponade |
| Cardiac cirrhosis |
| Line infection |
| Pulmonary complications |
| Cavitation |
| Infection |
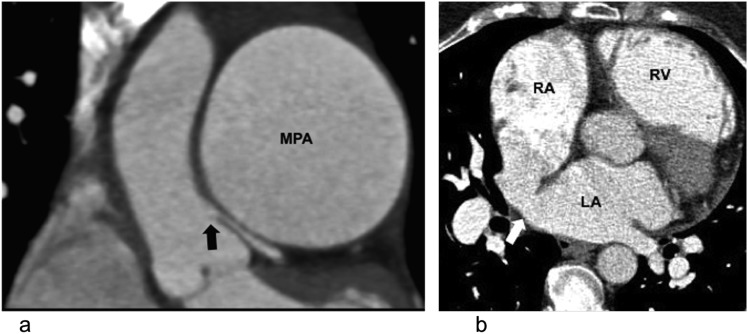
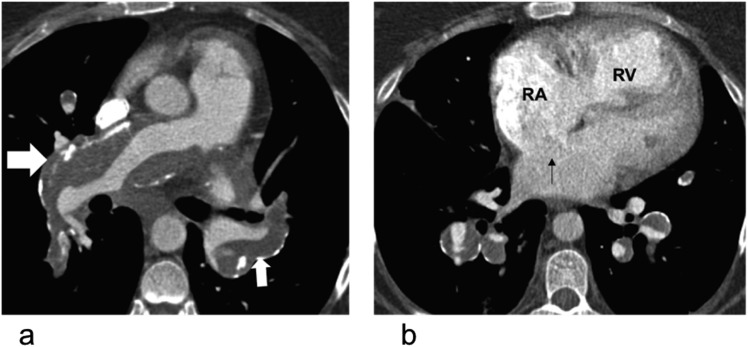
Figure 1.
Extrinsic compression of the left main stem coronary artery in a 37-year-old female with pulmonary hypertension with typical angina: (a) electrocardiogram-gated coronal CT is demonstrating extrinsic compression of the left main stem coronary artery (black block arrow) by the aneurysmal main pulmonary artery (MPA). (b) The axial CT image of the same patient is showing a sinus venosus-type atrial septal defect (white block arrow) and dilated right ventricle (RV). LA, left atrium; RA, right atrium.
Extrinsic compression of proximal airways from pulmonary artery dilatation is common in congenital heart disease (Figure 2). This may lead to asthma-like symptoms of wheezing and coughing and is unlikely to respond to conventional treatment. In addition, the same process may be happening at the level of the distal small airways, owing to their proximity to the distal pulmonary arteries.7 It is unclear whether this is a purely mechanical process, or if an imbalance of vascoconstrictive and bronchoconstrictive agents is at play.
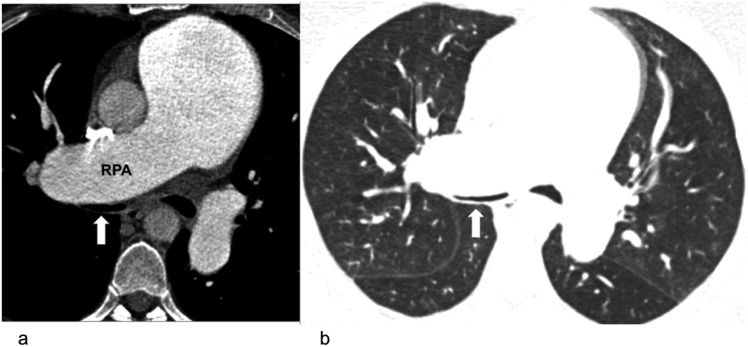
Figure 2.
Extrinsic compression of the airways in a 27-year-old female with pulmonary hypertension and worsening dyspnoea: compression of the right main bronchus (block arrows) by the dilated right pulmonary artery (RPA) on mediastinal (left) and lung window (right).
Pulmonary artery dissection
Pulmonary artery dissection is a rare and potentially catastrophic complication, the majority of which are diagnosed post-mortem. It is associated with congenital heart disease and may occur idiopathically from chronic PH, or iatrogenically from catheterization. The pulmonary artery dissection tends to rupture into the pericardium causing tamponade, rather than extending further downstream with a re-entrance site.9 The commonest location of dissection is the main pulmonary artery.10,11 On CT, its morphology may be similar to that of a classic aortic dissection, with an intimal flap and higher contrast attenuation within the true lumen (Figure 3). There have been case reports where this is the first presentation of chronically undiagnosed PH.12 Symptoms are non-specific and range from chest pain to dyspnoea.
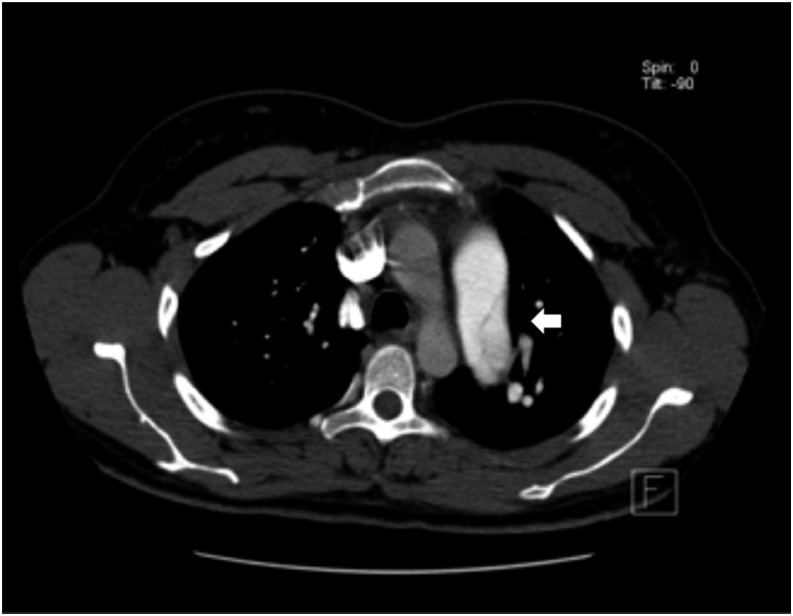
Figure 3.
Pulmonary artery dissection involving the left pulmonary artery (arrow). There is also a short movie available online.
Alveolar haemorrhage
Alveolar haemorrhage in patients with PAH is multifactorial and may be due to anticoagulation therapy and a continuous i.v. infusion of pulmonary vasodilator drugs.13 There is a controversy over the use of anticoagulants in patients with PH, and a recent analysis using of the European PH registry Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension supports the use of anticoagulation in patients with idiopathic PAH, but not for other subgroups.14
Haemorrhage can result from a pulmonary artery aneurysm rupture, and when the latter is present, it exists mainly in the main pulmonary artery.15 It may cause ground glass and consolidative changes on CT and dynamic extravasation of contrast during invasive angiogram (Figure 4). Cabezas' group studied a large cohort of patients with Group 1 PH, and the prevalence of pulmonary artery aneurysm was 12.2%.8 This used to be a diagnosis at necropsy, but is increasingly diagnosed owing to the growing use of cross-section imaging. The treatment of pulmonary artery aneurysm, by either surgery or medical optimization, is controversial in the literature.
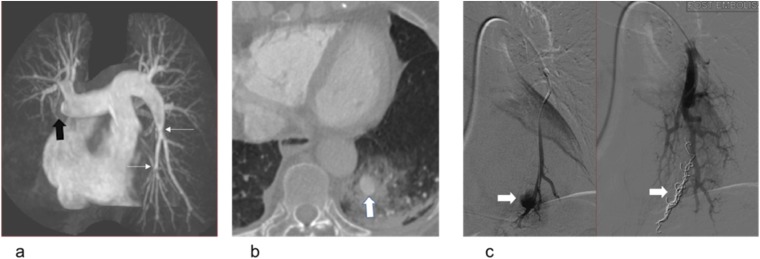
Figure 4.
Alveolar haemorrhage in a 45-year-old female with pulmonary hypertension post-right heart catheterization: (a) coronal maximum intensity projection of an MR pulmonary angiogram is demonstrating a proximal occlusion of the right middle and lower lobes (black block arrow) and proximal webs in the left lower lobe (thin white arrows) in keeping with chronic thromboembolic disease. (b) The patient developed haemoptysis following right heart catheterization. The axial CT image is showing a pseudoaneurysm of the posterior basal left lower lobe artery (white block arrow) and surrounding alveolar haemorrhage that developed as a consequence of forced passage of the catheter across the intraluminal webs. (c) Selected images of catheter pulmonary angiography before (left) and after (right) embolization of the left lower lobe pseudoaneurysm (block white arrows).
In situ thrombosis
In situ thrombosis is a concept of thrombus formation within the pulmonary arteries, without embolism from a distal deep-vein thrombosis. Thrombus formation is common in severe hypertension from any cause. Proposed mechanisms vary, and this may be due to a combination of wall shear stress from turbulent flow, vascular dilatation leading to stasis and vascular endothelial injury in PH. It may also be secondary to an increase in thrombin activity and disturbance of the thrombolytic pathway.16 In situ thrombosis is common in congenital heart disease and up to one-fifth of the patients with Eisenmenger's syndrome may have pulmonary thrombi. They can calcify and may be part of a partially thrombosed pulmonary artery (Figure 5). Unlike acute thrombi, in situ thrombi are located peripherally with lobulated contours and tend to form an obtuse angle with the vessel wall. They may also contain coarse calcification. Paradoxically, patients with Eisenmenger's syndrome also have abnormal coagulation and are prone to haemorrhage. This makes it difficult to manage their treatment.
Figure 5.
In situ thrombus in a 57-year-old female with long-standing pulmonary hypertension secondary to congenital heart disease: (a) the axial non-electrocardiogram-gated CT pulmonary angiogram with calcified thrombus lining the proximal pulmonary arteries (white block arrows). (b) There is dilatation of the right atrium (RA) and right ventricle (RV) with right ventricular hypertrophy and an ostium secondum atrial septal defect (black thin arrow). The segmental vessels are large and contain partially occlusive calcified thrombus.
Balloon angioplasty complications
The history of balloon pulmonary angioplasty (BPA) as a treatment of chronic thromboembolic pulmonary hypertension (CTEPH) dates back to 1983.17 The conventional treatment is pulmonary endarterectomy (PEA), which demonstrates immediate post-operative improvement in haemodynamics and improved survival.18 BPA may be an alternative for patients unsuitable for surgery. Studies have shown no immediate improvement in haemodynamics, but during follow-up, there is evidence of a decrease in right atrial pressure and pulmonary arterial pressure and increase in cardiac output.19,20 BPA could target distal lesions which could not be reached by PEA, usually the segmental and subsegmental pulmonary arteries. However, the lesions are still under high pressure, and patients with very poor baseline haemodynamics are prone to reperfusion oedema. The reported rate of this complication is 53–61%.19,21 A potentially fatal complication is perforation of the pulmonary arteries, resulting in haemorrhage and exanguination. This complication is less common and is reported to be up to 7%.22
Patients who have undergone BPA still receive PH-specific treatment, and whether this has an impact on the improvement in haemodynamics is yet to be determined. Further studies and long-term data are required to determine the effectiveness of BPA as an alternative to PEA.
Cardiac complications
Right heart failure
With increasing pulmonary artery resistance and pressure, the right ventricle responds to pressure overload initially with hypertrophy and eventually dilates to increase the preload to maintain stroke volume (Figure 6a,b). The right ventricle eventually ceases to adapt, although the mechanism is not fully understood. This may result in functional tricuspid regurgitation, pericardial effusions and cardiac cirrhosis.
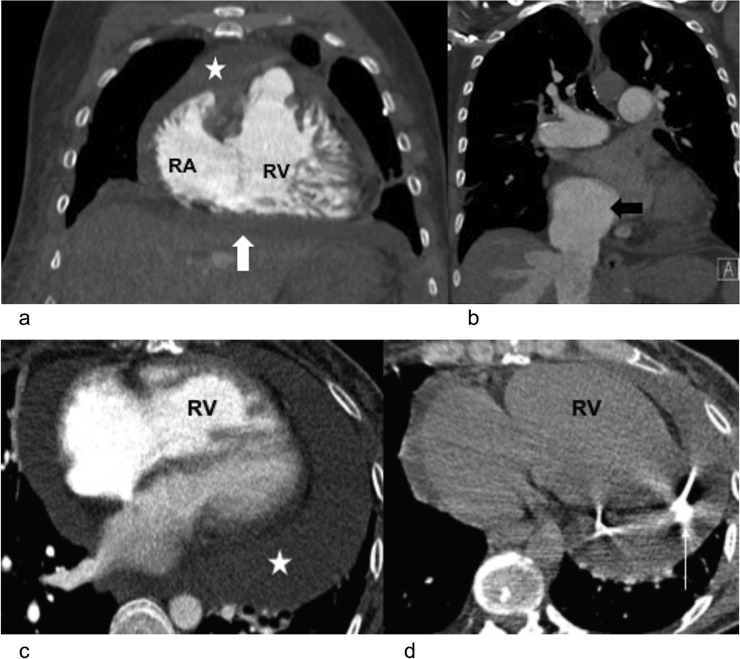
Figure 6.
Right heart failure and pericardial tamponade in two different patients with pulmonary hypertension: (a) coronal CT pulmonary angiogram is demonstrating dilatation of the right ventricle (RV) and hypertrophy with pericardial effusion (star) and ascites (block white arrow). There is also mild subcutaneous oedema. (b) The same patient also had a dilated inferior vena cava (block black arrow) and distended hepatic veins. (c, d) Axial CT pulmonary angiogram in a different case is demonstrating right ventricular dilatation and hypertrophy with a moderately large circumferential pericardial effusion (star). Clinically, the patient had signs of tamponade. A CT-guided pericardial drain (thin white arrow) was inserted with good improvement in symptoms. RA, right atrium.
Pericardial effusion and tamponade
Pericardial effusions are associated with up to half the patients with PH23,24 (Figure 6c,d). Mechanisms are unclear, and it has been hypothesized that it is due to the increased right chamber pressures causing increased myocytic transudation and decreased resorption. Its presence on echocardiography is a strong indicator of mortality in a multicentre trial.25 Pericardiocentesis in the PH setting is controversial and is associated with a high mortality.26 Mechanisms are unclear, but it may be due to the presentation of pericardial effusions in the late stages of PH. The normal echocardiography signs of right atrial and ventricular wall collapse may not be present, owing to the increase in right chamber pressures.
Cardiac cirrhosis
Congestive hepatopathy is a pattern of chronic liver injury due to raised right atrial pressures from any cause, including PH. The liver may have a lobulated contour, which can be associated with ascites and distended hepatic veins and inferior vena cava (Figure 7). Right heart failure increases the hydrostatic pressure within the sinusoids, leading to swelling and haemorrhage. Secondly, the reduced cardiac output causes acute hepatic necrosis. Eventually, this leads to fibrosis/cirrhosis. The fibrosis tends to spare the portal tracts, unlike other causes of cirrhosis.27 Patients may present with right upper quadrant pain, ascites and a pulsatile liver. Paradoxically, the loss of hepatic pulsation may signify more severe disease. However, the incidence of cirrhosis does not correlate with the severity of right heart failure. Hepatocellular carcinoma is a rare but reported complication of cardiac cirrhosis.28,29
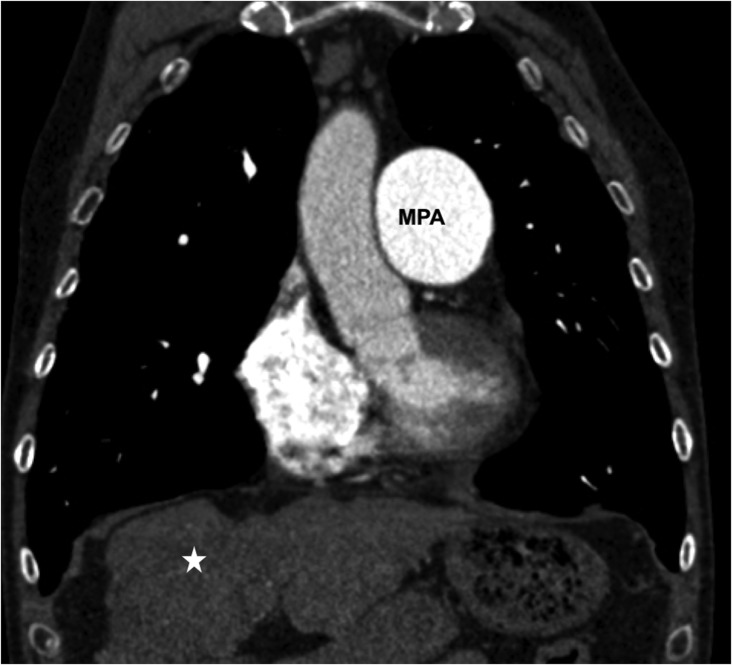
Figure 7.
Cardiac cirrhosis in a 40-year-old patient with long-standing idiopathic pulmonary hypertension: coronal CT is showing a dilated main pulmonary artery (MPA) and cirrhotic liver (star).
Line infection
Patients within the PAH and CTEPH may be treated with prostacyclin analogues, e.g. epoprostenol or trepostinil, to increase life expectancy and improve the quality of life. However, these drugs require long-term infusions via a central venous catheter, and secondary infections are not uncommon. Patients do not necessarily present with overwhelming sepsis, but the mortality may be as high as 30%.30 When compared with i.v. epoprostenol, i.v. trepostinil is associated with a higher rate of blood stream infections by gram-negative organisms.31,32 Long-term indwelling catheters may also lead to thrombi and fungal balls. Both bacterial and fungal infections could lead to pulmonary artery pseudoaneurysms, which are rare when compared with thoracic aortic aneurysms, but could rupture with devastating haemoptysis and haemorrhage (Figure 8). Conventional treatment is surgical resection including lobectomy, although selective angiography and endovascular treatment has also been reported as safe and effective.33,34 The latter remains controversial, as it introduces a foreign body into an infected field.
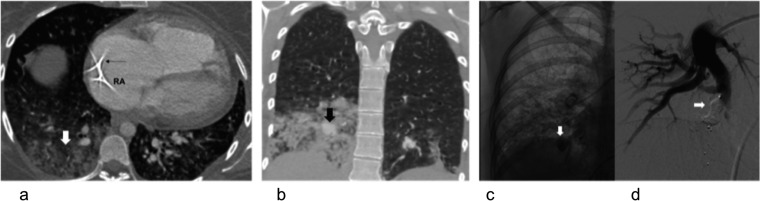
Figure 8.
A recurrent line infection in a 43-year-old patient with pulmonary hypertension with long line for i.v. prostacycline infusion: (a) axial CT with the tip of a Groshong line (black thin arrow) in the right atrium (RA). There is right lower lobe pneumonia (block white arrow). (b) In spite of 4 weeks of antibiotics, there was persistent consolidation. She developed massive haemoptysis and CT showed dense consolidation with a pseudoaneurysm formation (block black arrow). (c, d) Selected images of catheter pulmonary angiography before (c) and after (d) embolization of the right lower lobe pseudoaneurysm (block white arrows).
Pulmonary complications
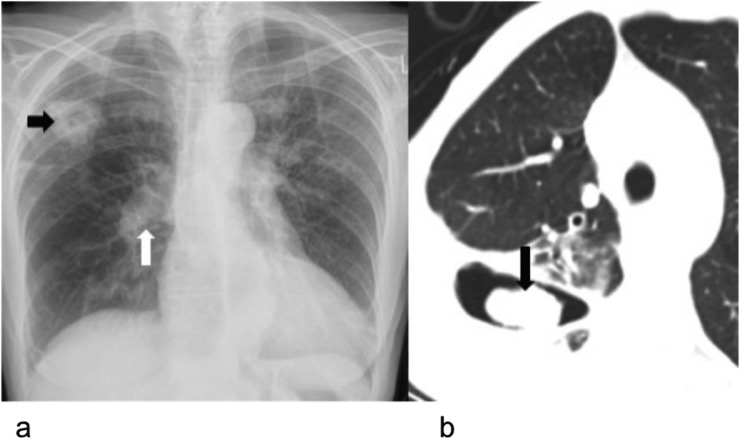
Cavitation secondary to infarcts is a known complication in acute pulmonary emboli and similarly, patients with CTEPH may present as such. These are initially aseptic, but could be secondarily infected and colonized by aspergillus (Figure 9). Cavitation is more common within mid and upper zones with the cavity wall generally being a poor discriminator of the underlying aetiology.35 Superinfection with clostridium species has been reported to be especially common, resulting in a necrotizing, cavitatory pneumonia.36
Figure 9.
Cavitating infarct with mycetoma in a 55-year-old male with pulmonary hypertension secondary to chronic thromboembolic disease: (a) chest radiograph with cardiomegaly and proximal pulmonary artery dilatation (block white arrow) and peripheral pruning. There is a thick-walled cavity (block black arrow) in the right upper lobe. (b) Axial CT is demonstrating an intracavitary soft-tissue material (block black arrow) within the thick-walled cavity that was a mycetoma forming within a cavitating infarct.
CONCLUSION
PH is a rare disease with a significant impact on the patient quality of life and survival. The radiologist plays a vital role in identifying complications at diagnosis and surveillance imaging.
Contributor Information
Sze M Mak, Email: makszemun@doctors.org.uk.
Nicola Strickland, Email: nicola.strickland@imperial.nhs.uk.
Deepa Gopalan, Email: deepa.gopalan@imperial.nhs.uk.
REFERENCES
- 1.Simonneau G, Gatzoulis M, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–41. doi: https://doi.org/10.1016/j.jacc.2013.10.029 [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–75. doi: https://doi.org/10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 3.Truong QA, Massaro JM, Rogers IS, Mahabadi AA, Kriegel MF, Fox CS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography. Circ Cardiovasc Imaging 2012; 5: 147–54. doi: https://doi.org/10.1161/CIRCIMAGING.111.968610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devaraj A, Wells AU, Meister MG, Corte TJ, Hansell DM. The effect of diffuse pulmonary fibrosis on the reliability of CT signs of pulmonary hypertension. Radiology 2008; 249: 1042–9. doi: https://doi.org/10.1148/radiol.2492080269 [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara K, Naito Y, Higashiue S, Takagaki Y, Goto Y, Okamoto M, et al. Left main coronary trunk compression by dilated main pulmonary artery in atrial septal defect. Report of three cases. J Thorac Cardiovasc Surg 1992; 104: 449–52. [PubMed] [Google Scholar]
- 6.Morjaria S, Grinnan D, Voelkel N. Massive dilatation of the pulmonary artery in association with pulmonic stenosis and pulmonary hypertension. Pulm Circ 2012; 2: 256–7. doi: https://doi.org/10.4103/2045-8932.97640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez-Bonetti P, Lupi-Herrera E, Martinez-Guerra ML, Barrios R, Seoane M, Sandoval J. Peripheral airways obstruction in idiopathic pulmonary artery hypertension (primary). Chest 1983; 83: 732–8. [DOI] [PubMed] [Google Scholar]
- 8.Cabezas J, Cano M, Acosta L. Demythologizing pulmonary artery aneurysm: prevalence and associated complications in a large pulmonary arterial hypertension population. Eur Heart J 2013; 34:(Suppl. 1). [Google Scholar]
- 9.Senbaklavaci O, Kaneko Y, Bartunek A. Rupture and dissection in pulmonary artery aneurysms: incidence, cause, and treatment—review and case report. J Thorac Cardiovasc Surg 2001; 121: 1006–8. doi: https://doi.org/10.1067/mtc.2001.112634 [DOI] [PubMed] [Google Scholar]
- 10.Lüchtrath H. Dissecting aneurysm of the pulmonary artery. Virchows Arch A Pathol Anat Histol 1981; 391: 241–7. [DOI] [PubMed] [Google Scholar]
- 11.Inayama Y, Nakatani Y, Kitamura H. Pulmonary artery dissection in patients without underlying pulmonary hypertension. Histopathology 2001; 38: 435–42. doi: https://doi.org/10.1046/j.1365-2559.2001.01129.x [DOI] [PubMed] [Google Scholar]
- 12.Arenaa V, De Giorgiob F, Abbatec A, Capellia A, De Mercuriob D, Carbone A. Fatal pulmonary arterial dissection and sudden death as initial manifestation of primary pulmonary hypertension: a case report. Cardiovasc Pathol 2004; 13: 230–2. doi: https://doi.org/10.1016/j.carpath.2004.02.003 [DOI] [PubMed] [Google Scholar]
- 13.Ogawa A, Matsubara H, Fujio H, Miyaji K, Nakamura K, Morita H. Risk of alveolar hemorrhage in patients with primary pulmonary hypertension—anticoagulation and epoprostenol therapy. Circ J 2005; 69: 216–20. doi: https://doi.org/10.1253/circj.69.216 [DOI] [PubMed] [Google Scholar]
- 14.Olsson KM, Delcroix M, Ghofrani HA, Tiede H, Huscher D, Speich R, et al. Anticoagulation and survival in pulmonary arterial hypertension: results from the comparative, prospective registry of newly initiated therapies for pulmonary hypertension (COMPERA). Circulation 2014; 129: 57–65. doi: https://doi.org/10.1161/CIRCULATIONAHA.113.004526 [DOI] [PubMed] [Google Scholar]
- 15.Seguchi M, Wada H, Sakakura K. Idiopathic pulmonary artery aneurysm. Circulation 2011; 124: e369–70. doi: https://doi.org/10.1161/CIRCULATIONAHA.111.029033 [DOI] [PubMed] [Google Scholar]
- 16.Chaouat A, Weitzenblum E, Higenbottam T. The role of thrombosis in severe pulmonary hypertension. Eur Respir J 1996; 9: 356–63. doi: https://doi.org/10.1183/09031936.96.09020356 [DOI] [PubMed] [Google Scholar]
- 17.Lock JE, Castaneda-Zuniga WR, Fuhrman BP, Bass JL. Balloon dilation angioplasty of hypoplastic and stenotic pulmonary arteries. Circulation 1983; 67: 962–7. doi: https://doi.org/10.1161/01.cir.67.5.962 [DOI] [PubMed] [Google Scholar]
- 18.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, et al. Improved outcomes in medically and surgically treated chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2008; 177: 1122–7. doi: https://doi.org/10.1164/rccm.200712-1841OC [DOI] [PubMed] [Google Scholar]
- 19.Kataoka M, Inami T, Hayashida K, Shimura N, Ishiguro H, Abe T, et al. Percutaneous transluminal pulmonary angioplasty for the treatment of chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 756–62. doi: https://doi.org/10.1161/CIRCINTERVENTIONS.112.971390 [DOI] [PubMed] [Google Scholar]
- 20.Andreassen AK, Ragnarsson A, Gude E, Geiran O, Andersen R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013; 99: 1415–20. doi: https://doi.org/10.1136/heartjnl-2012-303549 [DOI] [PubMed] [Google Scholar]
- 21.Feinstein JA, Goldhaber SZ, Lock JE, Ferndandes SM, Landzberg MJ. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation 2001; 103: 10–13. doi: https://doi.org/10.1161/01.CIR.103.1.10 [DOI] [PubMed] [Google Scholar]
- 22.Mizoguchi H, Ogawa A, Munemasa M. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv 2012; 5: 748–55. doi: https://doi.org/10.1161/circinterventions.112.971077 [DOI] [PubMed] [Google Scholar]
- 23.Shimony A, Fox BD, Langleben D, Rudski LG. Incidence and significance of pericardial effusion in patients with pulmonary arterial hypertension. Can J Cardiol 2013; 29: 678–82. doi: https://doi.org/10.1016/j.cjca.2012.04.009 [DOI] [PubMed] [Google Scholar]
- 24.Akinci SB, Gaine SP, Post W, Merrit WT, Tan HP, Winters B. Cardiac tamponade in an orthotopic liver recipient with pulmonary hypertension. Crit Care Med 2002; 30: 699–701. doi: https://doi.org/10.1097/00003246-200203000-00035 [DOI] [PubMed] [Google Scholar]
- 25.Raymond RJ, Hinderliter AL, Willis PW. Echocardiographic predictors of adverse outcomes in primary pulmonary hypertension. J Am Coll Cardiol 2002; 39: 1214–19. doi: https://doi.org/10.1016/s1062-1458(02)00790-0 [DOI] [PubMed] [Google Scholar]
- 26.Hemnes AR, Gaine SP, Wiener CM. Poor outcomes associated with drainage of pericardial effusions in patients with pulmonary arterial hypertension. South Med J 2008; 101: 490–4. doi: https://doi.org/10.1097/smj.0b013e31816c0169 [DOI] [PubMed] [Google Scholar]
- 27.Shah SC, Sass DA. Cardiac hepatopathy: a review of liver dysfunction in heart failure. Liver Res Open J 2015; 1: 1–10. [Google Scholar]
- 28.Song P, Koh K, Yoo B, Paik S, Lee J. A case of hepatocellular carcinoma complicating cardiac cirrhosis caused by constrictive pericarditis. [In Korean.] Korean J Gastroenterol 2005; 45: 436–40. [PubMed] [Google Scholar]
- 29.Saliba T, Dorkhom S, O'Reilly EM, Ludwig E, Gansukh B, Abou-Alfa GK. Hepatocellular carcinoma in two patients with cardiac cirrhosis. Eur J Gastroenterol Hepatol 2010; 22: 889–91. doi: https://doi.org/10.1097/MEG.0b013e32832e2bec [DOI] [PubMed] [Google Scholar]
- 30.Elliot C, Kiely D. Pulmonary hypertension. Contin Educ Anaesth Crit Care Pain 2006; 6: 17–22. doi: https://doi.org/10.1093/bjaceaccp/mki061 [Google Scholar]
- 31.Kitterman N, Poms A, Miller DP, Lombardi S, Farber HW, Barst RJ. Bloodstream infections in patients with pulmonary arterial hypertension treated with intravenous prostanoids: insights from the Reveal Registry®. Mayo Clin Proc 2012; 87: 825–34. doi: https://doi.org/10.1016/j.mayocp.2012.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Medrano F, Fernández-Ruiz M, Ruiz-Cano MJ. High incidence of bloodstream infection due to gram-negative bacilli in patients with pulmonary hypertension receiving intravenous treprostinil. [In Spanish.] Arch Bronconeumol 2012; 48: 443–7. doi: https://doi.org/10.1016/j.arbres.2012.06.005 [DOI] [PubMed] [Google Scholar]
- 33.Shin TB, Yoon SK, Lee KN. The role of pulmonary CT angiography and selective pulmonary angiography in endovascular management of pulmonary artery pseudoaneurysms associated with infectious lung diseases. J Vasc Interv Radiol 2007; 18: 882–7. doi: https://doi.org/10.1016/j.jvir.2007.04.023 [DOI] [PubMed] [Google Scholar]
- 34.Ghaye B, Trotteur G, Dondelinger RF. Multiple pulmonary artery pseudoaneurysms: intra-saccular embolization. Eur Radiol 1997; 7: 176–9. doi: https://doi.org/10.1007/s003300050130 [DOI] [PubMed] [Google Scholar]
- 35.Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E, Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep 2008; 2: 11–12. doi: https://doi.org/10.3941/jrcr.v2i3.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cannon JW. Necrotizing clostridial pneumonia: a case report and review of the literature. Mil Med 2002; 167: 85–6. [PubMed] [Google Scholar]