Abstract
Background
Preclinical findings suggest that combination treatment with bevacizumab and temsirolimus could be effective against malignant pediatric central nervous system (CNS) tumors.
Patients and Methods
Six pediatric patients were treated as part of a phase I trial with intravenous temsirolimus 25 mg on days 1, 8, 15, and bevacizumab at 5, 10, or 15 mg/kg on day 1 of each 21-day cycle until disease progression or patient withdrawal.
Results
The median patient age was six years (range, 3–14 years). The primary diagnoses were glioblastoma multiforme (n=2), medullobalstoma (n=2), pontine glioma (n=1) and ependymoma (n=1). All patients had disease refractory to standard of care (2–3 prior systemic therapies). Grade 3 toxicities possibly related to drugs used occurred in two patients: anorexia, nausea, and weight loss in one, and thrombocytopenia and alanine aminotransferase elevation in another. One patient with glioblastoma multiforme achieved a partial response (51% regression) and two patients (with medulloblastoma and pontine glioma) had stable disease for four months or more (20 and 47 weeks, respectively). One other patient (with glioblastoma multiforme) showed 18% tumor regression (duration=12 weeks).
Conclusion
The combination of bevacizumab with temsirolimus was well tolerated and resulted in stable disease of at least four months/ partial response in three out of six pediatric patients with chemorefractory CNS tumors.
Keywords: Temsirolimus, bevacizumab, pediatric, CNS tumors
Primary central nervous system (CNS) malignancies are the second most frequent tumors in pediatric patients, with 3.4 cases per 100,000 person-years of the estimated incidence for children and adolescents at or below 19 years of age in the United States (1, 2). Advances in diagnostic and therapeutic modalities have improved the 5-year survival rates of children with primary CNS tumors (3). However, due to the emergence of drug-resistant clones, the outcome is poor in progressive disease. During the past decade, targeted therapies have been increasingly identified for the most common adult malignancies. In contrast, very few targeted therapies have been developed for children with solid tumors.
CNS tumors, especially glioblastoma multiforme (GBM), are highly vascularized and characterized by abnormal blood vessels and the ability to invade normal brain structures (4). The binding of vascular endothelial growth factor (VEGF) to its various receptors plays an important role in angiogenesis and the pathogenesis of various cancers (5). Expression of pro-angiogenic factors and increased number of blood vessels has been associated with progressive disease and an unfavorable prognosis. Bevacizumab, a fully humanized monoclonal antibody against VEGF, normalizes tumor vascularity through antiangiogenic effects (6). Bevacizumab in combination with irinotecan demonstrated a response rate of 63% and a median progression-free survival (PFS) of 23 weeks in a study of adult patients with recurrent high-grade glioma (7). Therefore, the U.S. Food and Drug Administration (FDA) recently approved bevacizumab to treat GBM that has not responded to other chemotherapies. Despite such successes, treatment with antiangiogenic agents has resulted in only modest durable responses.
Increased expression of hypoxia-inducible factor 1α (HIF-1α) leads to adaptive tumor responses under hypoxic conditions, which is a known mechanism of tumor resistance to antiangiogenic therapy. (8). In addition to angiogenesis, GBM is characterized by increased phosphotidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway activity, with altered phosphatase and tensin homolog (PTEN) gene suppression (9). This subsequently activates mTOR, which increase the expression of key proteins associated with cell cycle regulation.
Regarding the PI3K/AKT/mTOR pathway, oncogenic mutations in PIK3CA have recently been identified in diffuse intrinsic pontine glioma (2). Furthermore, medulloblastomas overexpress the insulin-like grow factor-1 receptor (IGF-1R) and ERBB4, which also activate PI3K/AKT signaling (10–12). Finally, a number of genetic syndromes, including neurofibromatosis type 2 (NF2), are associated with the development of ependymomas. Inactivation of the tumor suppressor gene NF2 (encoding merlin) induces the development of nervous system tumors, at least in part, through activation of PI3K/AKT/mTOR signaling (13).
Temsirolimus is an mTOR inhibitor, which abrogates the growth of numerous human tumor cell lines in xenograft models, including medulloblastoma and glioblastoma (14). Its highly lipophilic nature allows it to penetrate the blood–brain barrier, whose existence is often a major impediment to treating these tumor types (15). Importantly, mTOR inhibitors can also inhibit HIF-1α, down-regulating tumor and endothelial cell proliferative and survival functions, including compensatory responses to hypoxia (16). Because up-regulation of HIF-1α mediates chemoresistance to bevacizumab, we hypothesized that combination treatment with bevacizumab and temsirolimus might be effective against pediatric malignant gliomas. Here, we report our experience with toxicity and clinical response in pediatric patients with CNS tumors treated with the combination of these two targeted agents.
Patients and Methods
Study design and treatment plan
The study reported is part of a single institution, phase I, open-label, dose-escalation clinical trial (NCT00610493). The primary objective of the trial was to determine the maximum tolerated dose and dose-limiting toxicities (DLTs) of combination treatment with bevacizumab and temsirolimus. Secondary objectives included preliminary descriptive assessment of antitumor efficacy and assessment of correlates of antiangiogenesis. This trial successfully completed dose escalation to the highest specified dose level, that is dose level 13, which consisted of the highest FDA-approved doses of both drugs (bevacizumab 15 mg/kg i.v. every three weeks and temsirolimus 25 mg i.v. weekly) (17). This article focuses only on a subset of six pediatric patients with primary CNS tumors in order to provide information on the side effects, tolerability and efficacy of this combination of targeted therapies in these patients.
Treatment was administered on an outpatient basis at The University of Texas MD Anderson Cancer Center. Treatment cycles were of 21 days’ duration. No commercial agents or therapies other than those described here were administered with the intent to treat the patient’s malignancy. For this subset of patients, temsirolimus was administered at a dose of 25 mg i.v. on days 1, 8 and 15, and bevacizumab was given at either 5, 10, or 15 mg/kg on day 1. The same schedules and doses of bevacizumab and temsirolimus were administered in subsequent cycles barring evidence of tumor progression or prohibitive toxicity.
For these pediatric patients, all biopsies except for one pontine glioma (Table I) were reviewed by an MD Anderson Cancer Center pathologist and classified using the World Health Organization classification (18). Consent was obtained and patients were treated in accordance with MD Anderson Cancer Center Institutional Review Board guidelines.
Table I.
Baseline demographics and clinical characteristics of study patients.
| Characteristic | Total (%) |
|---|---|
| No. of patients | 6 |
|
| |
| Median age (range), years | 6 (3–14) |
|
| |
| Median (range) no. prior systemic therapies | 2 (2–3) |
|
| |
| Lansky performance status | |
|
| |
| 80–100% | 1 (17) |
| 60–79% | 2 (33) |
| 40–59% | 3 (50) |
|
| |
| Prior treatment | |
|
| |
| Surgery | 5 (83) |
| Radiation | 6 (100) |
| Chemotherapy | 6 (100) |
|
| |
| Primary diagnosis | |
|
| |
| Glioblastoma multiforme | 2 |
| Pontine glioma | 1 |
| Ependymoma | 1 |
| Medulloblastoma | 2 |
Eligibility criteria
Patients were eligible for enrollment if they had histologically documented, advanced or metastatic solid tumors refractory to standard therapy or for which no standard therapy was available that would induce a complete response (CR) rate of at least 10% or improve survival by at least three months. Other key inclusion criteria were: absolute neutrophil count ≥ 1,000/ml; platelets ≥ 50,000/ml; creatinine ≤3 times the upper limit of normal (ULN); total bilirubin ≤3.0 mg/dl; alanine aminotransferase (ALT) ≤5 times the ULN; fasting level of total cholesterol <350 mg/dl; and, triglyceride <400 mg/dl. Key exclusion criteria were: hemoptysis or clinically significant unexplained bleeding within 28 days of study entry; uncontrolled systemic hypertension; hypersensitivity to bevacizumab or temsirolimus and their metabolites. Prior exposure to mTOR and VEGF inhibitors were not exclusion criteria for study entry, nor were patients with a history of venous thromboembolism excluded. Because temsirolimus metabolism significantly increases with concurrent use of cytochrome P450 3A4-inducing medications, such as dexamethasone, or anti-epileptic drugs (e.g. phenytoin, carbamazepine, phenobarbital, oxcarbazepine and primidone), it was strongly recommended to treating physicians that these medications be discontinued and five elimination half-lives of such medication be allowed to pass before enrollment on this trial.
Evaluation of safety and efficacy
Adverse events were graded based on the Common Terminology Criteria for Adverse Events v.3.0 (19). Side-effects were monitored in a prospective manner. History and physical examinations were done approximately weekly during the first cycle and then at the start of each subsequent cycle as per protocol. Laboratory analysis, including urinalysis, complete blood count with differential leukocyte count, renal function, hepatic enzymes and serum electrolytes, were performed weekly during the first cycle and then at the start of each subsequent cycle as per protocol.
Imaging evaluation
Magnetic resonance imaging (MRI) of the brain was performed within four weeks before initiation of treatment and at 6-week intervals thereafter, or earlier with clinical indications per the discretion of the treating physician. Tumor measurement for determining treatment response was according to the modified MacDonald criteria (20). For the purposes of this article, complete response (CR) is defined as resolution of all enhancing tumor, partial response (PR) as a decrease in tumor size of at least 50%, progressive disease (PD) as an increase in tumor size of 25% or more, stable disease defines all of the other patients. All responses were reviewed by our neuroradiologist (NGT).
Results
Patient characteristics
Between June 2009 and April 2010, six pediatric patients with refractory CNS tumors (median age=6 years; range=3–14 years) who met the inclusion and exclusion criteria were enrolled on this phase I clinical trial with combined temsirolimus and bevacizumab. The primary diagnoses included glioblastoma multiforme (n=2), medulloblastoma (n=2), pontine glioma (1) and ependymoma (n=1) (Table I). Most patients had a Lansky score ≤80% (21).
Patient characteristics are shown in Table I. Five patients had experienced relapse after surgery and subsequent radiation therapy. Patients had a median of two prior chemotherapy regimens (range=2–3). No patient had received a prior mTOR inhibitor. Four patients had previously been treated with a bevacizumab-containing regimen.
Toxicity
Therapy was generally well tolerated with manageable toxicities. Two out of six patients experienced grade 3 toxicity possibly related to drugs used: (i) grade 3 anorexia, nausea and weight loss; and (ii) thrombocytopenia and ALT elevation, delaying treatment for two weeks in this patient. In both cases, treatment was stopped following disease progression. No patients experienced grade 4 toxicities. At a dose of 15 mg/kg of bevacizumab, one patient experienced grade 2 hypertension which was well controlled with antihypertensive medication. Two patients had grade 2 skin toxicity, including complications of wound healing (superficial skin ulceration around skin folds and dehiscence of central venous catheter insertion site). There was no intracranial bleeding or other serious complications. All other side-effects were grade 2 or less, including neutropenia in two out of six patients (grade 1, n=1; grade 2, n=1), thrombocytopenia in three patients (grade 1, n=1; grade 2, n=2), grade 1 nausea in one patients, grade 2 diarrhea in one patient, hyponatremia in three patients (grade 1, n=1; grade 2, n=2), grade 1 hypokalemia in one patient, grade 2 hypophosphatemia in one patient, grade 1 hyperglycemia in two patients, and aspartate aminotransferase (AST) and ALT elevations in four patients (grade 1, n=1, grade 2, n=3). Five patients developed abnormal lipid profiles (grade 2 hypercholesterolemia/grade 2 hypertriglyceridemia). There were no dose reductions of either bevacizumab or temsirolimus due to toxicity.
Evaluation of antitumor activity
Median time to disease progression was 14 weeks (range=7–47 weeks; Table II). Although patients were heavily pretreated, a PR was achieved in one patient. In this patient with GBM, restaging studies after four cycles of therapy show a 51% decrease in the size of the tumor (Figure 1). Stabilization of disease for a median of 16 weeks (range=8–47 weeks) was achieved in four other patients (one each with GBM, medulloblastoma, ependymoma, and pontine glioma). In fact, the patient with pontine glioma received 47 weeks of therapy before clinical deterioration. This patient was treated with therapy for 36 weeks before therapy was halted for radiation to the pontine lesion. Therapy was continued for 11 more weeks after radiation was completed for clinical benefit. One patient with medulloblastoma was taken off of treatment without progression after 20 weeks secondary to peritonitis associated with his ventriculoperitonial shunt. Each patient’s best response by the modified MacDonald criteria is illustrated by waterfall plot shown in Figure 2.Discussion
Table II.
Modified McDonald response evaluation and characterization by patient.
| Disease site/histology | No. of patients treated | Bevacizumab dose (mg/kg) | PR | SD | Time to progression (weeks) | Prior bevacizumab therapy (no.) | Dexamethasone use (no.) |
|---|---|---|---|---|---|---|---|
| Posterior fossa | 5 | 11 (7–20) | 3 | 1 | |||
|
| |||||||
| Glioblastoma multiforme | 1 | 5 | 1 | 12 | 1 | 0 | |
| Glioblastoma multiforme | 1 | 10 | 1 | 18 | 1 | 0 | |
| Epyndemoma | 1 | 15 | 1 | 8 | 0 | 0 | |
| Medulloblastoma | 1 | 15 | 1† | 20 | 0 | 0 | |
| Medulloblastoma | 1 | 15 | 7 | 1 | 1‡ | ||
|
| |||||||
| Mid brain | 1 | 47 | 1 | 0 | |||
|
| |||||||
| Pontine glioma | 1 | 15 | 1† | 47* | 1 | 0 | |
PR, partial response; SD, stable disease.
Treated with therapy for 12 cycles before therapy was halted for radiation to the pontine lesion. At the discretion of the treating physician therapy was continued for three more cycles after radiation was completed for clinical benefit.
Stable disease lasting four months or more;
stable dosing of dexamethasone at 1 mg orally twice daily.
Figure 1.
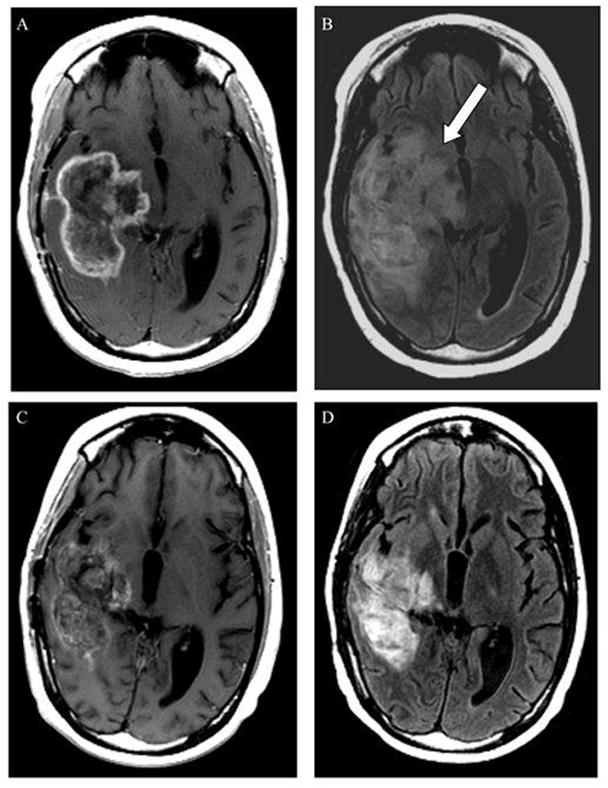
Magnetic resonance imaging (MRI) scan obtained in a 14-year-old female with recurrent glioblastoma multiforme. Prior to initiating bevacizumab and temsirolimus therapy, MRI demonstrated an approximately 7-cm rim-enhancing, centrally necrotic right temporal mass involving the basal ganglia and thalamus on an axial T1-weighted post-contrast image (A), and accompanying hyperintensity on the axial FLAIR T2-weighted image (arrow in B). After 13 weeks of treatment, follow-up imaging demonstrates attenuation in the enhancement pattern (C) with a pronounced decrease in the associated FLAIR signal abnormality (D) −51% from baseline.
Figure 2.
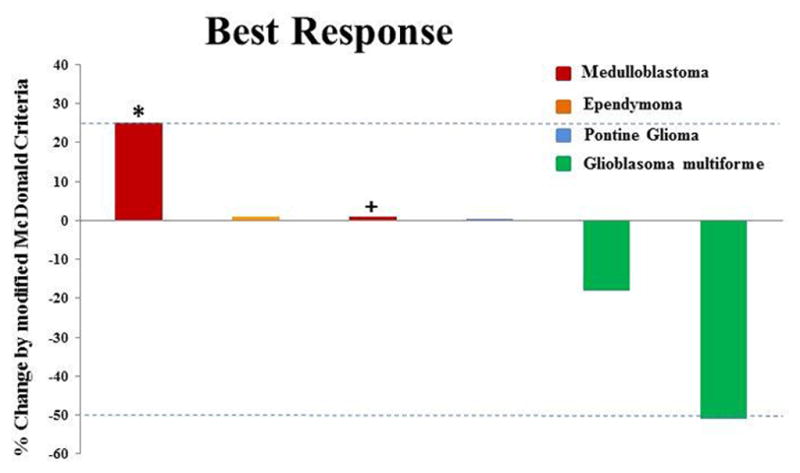
Individual patients/disease sites are represented by vertical bars on the X-axis. The best response per modified MacDonald criteria (%) is depicted on the Y-axis. Disease in four of the six patients was measurable. Of the two patients who were not measurable, one patient with stable disease (for 20 weeks) was assigned a value of +1% (+) and one patient was assigned a value of +25% for clinical progression (*).
Treatment planning for pediatric patients with chemorefractory CNS tumors is challenging because: i) definitive data regarding optimal therapy are lacking; ii) it is difficult to enroll patients in dose-defining pediatric studies; and iii) toxicities and tumor resistance are associated with prior conventional treatment. For these reasons, successful novel treatment regimens are needed.
A bevacizumab-containing regimen resulted in a 6-month PFS of 38% in pediatric patients with recurrent high-grade glioma (22), which is similar to the outcomes of adult patients (23). The implication is that combining bevacizumab with a chemotherapeutic agent could also delay disease progression in pediatric patients with predominant contrast-enhancing disease on imaging. The common adverse effects of bevacizumab and other VEGF inhibitors are hypertension and proteinuria, and patients may be at risk for wound complications, hemorrhage, gastrointestinal perforation and thromboembolic events (24). However, the largest phase II study thus far was in 167 adult patients with recurrent malignant glioma treated with bevacizumab, with or without irinotecan. In that study, there were three cases of intracranial hemorrhage (1.8%), three arterial thromboembolic events (1.8%), three complications of wound healing (1.8%), and one patient had gastrointestinal perforation (0.6%) (25). In our study, one out of four patients receiving 15 mg/kg of bevacizumab had grade 2 hypertension and another had a grade 2 complication of wound healing associated with the central venous catheter insertion site. The remaining patients at this dose level had no bevacizumab-associated complications.
The FDA recently approved the mTOR inhibitors temsirolimus and everolimus for the treatment of patients with refractory renal cell cancer (26, 27). Inhibitors of mTOR have shown single-agent activity in other types of cancer and clinical trials are currently ongoing in several tumor types (28). In 18 pediatric patients with recurrent and refractory solid tumors, temsirolimus of 150 mg/m2 weekly as the highest dose level was tolerable in a phase I study (29). One patient experienced dose-limiting anorexia at this dose level. Otherwise, toxicities were mild and tolerable. This was attributed to a shorter half-life and lower area under the curve (AUC) in pediatric compared with adult patients with solid tumors. Therefore, pediatric patients were administered more than 95% of the median dose intensity for all dose levels tested (29).
To better characterize the relationship between dose and tolerability to temsirolimus, 111 patients with refractory renal cell carcinoma were randomly assigned to a phase II study of fixed doses of 25, 75, or 250 mg i.v. weekly (30). Overall, there was are CR, seven PRs, and 29 minor responses, but toxicity and efficacy were similar at each dose level. This observation, together with the greater number of dose reductions and treatment interruptions at the higher dose levels, led to 25 mg i.v. weekly as the optimum dose level being recommended for future temsirolimus studies. In our study, five out of six patients whose disease was refractory to prior chemotherapy, including bevacizumab in three patients, showed disease control with weekly i.v. temsirolimus at 25 mg. These results suggest the potential efficacy of this dose of temsirolimus in treating pediatric solid cancer. One important consideration is that patients with malignant glioma can require enzyme-inducing antiepileptic drugs or dexamethasone. Such patients exhibit increased drug metabolism by the CYP450 enzyme system, thereby being able to tolerate higher doses than patients not taking these agents. Accordingly, patients taking enzyme-inducing agents demonstrated a maximum tolerated dose of i.v. temsirolimus at 250 mg weekly in a phase I study (14). In a phase II study with glioma patients not on enzyme-inducing antiepileptic drugs, 250 mg led to excessive toxicity, mainly stomatitis, so the dose was reduced to 170 mg (31). Therefore, one question to be answered is whether the dose of temsirolimus should be elevated to greater than 25 mg in pediatric patients taking enzyme-inducing antiepileptic drugs or steroids. Of note, none of the pediatric patients treated on this study were taking enzyme-inducing antiepileptic drugs and only one patient with medulloblastoma was taking dexamethasone at a dose of 1 mg orally twice daily.
Many mechanisms of resistance to VEGF inhibitors have been described, with recent emphasis on hypoxic responses. In cell culture models, rapamycins have also been shown to inhibit HIF-1α, a transcription factor that regulates the expression of VEGF, suggesting that combined VEGF and mTOR inhibition could have greater antiangiogenic and antitumor activity than either agent given as monotherapy (32–34). In our study, the fact that three out of four patients received previous bevacizumab treatment, and had stable disease for 8, 12 and 47 weeks, respectively, implies that temsirolimus may assist in overcoming resistance to VEGF inhibitors.
In conclusion, the combination of bevacizumab with temsirolimus was well tolerated at the maximum FDA-approved doses and showed PR (51%) in one patient with glioblastoma multiforme and SD in four patients, with two of the latter patients having stable disease for larger than four months. Side-effects that were seen were expected and easily managed. There were no dose reductions of either bevacizumab or temsirolimus due to toxicity. Further study of this combination in larger populations of children with primary CNS tumors is warranted.
Acknowledgments
Funding: No disclosures
Sharon Rice, a senior data coordinator at MD Anderson Cancer Center, provided assistance in data compilation.
Footnotes
Disclosure of Potential Conflicts of Interest
None of the Authors have any conflict of interest relevant to the subject of this article.
References
- 1.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004) Cancer. 2008;112:416–432. doi: 10.1002/cncr.23169. [DOI] [PubMed] [Google Scholar]
- 2.Grill J, Puget S, Andreiuolo F, Philippe C, MacConaill L, Kieran MW. Critical oncogenic mutations in newly diagnosed pediatric diffuse intrinsic pontine glioma. Pediatr Blood Cancer. 2012;58:489–491. doi: 10.1002/pbc.24060. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 4.Miletic H, Niclou SP, Johansson M, Bjerkvig R. Anti-VEGF therapies for malignant glioma: treatment effects and escape mechanisms. Expert Opin Ther Targets. 2009;13:455–468. doi: 10.1517/14728220902806444. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology. 2005;69(Suppl 3):4–10. doi: 10.1159/000088478. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK. Normalization of tumor vasculature: An emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 7.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011–1027. doi: 10.1200/JCO.2005.06.081. [DOI] [PubMed] [Google Scholar]
- 9.Knobbe CB, Merlo A, Reifenberger G. Pten signaling in gliomas. Neuro Oncol. 2002;4:196–211. [PMC free article] [PubMed] [Google Scholar]
- 10.Wang JY, Del Valle L, Gordon J, Rubini M, Romano G, Croul S, Peruzzi F, Khalili K, Reiss K. Activation of the IGF-IR system contributes to malignant growth of human and mouse medulloblastomas. Oncogene. 2001;20:3857–3868. doi: 10.1038/sj.onc.1204532. [DOI] [PubMed] [Google Scholar]
- 11.Gilbertson RJ, Clifford SC, MacMeekin W, Meekin W, Wright C, Perry RH, Kelly P, Pearson AD, Lunec J. Expression of the ErbB-neuregulin signaling network during human cerebellar development: Implications for the biology of medulloblastoma. Cancer Res. 1998;58:3932–3941. [PubMed] [Google Scholar]
- 12.Elenius K, Choi CJ, Paul S, Santiestevan E, Nishi E, Klagsbrun M. Characterization of a naturally occurring ErbB4 isoform that does not bind or activate phosphatidyl inositol 3-kinase. Oncogene. 1999;18:2607–2615. doi: 10.1038/sj.onc.1202612. [DOI] [PubMed] [Google Scholar]
- 13.James MF, Han S, Polizzano C, Plotkin SR, Manning BD, Stemmer-Rachamimov AO, Gusella JF, Ramesh V. NF2/merlin is a novel negative regulator of mTOR complex 1, and activation of mTORC1 is associated with meningioma and schwannoma growth. Mol Cell Biol. 2009;29:4250–4261. doi: 10.1128/MCB.01581-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang SM, Kuhn J, Wen P, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, Cloughesy T, De Angelis L, Razier J, Hess K, Dancey J, Prados MD. Phase I/pharmacokinetic study of CCI-779 in patients with recurrent malignant glioma on enzyme-inducing antiepileptic drugs. Invest New Drugs. 2004;22:427–435. doi: 10.1023/B:DRUG.0000036685.72140.03. [DOI] [PubMed] [Google Scholar]
- 15.Geoerger B, Kerr K, Tang CB, Fung KM, Powell B, Sutton LN, Phillips PC, Janss AJ. Antitumor activity of the rapamycin analog CCI-779 in human primitive neuroectodermal tumor/medulloblastoma models as single agent and in combination chemotherapy. Cancer Res. 2001;61:1527–1532. [PubMed] [Google Scholar]
- 16.Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA. Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res. 2003;9:4641–4652. [PubMed] [Google Scholar]
- 17.Piha-Paul S. An Innovative Phase I Trial Design Allowing for the Identification of Multiple Potential Maximum Tolerated Doses with Combination Therapy of Targeted Agents. UT GSBS Dissertations and Theses. 2010 Paper 72. http://digitalcommons.library.tmc.edu/utgsbs_dissertations/72.
- 18.Louis DNOH, Wiestler OD, Cavenee WK. Classification of Tumours of the Nervous System. IARC Press; Lyon, France: 2007. [Google Scholar]
- 19.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. Bethesda, MD: 2003. [Google Scholar]
- 20.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 21.Lansky SB, List MA, Lansky LL, Ritter-Sterr C, Miller DR. The measurement of performance in childhood cancer patients. Cancer. 1987;60:1651–1656. doi: 10.1002/1097-0142(19871001)60:7<1651::aid-cncr2820600738>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 22.Parekh C, Jubran R, Erdreich-Epstein A, Panigrahy A, Bluml S, Finlay J, Dhall G. Treatment of children with recurrent high-grade gliomas with a bevacizumab containing regimen. J Neurooncol. 2011;103:673–680. doi: 10.1007/s11060-010-0444-x. [DOI] [PubMed] [Google Scholar]
- 23.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 24.Verheul HM, Pinedo HM. Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer. 2007;7:475–485. doi: 10.1038/nrc2152. [DOI] [PubMed] [Google Scholar]
- 25.Drappatz J, Schiff D, Kesari S, Norden AD, Wen PY. Medical management of brain tumor patients. Neurol Clin. 2007;25:1035–1071. ix. doi: 10.1016/j.ncl.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grunwald V, Thompson JA, Figlin RA, Hollaender N, Urbanowitz G, Berg WJ, Kay A, Lebwohl D, Ravaud A. Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 27.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf IG, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ. Temsirolimus, interferon alpha, or both for advanced renal cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 28.Altomare I, Bendell JC, Bullock KE, Uronis HE, Morse MA, Hsu SD, Zafar SY, Blobe GC, Pang H, Honeycutt W, Sutton L, Hurwitz HI. A phase II trial of bevacizumab plus everolimus for patients with refractory metastatic colorectal cancer. Oncologist. 2011;16:1131–1137. doi: 10.1634/theoncologist.2011-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spunt SL, Grupp SA, Vik TA, Santana VM, Greenblatt DJ, Clancy J, Berkenblit A, Krygowski M, Ananthakrishnan R, Boni JP, Gilbertson RJ. Phase I study of temsirolimus in pediatric patients with recurrent/refractory solid tumors. J Clin Oncol. 2011;29:2933–2940. doi: 10.1200/JCO.2010.33.4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, Park Y, Liou SH, Marshall B, Boni JP, Dukart G, Sherman ML. Randomized phase II study of multiple dose levels of CCI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 31.Chang SM, Wen P, Cloughesy T, Greenberg H, Schiff D, Conrad C, Fink K, Robins HI, De Angelis L, Raizer J, Hess K, Aldape K, Lamborn KR, Kuhn J, Dancey J, Prados MD. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 32.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–7014. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1α expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 34.George DJ, Kaelin WG., Jr The von Hippel-Lindau protein, vascular endothelial growth factor, and kidney cancer. N Engl J Med. 2003;349:419–421. doi: 10.1056/NEJMp030061. [DOI] [PubMed] [Google Scholar]


