Abstract
Background/Objectives
The association between weight change and cognition is controversial. We examined the association between 20-year weight change and cognitive function in late life.
Design
Cohort study.
Setting
Study of Osteoporotic Fractures (SOF).
Participants
1,289 older, community-dwelling women (mean baseline age 68 [65–81] and 88 [82–102] at cognitive testing).
Measurements
SOF participants had body weight measured repeatedly over 20 years (mean 8 weights). Adjudicated cognitive status was classified as normal (n=775) or mild cognitive impairment (MCI)/dementia (n=514) at Year 20. Logistic models were used to evaluate whether absolute weight change, rate of weight loss per year, presence of abrupt, unrecovered weight loss, and weight variability were associated with MCI or dementia.
Results
Women with greater rate of weight loss over 20 years had increased chance of developing MCI or dementia. In age/education/clinic-adjusted “base” models, each 0.5 kg/year decrease resulted in 30% increased odds of MCI/dementia (OR=1.30 [95% CI: 1.14, 1.49]). After adjustment for age, education, clinic, depression, and walking speed, there was 17% (OR=1.17 [95% CI: 1.02, 1.35]) increased odds of MCI/dementia for each 0.5 kg/year decrease in weight. In base models, variability in weight was significant. Each 1% average deviation from each woman’s predicted weight curve was associated with 11% increased odds of MCI/dementia (OR=1.11 [95% CI: 1.04, 1.18]). The estimate was attenuated after full adjustment (OR=1.06 [95% CI: 0.99, 1.14]). The presence of an abrupt weight decline was not associated with MCI/dementia.
Conclusions
Rate of weight loss over 20 years was associated with development of MCI or dementia in women surviving past 80 years, suggesting that nutritional status, social-environmental factors, and/or adipose tissue function and structure may affect cognitive function with aging.
Keywords: Weight trajectory, dementia, cognitive dysfunction
INTRODUCTION
Weight loss in late life has been linked to cognitive decline and development of dementia.1–12 Whether this weight loss is a result of or contributes to that decline is controversial.1,13 Weight loss may be an early manifestation of dementia,14 which could explain the association. However, weight loss has been shown to precede cognitive decline,4 suggesting that weight loss itself or the pathological processes behind it could contribute to cognitive decline. There is a paucity of data on whether other aspects of the late-life weight trajectory are associated with cognitive health, among them weight variability and an abrupt decline in weight.
Measuring weight is a simple tool available to all health care providers. Defining whether weight trajectories in older adults are associated with MCI and dementia could inform efforts to maintain cognitive function. For example, cognitive maintenance interventions could target nutritional status, social-environmental factors, and/or adipose tissue function and structure.
In this study, we sought to determine whether weight trajectories among 1,289 community-dwelling, ambulatory women ages 65 and older were associated with cognitive function after an average of 20 years. We utilized the Study of Osteoporotic Fractures (SOF) cohort, which allowed us to focus our analysis on the oldest old, those who survived into their 80s, 90s and beyond. We hypothesized that among these oldest old women, those with greater weight loss, greater weight variability, and/or an abrupt decline in weight would be more likely to have MCI/dementia.
METHODS
Study Overview and Study Sample
In 1986–88, the Study of Osteoporotic Fractures recruited 9,704 community-dwelling women, ages 65 and older (>99% non-Hispanic white) in four US regions: Baltimore County, Maryland; Minneapolis, Minnesota; Portland, Oregon; and the Monongahela Valley near Pittsburgh, Pennsylvania.15 Women unable to walk without assistance and those with bilateral hip replacements were not eligible for inclusion.
The women attended clinic visits, where their weight was measured, every 2 to 4 years. All surviving participants were invited to attend a Year 20 examination between 2006 and 2008; 2,121 women completed the Year 20 clinic or home visit. At the Year 20 visit, 3 of the 4 SOF sites participated in an ancillary study regarding clinical cognitive status.1,495 women had complete cognitive testing at Year 20. Because there were only 5 women of nonwhite race, they were excluded. 1,289 women (86%) also had weight measurements (mean 8 measurements) taken at baseline and the Year 20 examinations so that a weight trajectory could be determined. These women are the subject of this report (Figure 1).
Figure 1.
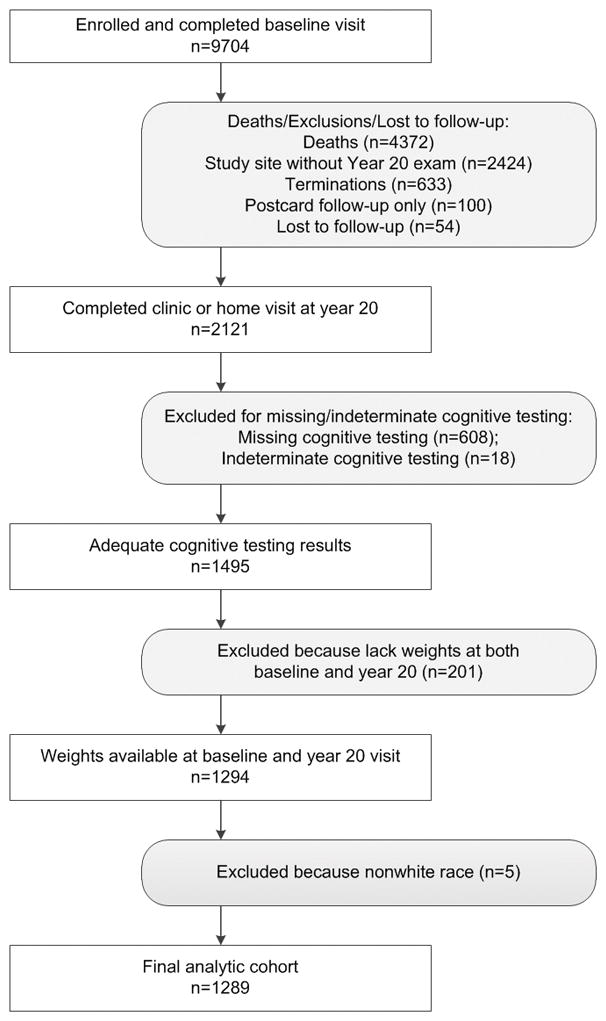
Consort diagram
All women provided written consent, and the SOF was approved by each site’s Institutional Review Board.
Weight
Body weight was measured in light clothing with a standard balance beam or digital scale at all 8 visits over the 20-year period (mean 8 visits). A total of 1,068 (83%) women had all measurements; 97% had at least 6 measurements between baseline and the Year 20 exam.
Other Characteristics
Height was measured with a wall-mounted Harpenden stadiometer (Holtain, Dyved, UK). These measures were then used to compute body mass index (BMI).15 Participants completed questionnaires assessing basic demographics, medical history, and educational history at baseline. Smoking and alcohol consumption, medical history, health behaviors (e.g., walking for exercise), independent activities of daily living, and self-reported health were determined by questionnaire and interview at Year 20. A comorbidity index at Year 20 was calculated by summing self-reported medical conditions (e.g. stroke, diabetes, congestive heart failure). Depressive symptoms were assessed at the Year 20 visit with the 15-item Geriatric Depression Scale (GDS).16 Walking speed was assessed at the Year 20 visit as the average time to complete two trials on a 6-meter course. At Year 16, visual acuity was measured with letter charts of Bailey and Lovie.17
Cognition and Cognitive Diagnosis
At Year 20, centrally trained clinic staff administered the cognitive battery described previously.18 Briefly, the battery included the Modified Mini-Mental State Examination (3MS),19 a 100-point extended version of the Mini-Mental State Examination (MMSE), California Verbal Learning Test–II Short Form Immediate and Delayed (CVLT-I and CVLT-D),20 Digit Span—Forward and Digit Span--Backward,21 Trail Making Test Part B (Trails B),22 Verbal Associative Fluency (Verbal Fluency) and Category Fluency,23 and Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE).24
Clinical cognitive diagnosis of normal cognition, MCI, and dementia were adjudicated from the Year 20 exam testing as previously described.25,26 Mild cognitive impairment (MCI) represents an early stage in the neurobiological and neuropathological changes that may culminate in dementia.27,28 Therefore, the decision was made a priori to combine MCI and dementia in order to be adequately powered. Women were considered cognitively normal if they did not meet any of 5 criteria for more detailed adjudication. The criteria were: 1) 3MS score <88, 2) CVLT delayed recall score <4, 3) IQCODE24 score ≥3.6, 4) self-reported physician diagnosis of dementia, or 5) nursing home residency. Next, data from women who met any of the above criteria were examined by a team of expert clinicians, who reviewed individuals’ cognitive test results from Year 20 and all prior cognitive assessment data, functional status, medical history, medications, and depressive symptoms, and then diagnosed individuals as having MCI (based on modified Peterson criteria)29 or dementia (based on DSM-IV criteria), or being cognitively normal.
Statistical Analyses
Baseline characteristics were compared using Chi-square for categorical variables and T-test for continuous variables. For the primary analyses, we examined the association between 20-year weight trajectory and cognition at the Year 20 visit. We considered a number of measures characterizing weight trajectories. Absolute weight change over time was calculated as the difference in weight from baseline to Year 20 (kilogram decrease in weight). For the MCI/dementia outcome, we evaluated weight change continuously, per 10 kg of weight loss. Ten kilograms was chosen based on the standard deviation (SD) (8.99 kg) of the overall population weight change and consideration of clinical relevance. For cognitive testing outcomes we evaluated weight change categorically as: 1) no weight loss (weight gain or no change [change ≥ 0]); 2) minimal weight loss (amount of weight loss the same as or slightly greater [within 1 SD] than the population change [1 SD ≤ change <0]); or 3) moderate weight loss (amount of weight loss greater than the SD for the population change [change <1 SD]).
Rate of weight loss (kilogram per year) was calculated using the slope estimate from a linear model fit to each woman’s weight measures over time. We evaluated rate of loss both continuously and in categories. For MCI/dementia, we examined Odds Ratio (OR) per 0.5 kg per year decrease in weight. This value was based on the population SD (0.44 kg per year decrease in weight) and clinical relevance. We also compared slope of weight change to the overall population slope: 1) positive slope ([slope >=0] indicating weight gain); 2) minimal negative slope (rate of weight loss the same as or slightly greater [within 1 SD] than the population rate [1 SD <= slope <0]); or 3) moderate negative slope (rate of weight loss greater than the SD for the population rate [slope <1 SD]).
Presence of an unrecovered, abrupt decline in weight indicated a marked loss of weight that was inconsistent with the woman’s prior weight trajectory. For this measure, we determined whether the weight trajectory fit a quadratic equation, with a relative maximum, and whether the weight loss continued to Year 20. This measure was evaluated as a dichotomous variable (yes/no).
Variability of weight over time was calculated using the root mean square percentage error (RMSPE) of the estimated linear and quadratic weight curves for each woman. The RMSPE represents the variability around the predicted curve. We used the RMSPE from the linear or quadratic equation that best fit the woman’s weight curve. We examined this as a continuous measure as well as quartile categories.
Using logistic regression, we examined the likelihood of developing MCI or dementia (categorized together as the measure of cognitive decline) for each of the individual weight trajectory measures. We evaluated these relationships in unadjusted and age/education/clinic adjusted “base” models.
For our multivariable models, we considered several potentially confounding factors, including baseline body composition (baseline height and body mass index [BMI]), Year 20 lifestyle (alcohol use, smoking), Year 20 health status (self-reported health, depression, walking for exercise, walking speed, comorbidity index), Year 16 visual impairment, and baseline oral estrogen use.30–42 These factors were chosen because they have been shown to influence cognition and weight. Each potential covariate was added individually to the base model. Covariates individually associated with Year 20 cognition in base models (p<0.10) were further evaluated together in a logistic regression model using manual backwards stepwise selection, where age, education, clinic, and weight variables were forced. Statistically significant variables remained in the final model (p<0.05).43,44 Our final, fully adjusted model consisted of the covariates identified above, plus the individual weight measures that remained independently significant at p<0.05.
Finally, we examined the association between weight trajectory measures and individual cognitive testing scores at the Year 20 exam. We used generalized linear models to compare mean scores and test for trend among different categorical weight trajectory measures, adjusting for the same covariates as in the MCI and dementia models. Cognitive measures not normally distributed (MMSE, 3MS and Trails B) were log-transformed for these analyses. Data were back transformed for display of results.
Statistical analyses were completed using SAS v9.4 (SAS, Inc., Cary, NC). We considered p<0.05 to be significant.
RESULTS
Sample Characteristics
SOF participants who survived to the Year 20 exam and had cognitive testing as well as baseline-to-Year-20 weight measurements over the study period were evaluated (n=1,289). Of these, 775 were cognitively normal and 514 were considered to have MCI/dementia. Those who were cognitively normal at Year 20 were younger and more educated at baseline; there was no difference in baseline BMI (26.57 vs 26.90 kg/m2) (Table 1). At the Year 20 exam, women who were cognitively normal were heavier, walked faster, drank more alcohol, and had lower GDS depression scores. They did not differ significantly on baseline estrogen use or on Year 20 comorbidity index, Year 16 visual acuity, baseline smoking history, Year 20 self-reported health, or Year 20 walking for exercise. Women who developed MCI/dementia had greater weight loss, steeper slope of weight loss, and more variability in weight between baseline to Year 20. They did not have a greater likelihood of an abrupt decline in weight. After adjusting for age, education, and clinic, walking speed and depressive symptoms were the only non-weight measures independently associated with MCI/dementia and therefore included in the fully adjusted models.
Table 1.
Overall Characteristics
| Normal at Year 20 | MCI/dementia at Year 20 | p-value | |
|---|---|---|---|
| N | 775 | 514 | |
| Baseline age, yrs (mean, se) | 67.87 (0.09) | 68.89 (0.14) | <.0001 |
| Education, =>12 yrs (n, %) | 687 (88.65) | 413 (80.35) | <.0001 |
| Baseline BMI, kg/m2 (mean, se) | 26.57 (0.15) | 26.9 (0.20) | 0.18 |
| Baseline height, cms (mean, se) | 160.5 (0.21) | 160.0 (0.25) | 0.09 |
| Baseline estrogen use, ever (n, %) | 386 (50.26) | 230 (45.36) | 0.09 |
| Year 20 weight, kgs (mean, se) | 63.76 (0.41) | 61.72 (0.52) | 0.002 |
| Year 20 walking speed, m/sec (mean, se) | 0.75 (0.01) | 0.57 (0.01) | <.0001 |
| Year 20 comorbidity scorea (n, %) | 0.57 | ||
| 1 | 236 (30.45) | 165 (32.29) | |
| 2 or more | 132 (17.03) | 93 (18.20) | |
| Year 16 visual acuity, poor (n, %) | 105 (15.46) | 80 (19.95) | 0.06 |
| Year 20 ever smoker (n, %) | 248 (32.00) | 180 (35.02) | 0.26 |
| Year 20 alcohol use (n, %) | <.0001 | ||
| <3 days/ week | 236 (30.49) | 122 (23.87) | |
| 3–7 days/ week | 98 (12.66) | 39 (7.63) | |
| Year 20 self-reported health, good/excellent (n, %) | 622 (80.26) | 396 (77.65) | 0.26 |
| Year 20 walks for exercise (n, %) | 340 (44.91) | 204 (40.88) | 0.16 |
| Year 20 depressionb (n, %) | 53 (6.86) | 91 (18.42) | <.0001 |
| Weight change baseline to Year 20, kgs (mean, se) | −4.44 (0.30) | −6.80 (0.43) | <.0001 |
| Slope of weight change from baseline to year 20, kg/year (mean, se) | −0.20 (0.01) | −0.31 (0.02) | <.0001 |
| Weight variability, RMSPEc (mean, se) | 2.78 (0.06) | 3.09 (0.08) | 0.003 |
| Weight decline (n, %)d | 218 (28.13) | 131 (25.49) | 0.30 |
Abbreviation: se refers to standard error
Comorbidity index was calculated using the sum (0–7) of seven possible self-reported medical conditions, including ever having a stroke, diabetes, COPD, Parkinson’s disease, heart attack/angioplasty, congestive heart failure, or peripheral vascular disease.
Depressive symptoms were assessed with the 15-item Geriatric Depression Scale (GDS). Those with scores >=6 were considered to have depression.
Variability of weight calculated using the root mean square percentage error (RMSPE) of the estimated linear and quadratic weight curves for each woman.
N, % refers to the number and percentage of women with an unrecovered abrupt decline in weight based on whether the weight trajectory fit a quadratic equation with a relative maximum and weight loss continued to the end of weight trajectory.
Association between weight trajectory and MCI/dementia
Overall, the average weight change between baseline and 20-year exams was a loss of 5.38 kilograms (SD=8.99; range loss of 42.50 kgs to gain of 27.50 kgs). Compared to women who had MCI/dementia at the Year 20 visit, women who were cognitively normal at Year 20 had less weight loss from baseline to the Year 20 visit (−4.44 kgs [SD=8.39 kgs] vs −6.80 kgs [SD=9.65 kgs]; p<0.0001), which translated into a less steep slope of weight loss (−0.20 kgs/year [SD=0.41] vs −0.31 kgs/year [SD=0.47]; p<0.0001). In unadjusted models, each 0.5 kg/year decrease in weight (10 kg loss over 20 years) resulted in a 35% increase in odds of developing MCI/dementia (OR=1.35 [95% CI: 1.19, 1.54]; Table 2). These estimates were similar after adjustment for age, education, and clinic (30% increase in odds of developing MCI/dementia for each 0.5 kg/year weight loss (OR=1.30 [95% CI: 1.14, 1.49])). Although estimates were attenuated after full adjustment, women still had a significant, 17% increase in odds of developing MCI/dementia for each 0.5 kg/year weight loss (OR=1.17 [95% CI: 1.02, 1.35]).
Table 2.
Odds of Developing Dementia or MCI According to Individual Characteristics of Weight Trajectory
| Weight Measurea | Unadjusted OR (95% CI) |
Age, education, clinic adjusted OR (95% CI) |
Fully adjustedb OR (95% CI) |
|---|---|---|---|
| Change in weight from baseline to Year 20 | |||
| Per 10 kg decrease in weight | 1.35 (1.19,1.53) | 1.30 (1.14, 1.47) | 1.18 (1.03, 1.35) |
| Slope of weight change from baseline to Year 20 | |||
| Per 0.5 kg/year decrease in weight | 1.35 (1.19, 1.54) | 1.30 (1.14, 1.49) | 1.17 (1.02, 1.35) |
| Slope of weight change categoryc | |||
| Positive slope | 1.0(ref) | 1.0(ref) | 1.0(ref) |
| Minimal negative slope | 1.33 (1.02, 1.72) | 1.22 (0.93, 1.59) | 1.09 (0.82, 1.44) |
| Moderate negative slope | 2.25 (1.57, 3.24) | 2.02 (1.39, 2.93) | 1.46 (0.98, 2.17) |
| Abrupt weight declined | |||
| No | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Yes | 0.87 (0.68, 1.13) | 0.82 (0.63, 1.07) | 0.78 (0.59, 1.03) |
|
Weight variability (RMSPE)e Per ≈1% average deviation from predicted curve |
1.10 (1.03, 1.17) | 1.11 (1.04, 1.18) | 1.06 (0.99, 1.14) |
| Weight variability (RMSPE) quartilee | |||
| Q1 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Q2 | 1.12 (0.81, 1.54) | 1.15 (0.83, 1.60) | 1.24 (0.87, 1.75) |
| Q3 | 1.29 (0.94, 1.77) | 1.33 (0.96, 1.84) | 1.16 (0.82, 1.63) |
| Q4 | 1.51 (1.10, 2.07) | 1.60 (1.15, 2.21) | 1.33 (0.94, 1.88) |
Each model includes only the weight measure of interest and the non-weight covariates.
Includes age, education, clinic, depression, and walking speed (quartiles).
Categories are relative to overall population slope (0.44 kg per year decrease in weight); positive slope (slope >=0) indicates weight gain; minimal negative slope indicates rate of weight loss the same as or slightly greater (within 1 SD) than the population rate (1 SD <= slope <0); moderate negative slope indicates rate of weight loss greater than the SD for the population rate (slope <1 SD).
Presence of an unrecovered abrupt decline in weight based on whether the weight trajectory fit a quadratic equation with a relative maximum and weight loss continued to the end of weight trajectory (yes/no).
Variability of weight calculated using the root mean square percentage error (RMSPE) of the estimated linear and quadratic weight curves for each woman.
When we examined slope of weight change categories and MCI or dementia at Year 20, we found associations similar to the continuous models. Women who had moderate negative slopes of weight loss had more than a two-fold (OR=2.25 [95% CI: 1.57, 3.24]) greater unadjusted odds of developing MCI or dementia at the Year 20 exam compared to those with positive slopes. The estimate was similar after adjustment for age, education, and clinic (OR=2.02 [95% CI: 1.39, 2.93]) but was attenuated after adjustment for walking speed and depressive symptoms (OR=1.46 [95% CI: 0.98, 2.17]).
The presence of an abrupt, unrecovered weight decline was not associated with development of MCI or dementia in unadjusted models (OR=0.87 [95% CI: 0.68, 1.13]) or adjusted models (OR=0.82 [95% CI: 0.63, 1.07] and OR=0.78 [95% CI: 0.59, 1.03] in age/education/clinic-adjusted and fully adjusted models, respectively).
Women who were cognitively normal at Year 20 also had less variability around their weight trajectory compared to women who developed MCI/dementia (RMSPE mean 2.78 [SD=1.71] compared to 3.09 [SD=1.92]; p=0.003). In unadjusted models, there was a 10% greater odds of developing MCI/dementia for each 1% average deviation from predicted weight curve (OR=1.10 [95% CI: 1.03, 1.17]). The estimate remained similar after adjustment for age, education, and clinic (OR=1.11 [95% CI: 1.04, 1.18]) but was attenuated after further adjustment for walking speed and depression (OR=1.06 [95% CI: 0.99, 1.14]).
Weight characteristics included simultaneously
Higher rate of weight loss and greater weight variability were both determined to be significant, independent predictors of MCI/dementia. Table 3 shows the likelihood of MCI/dementia when these are examined simultaneously in unadjusted and adjusted models. In unadjusted models, each 0.5 kg per year decrease in weight was associated with a 32% increase in odds (OR=1.32 [95% CI: 1.16, 1.51]) of developing MCI/dementia, and each 1% average deviation from predicted weight curves was associated with an 8% increase in odds (OR=1.08 [95% CI: 1.01, 1.15]) of developing MCI or dementia. Estimates were similar after adjustment for age, education, and clinic (OR=1.27 [95% CI: 1.11, 1.45] and OR=1.09 [95% CI: 1.02, 1.16] for slope and variability, respectively) but were attenuated after further adjustment for walking speed and depression (OR=1.16 [95% CI: 1.00, 1.33] and OR=1.05 [95% CI: 0.98, 1.13] for slope and variability, respectively).
Table 3.
Odds of Developing Dementia or MCI When Weight Trajectory Characteristics Examined Simultaneously
| Weight Measurea | Unadjusted score (95% CI) | Age, education, clinic adjusted OR (95% CI) | Fully adjustedb OR (95% CI) |
|---|---|---|---|
| Slope of weight change from baseline to Year 20 | |||
| Per 0.5 kg/year decrease in weight) | 1.32 (1.16, 1.51) | 1.27 (1.11, 1.45) | 1.16 (1.0, 1.33) |
| Weight variability (RMSPE) c | |||
| Per ≈1% average deviation from predicted curve | 1.08 (1.01, 1.15) | 1.09 (1.02, 1.16) | 1.05 (0.98, 1.13) |
Models include both weight measures simultaneously.
Includes age, education, clinic, depression, and walking speed (quartiles).
Variability of weight calculated using the root mean square percentage error (RMSPE) of the estimated linear and quadratic weight curves for each woman.
Individual cognitive tests
We examined the association between weight trajectory variables and individual cognitive test performance at Year 20. In age/education/clinic-adjusted “base” models, we found that women with greater weight loss over 20 years (as measured by either slope of weight change or total weight change) had poorer cognitive performance on MMSE, 3MS, Trails B, CVLT-I, CVLT-D, Verbal Fluency and Category Fluency (p<0.05 for all; data not shown). When adjusted for walking speed and depression, only CVLT-I remained significantly associated with absolute weight change (p=0.002), and CVLT-I and CVLT-D remained significantly associated with rate of weight loss (p’s=0.001 and 0.03, respectively; Table 4). Digit Span Forward and Backward were not associated with weight change over time in any models (total weight change adjusted p’s=0.33, and 0.55, respectively; slope of weight change adjusted p’s=0.19, and 0.85, respectively; Table 4). Abrupt weight decline was not associated with performance on any cognitive test.
Table 4.
Cognitive Function According to Individual Characteristics of Weight Trajectory in Fully Adjusted Models
| MMSE | 3MS | Trails B | CVLT-I | CVLT-D | Verbal Fluency |
Category Fluency |
Digit span Forward |
Digit Span Backward |
|
|---|---|---|---|---|---|---|---|---|---|
| Weight change characteristica,b |
Meanc (95% CI) |
Meanc (95% CI) |
Meanc (95% CI) |
Meanc (95% CI) |
Meanc (95% CI) |
Meanc (95% CI) |
Meanc (95% CI) |
Meanc (95% CI) |
Meanc (95% CI) |
| Weight change baseline to year 20d | |||||||||
| No weight loss | 22.06 (21.51, 22.62) | 85.42 (84.08, 86.78) | 174.57 (161.74, 188.42) | 22.78 (22.07, 23.49) | 4.76 (4.39, 5.12) | 9.83 (9.28, 10.38) | 10.22 (9.75, 10.70) | 7.05 (6.75, 7.34) | 5.02 (4.74, 5.29) |
| Minimal weight loss | 21.77 (21.33, 22.21) | 84.89 (83.81, 85.98) | 180.89 (169.59, 192.95) | 22.42 (21.85, 23.0) | 4.96 (4.66, 5.25) | 9.89 (9.45, 10.34) | 10.12 (9.73, 10.50) | 7.11 (6.87, 7.35) | 5.14 (4.92, 5.36) |
| Moderate Weight loss | 21.51 (20.85, 21.19) | 84.37 (82.73, 86.05) | 190.27 (173.04, 209.21) | 21.31 (20.43, 22.19) | 4.28 (3.84, 4.73) | 9.18 (8.49, 9.86) | 9.83 (9.24, 10.42) | 7.24 (6.88, 7.61) | 5.13 (4.79, 5.47) |
| Adjusted P value for trend | 0.14 | 0.25 | 0.09 | 0.002 | 0.06 | 0.08 | 0.23 | 0.33 | 0.55 |
| Slope of weight change baseline to year 20e | |||||||||
| Positive slope | 22.07 (21.54, 22.62) | 85.55 (84.23, 86.89) | 174.55 (161.96, 188.13) | 22.85 (22.15, 23.55) | 4.83 (4.48, 5.19) | 10.0 (9.46, 10.54) | 10.12 (9.65, 10.59) | 7.02 (6.74, 7.31) | 5.10 (4.83, 5.37) |
| Minimal negative slope | 21.78 (21.33, 22.23) | 84.81 (83.71, 85.91) | 182.64 (171.10, 194.95) | 22.43 (21.85, 23.01) | 4.93 (4.63, 5.23) | 9.81 (9.36, 10.26) | 10.14 (9.75, 10.53) | 7.11 (6.87, 7.35) | 5.10 (4.88, 5.33) |
| Moderate negative slope | 21.45 (20.79, 22.12) | 84.44 (82.81, 86.10) | 185.38 (168.60, 203.83) | 21.23 (20.35, 22.10) | 4.31 (3.87, 4.76) | 9.27 (8.59, 9.95) | 9.95 (9.36, 10.54) | 7.28 (6.92, 7.65) | 5.14 (4.80, 5.47) |
| Adjusted P value for trend | 0.09 | 0.22 | 0.24 | 0.001 | 0.03 | 0.05 | 0.59 | 0.19 | 0.85 |
| Abrupt weight declinef | |||||||||
| No | 21.74 (21.32, 22.17) | 84.77 (83.72, 85.84) | 181.46 (170.29, 193.37) | 22.31 (21.74, 22.87) | 4.75 (4.46, 5.04) | 9.76 (9.33, 10.20) | 9.98 (9.60, 10.35) | 7.16 (6.93, 7.39) | 5.14 (4.92, 5.36) |
| Yes | 21.95 (21.42, 22.49) | 85.35 (84.05, 86.67) | 179.55 (166.94, 193.12) | 22.44 (21.75, 23.13) | 4.92 (4.57, 5.27) | 9.80 (9.27, 10.33) | 10.40 (9.94, 10.86) | 7.01 (6.73, 7.30) | 5.04 (4.77, 5.30) |
| Adjusted P value for trend | 0.40 | 0.35 | 0.75 | 0.67 | 0.31 | 0.88 | 0.05 | 0.26 | 0.41 |
| Weight variability (RMSPE) quartileg | |||||||||
| Q1 | 21.92 (21.37, 22.47) | 85.46 (84.12, 86.83) | 173.47 (160.64, 187.33) | 22.38 (21.66, 23.10) | 4.94 (4.57, 5.31) | 10.01 (9.45, 10.56) | 10.48 (10.01, 10.96) | 7.04 (6.75, 7.34) | 4.96 (4.69, 5.24) |
| Q2 | 21.93 (21.37, 22.51) | 85.46 (84.07, 86.87) | 182.87 (168.89, 198.00) | 22.54 (21.79, 23.28) | 4.76 (4.39, 5.14) | 10.11 (9.54, 10.68) | 10.23 (9.74, 10.73) | 7.26 (6.96, 7.57) | 5.28 (5.0, 5.57) |
| Q3 | 21.89 (21.36, 22.44) | 84.86 (83.55, 86.20) | 178.93 (165.75, 193.16) | 22.39 (21.68, 23.09) | 4.80 (4.44, 5.16) | 9.60 (9.06, 10.14) | 9.72 (9.25, 10.18) | 6.98 (6.69, 7.27) | 5.01 (4.75, 5.28) |
| Q4 | 21.47 (20.93, 22.03) | 84.07 (82.72, 85.44) | 189.32 (174.94, 204.89) | 22.10 (21.37, 22.83) | 4.70 (4.33, 5.08) | 9.44 (8.87, 10.0) | 10.04 (9.55, 10.53) | 7.22 (6.92, 7.52) | 5.22 (4.94, 5.50) |
| Adjusted P value for trend | 0.17 | .05 | 0.07 | 0.44 | 0.31 | 0.03 | 0.03 | 0.64 | 0.31 |
Each model includes the single weight measure of interest and the non-weight covariates of age, education, clinic, depression, and walking speed (quartiles).
Sample size for analyses varied based on completion of cognitive tests and covariate assessment: MMSE (n=1267), 3MS (n=1267), Trails B (n=1035), CVLT – Immediate recall (n=1246), CVLT – Delayed recall (n=1246), Verbal fluency (n=1246), Category fluency (n=1246), Digit span – forward (n=1259), Digit span – backward (n=1254)
Means are adjusted.
Categories are relative to population change SD (8.99 kg); no weight loss signifies weight change >=0; minimal weight loss indicates weight change was <=1 SD but >0; moderate weight loss indicates weight change was >1 SD.
Categories are relative to overall population slope (0.44 kg per year decrease in weight); positive slope (slope >=0) indicates weight gain; minimal negative slope indicates rate of weight loss the same as or slightly greater (within 1 SD) than the population rate (1 SD <= slope <0); moderate negative slope indicates rate of weight loss greater than the SD for the population rate (slope <1 SD).
Presence of an unrecovered abrupt decline in weight determined from whether the weight trajectory fit a quadratic equation with a relative maximum and weight loss continued to the end of the weight trajectory (yes/no).
Variability of weight calculated using the root mean square percentage error (RMSPE) of the estimated linear and quadratic weight curves for each woman.
Greater weight variability was associated with poorer performance on several cognitive tests. In age/education/clinic-adjusted models, women with greater weight variability as measured by RMSPE had poorer performance on MMSE, 3MS, Trails B, CVLT-I, CVLT-D, Verbal Fluency, and Category Fluency (p<0.05 for all; data not shown). The association remained significant in models adjusted for walking speed and depression for 3MS (p=0.05), Verbal Fluency (p=0.03), and and Category Fluency (p=0.03). There was no association between weight variability and Digit Span Forward or Backward in any of these models (p’s=0.64 and 0.31, respectively; Table 4).
DISCUSSION
Among older women (ages 83–102) in the SOF cohort with measured weights over 20 years, greater than average weight loss over time (approximately >0.5 kg/year) was associated with development of MCI/dementia. Although weight variability was associated with development of MCI/dementia, rate of weight loss was a more important predictor than variability. An abrupt decline in weight was not associated with development of MCI/dementia.
This study offers a unique perspective into how women’s weight trajectories in the 20 years leading up to ages 83–102 are associated with cognitive function. Previous research that followed older persons for shorter periods (3–12 years) found that weight loss was associated with development of dementia.1–12 However, cognitive decline has been shown to precede dementia diagnosis by up to 9 years.45 Therefore, it was possible in these shorter studies that preclinical cognitive decline was causing weight loss. Our study, with its 20 years of follow-up, is less likely to be subject to this bias and suggests that losing weight in later decades is associated with greater likelihood of cognitive decline.
We also examined the association between weight trajectory variables and performance on individual cognitive tests. We found that women with more weight loss had poorer verbal memory and verbal fluency at Year 20 compared to those with less weight loss. The Women’s Health Initiative Study of Cognitive Aging (WHISCA) study also found that women who lost weight performed more poorly on verbal fluency at follow-up compared to those who had more stable weight; however, they did not find the difference in verbal memory that we observed, perhaps because women in the WHISCA study were younger.46 Although both weight loss and weight variability were associated with overall mental status, the association was attenuated with adjustment for walking speed and depression. There was no association between weight trajectory variables and tests of executive function and working memory, which was similar to the findings in WHISCA.
As far as we know, we are the first to examine weight variability in relation to cognitive function. Previous studies examining the association between weight cycling with mortality and physical function have shown conflicting results.47–49 These studies differ from the current work by relying on subjective weight history48,49 or following measured weights for only 7 years.47 Based on work from the Cardiovascular Health Study showing that variations in measured weight were associated with future physical limitations and mortality in older adults,47 we hypothesized that greater weight variability would be associated with accelerated cognitive aging. We hypothesized that variability in weight may reflect difficulties in maintaining homeostasis possibly secondarily to cognitive decline with aging. Although we found that greater weight variability was associated with a higher likelihood of MCI/dementia in univariable models, it was not as strong of a predictor as rate of weight loss when considered together.
As people age, they are at risk of weight loss because of changes in taste and smell, impaired digestion, and malabsorption/poor usage of nutrients due to chronic diseases or drug-nutrient interactions. In addition, social-environmental factors (e.g. solitude, institutionalization), psychological factors (e.g. depression), or a limited level of independence (e.g. difficulty in purchasing and preparing foods) can lead to weight loss.50 Weight loss and the associated alterations in adipose tissue function and structure could negatively affect brain health. For example, persons with adiposopathy51,52 or excessive hypertrophy of adipocytes have dysregulated paracrine and endocrine adipose tissue that contributes to the syndrome of weight loss, sarcopenia, frailty, and impaired cognitive function. Adipokines may play an important role in this association.4,53 Leptin has been called a potential cognitive enhancer/protector because higher levels have been associated with improved hypothalamic and hippocampal function,54 slower preclinical cognitive decline,55,56 and reduced incidence of dementia and AD.57 In the hippocampus, leptin facilitates presynaptic and postsynaptic transmitter release and sensitivity, affecting learning and memory.54 Experimental models suggest that leptin may either directly or indirectly, through its effect on lipid metabolism, help decrease AD pathology.58
Alternatively, both impaired cognition and decreased weight could result from age-related degenerative processes in the brain or other comorbidities. Low BMI is correlated with cerebral atrophy in dementia patients.59 Atrophy of the mesial temporal cortex, which is involved in feeding behavior and memory, is preferentially involved in AD.59 Weight loss with aging could potentially accelerate brain atrophy, as occurs during anorexia nervosa in younger adults.59–61 As the deficits seen with anorexia nervosa may not fully reverse after weight recovery62 and leptin’s effects on the brain could be cumulative, weight variability with its repeated episodes of weight loss could negatively affect the brain.
Our findings were attenuated after adjustment for walking speed and depressive symptoms, both of which are associated with cognition and weight.63–66 This may be due to episodes of illness that lead to lower walking speed and/or bouts of depression, both of which might affect weight trajectory and cognitive function. Given that we tested many other potential covariates, with only walking speed and depressive symptoms influencing the weight loss/variability and cognitive function relationship, our results suggest that walking speed and depression should be measured and examined during studies on the association between weight and cognition.
We did not find that an abrupt decline in weight was associated with poorer cognitive function. Although previous studies have not specifically looked at abrupt, unrecovered weight loss, several studies have noted that weight decreases in the years preceding dementia diagnosis.2,67 Our study differed in that our population consisted of exceptionally older women who had survived into their 80s, 90s and beyond. In addition, we examined weight trajectories starting after age 65, while previous studies followed subjects from midlife (50s).2,67,68
Our study had several strengths. We studied a large cohort of women with multiple weight measurements over 20 years. The mean age of our population at study’s end was 88 (range 83–102), so our observation period covered the period when risk of cognitive decline is greatest.69 Women were recruited irrespective of BMD status and measurements were rigorous,15 including detailed cognitive evaluation at the Year 20 examination. However, our study had some limitations. We relied on participants’ self-reports of medical conditions and functional limitations. Women must have been observed for 20 years to be measured for weight change and Year 20 cognitive function, so only survivors were included in analyses, potentially leading to survival bias. We did not have sufficient data on voluntary weight loss to be able to distinguish between intentional and unintentional weight loss, which are very different. We did not measure cognitive function at baseline, so we cannot exclude that early, preclinical cognitive decline resulted in greater weight loss over time in those who developed MCI/dementia. Finally, our study sample comprised Caucasian women; thus, our findings may not be generalizable to men or other racial/ethnic groups.
Conclusions
Women who survived into their 80s and 90s with normal cognitive function had more stable weight in the prior 20 years. Although the association seen with weight loss is smaller than that associated with traditional risk factors such as age69 and education,30 it could be of importance given the high prevalence of cognitive decline and dementia with aging, and because weight is a potentially modifiable risk factor in older age. Future research should target nutritional status, social-environmental factors, and/or adipose tissue function and structure as methods for preserving cognitive function into old age.
Acknowledgments
We thank Amie M. Weitz of the Kaiser Permanente Center for Health Research for help with manuscript preparation. We thank Katherine K. Essick of the Kaiser Permanente Center for Health Research for editorial assistance.
Funding Source: The Study of Osteoporotic Fractures (SOF) is supported by the National Institutes of Health. The National Institute on Aging (NIA) provides support under the following grant numbers: R01 ag005407, R01 ar35582, R01 ar35583, R01 ar35584, R01 ag005394, R01 ag027574, and R01 ag027576.
Conflict of Interest Checklist
| Elements of Financial/Personal Conflicts | ESL | JR | KP | KY | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts | KE | JC | PC | SC | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | x | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts | TH | |
|---|---|---|
| Yes | No | |
| Employment or Affiliation | x | |
| Grants/Funds | x | |
| Honoraria | x | |
| Speaker Forum | x | |
| Consultant | x | |
| Stocks | x | |
| Royalties | x | |
| Expert Testimony | x | |
| Board Member | x | |
| Patents | x | |
| Personal Relationship | x | |
For “yes”, provide a brief explanation: Dr. LeBlanc’s institute has received research funding from Amgen, Astrazeneca, and Bristol Meyers Squibb for unrelated projects on which she was investigator.
Footnotes
Author Contributions:
ESL: Conception and design and interpretation of data; drafting the article and revising it for important intellectual content; and final approval of the version to be published.
JR: Acquisition of data and analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; and final approval of the version to be published.
KP: Acquisition of data and analysis and interpretation of data; drafting the article and revising it critically for important intellectual content; and final approval of the version to be published.
KY: Conception and design and acquisition of data and interpretation of data; revising article for important intellectual content; and final approval of the version to be published.
KE: Conception and design and interpretation of data; revising article for important intellectual content; and final approval of the version to be published.
JC: Conception and design and interpretation of data; revising article for important intellectual content; and final approval of the version to be published.
PC: Interpretation of data; revising article critically for important intellectual content; and final approval of the version to be published.
SC: Interpretation of data; revising article critically for important intellectual content; and final approval of the version to be published.
TH: Conception and design and interpretation of data; revising article for important intellectual content; and final approval of the version to be published.
Sponsor’s role: The sponsor played no role in the design, methods, subject recruitment, data collections, analysis, or preparation of the paper.
References
- 1.Luchsinger JA, Patel B, Tang MX, Schupf N, Mayeux R. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64(3):392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustafson DR, Backman K, Waern M, Ostling S, Guo X, Zandi P, Mielke MM, Bengtsson C, Skoog I. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73(19):1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stewart R, Masaki K, Xue QL, Peila R, Petrovitch H, White LR, Launer LJ. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 4.Buchman AS, Wilson RS, Bienias JL, Shah RC, Evans DA, Bennett DA. Change in body mass index and risk of incident Alzheimer disease. Neurology. 2005;65(6):892–897. doi: 10.1212/01.wnl.0000176061.33817.90. [DOI] [PubMed] [Google Scholar]
- 5.Nourhashemi F, Deschamps V, Larrieu S, Letenneur L, Dartigues JF, Barberger-Gateau P. Body mass index and incidence of dementia: the PAQUID study. Neurology. 2003;60(1):117–119. doi: 10.1212/01.wnl.0000038910.46217.aa. [DOI] [PubMed] [Google Scholar]
- 6.Johnson DK, Wilkins CH, Morris JC. Accelerated weight loss may precede diagnosis in Alzheimer disease. Arch Neurol. 2006;63(9):1312–1317. doi: 10.1001/archneur.63.9.1312. [DOI] [PubMed] [Google Scholar]
- 7.Knopman DS, Edland SD, Cha RH, Petersen RC, Rocca WA. Incident dementia in women is preceded by weight loss by at least a decade. Neurology. 2007;69(8):739–746. doi: 10.1212/01.wnl.0000267661.65586.33. [DOI] [PubMed] [Google Scholar]
- 8.Hughes TF, Borenstein AR, Schofield E, Wu Y, Larson EB. Association between late-life body mass index and dementia: The Kame Project. Neurology. 2009;72(20):1741–1746. doi: 10.1212/WNL.0b013e3181a60a58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vidoni ED, Townley RA, Honea RA, Burns JM. Alzheimer disease biomarkers are associated with body mass index. Neurology. 2011;77(21):1913–1920. doi: 10.1212/WNL.0b013e318238eec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagger YZ, Tanko LB, Alexandersen P, Qin G, Christiansen C. The implications of body fat mass and fat distribution for cognitive function in elderly women. Obes Res. 2004;12(9):1519–1526. doi: 10.1038/oby.2004.189. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Yanez D, Harris T, Duxbury A, Enright PL, Fried LP. Weight change in old age and its association with mortality. J Am Geriatr Soc. 2001;49(10):1309–1318. doi: 10.1046/j.1532-5415.2001.49258.x. [DOI] [PubMed] [Google Scholar]
- 12.Wallace JI, Schwartz RS, LaCroix AZ, Uhlmann RF, Pearlman RA. Involuntary weight loss in older outpatients: incidence and clinical significance. J Am Geriatr Soc. 1995;43(4):329–337. doi: 10.1111/j.1532-5415.1995.tb05803.x. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Jr, Luchsinger JA. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336–342. doi: 10.1001/archneurol.2008.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burns JM, Johnson DK, Watts A, Swerdlow RH, Brooks WM. Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch Neurol. 2010;67(4):428–433. doi: 10.1001/archneurol.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, Cauley J, Black D, Vogt TM. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332(12):767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 16.Sheikh J, JY . Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. 1986. pp. 165–173. [Google Scholar]
- 17.Bailey IL, Lovie JE. New design principles for visual acuity letter charts. American journal of optometry and physiological optics. 1976;53(11):740–745. doi: 10.1097/00006324-197611000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Fine EM, Kramer JH, Lui LY, Yaffe K Study Of Osteoporotic Fractures Sof Research G. Normative data in women aged 85 and older: verbal fluency, digit span, and the CVLT-II short form. The Clinical neuropsychologist. 2012;26(1):18–30. doi: 10.1080/13854046.2011.639310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 20.Delis D, Kramer J, Ober B. California Verbal Learning Test. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- 21.Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; 2006. General cognitive functioning, neuropsychological batteries, and assessment of premorbid intelligence; pp. 283–285. [Google Scholar]
- 22.Reitan R. Manual for Administration and Scoring. Mesa, AZ: Reitan Neuropsycology Laboratory; 1992. [Google Scholar]
- 23.Strauss E, Sherman E, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; 2006. pp. 499–503. [Google Scholar]
- 24.Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19(4):1015–1022. doi: 10.1017/s0033291700005742. [DOI] [PubMed] [Google Scholar]
- 25.Yaffe K, Middleton LE, Lui LY, Spira AP, Stone K, Racine C, Ensrud KE, Kramer JH. Mild cognitive impairment, dementia, and their subtypes in oldest old women. Arch Neurol. 2011;68(5):631–636. doi: 10.1001/archneurol.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Metti AL, Yaffe K, Boudreau RM, Ganguli M, Lopez OL, Stone KL, Cauley JA. Change in inflammatory markers and cognitive status in the oldest-old women from the Study of Osteoporotic Fractures. J Am Geriatr Soc. 2014;62(4):662–666. doi: 10.1111/jgs.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haroutunian V, Hoffman LB, Beeri MS. Is there a neuropathology difference between mild cognitive impairment and dementia? Dialogues in clinical neuroscience. 2009;11(2):171–179. doi: 10.31887/DCNS.2009.11.2/vharoutunian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petersen RC, Roberts RO, Knopman DS, Boeve BF, Geda YE, Ivnik RJ, Smith GE, Jack CR., Jr Mild cognitive impairment: ten years later. Arch Neurol. 2009;66(12):1447–1455. doi: 10.1001/archneurol.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 30.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer’s disease. Jama. 1994;271(13):1004–1010. [PubMed] [Google Scholar]
- 31.Corrada MM, Brookmeyer R, Paganini-Hill A, Berlau D, Kawas CH. Dementia incidence continues to increase with age in the oldest old: the 90+ study. Ann Neurol. 2010;67(1):114–121. doi: 10.1002/ana.21915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rissanen AM, Heliovaara M, Knekt P, Reunanen A, Aromaa A. Determinants of weight gain and overweight in adult Finns. Eur J Clin Nutr. 1991;45(9):419–430. [PubMed] [Google Scholar]
- 33.Vasan RS, Pencina MJ, Cobain M, Freiberg MS, D’Agostino RB. Estimated risks for developing obesity in the Framingham Heart Study. Ann Intern Med. 2005;143(7):473–480. doi: 10.7326/0003-4819-143-7-200510040-00005. [DOI] [PubMed] [Google Scholar]
- 34.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med. 2002;347(22):1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 35.Montlahuc C, Soumare A, Dufouil C, Berr C, Dartigues JF, Poncet M, Tzourio C, Alperovitch A. Self-rated health and risk of incident dementia: a community-based elderly cohort, the 3C study. Neurology. 2011;77(15):1457–1464. doi: 10.1212/WNL.0b013e31823303e1. [DOI] [PubMed] [Google Scholar]
- 36.Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology. 2011;77(3):227–234. doi: 10.1212/WNL.0b013e318225c6bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katon W, Pedersen HS, Ribe AR, Fenger-Gron M, Davydow D, Waldorff FB, Vestergaard M. Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA psychiatry. 2015;72(6):612–619. doi: 10.1001/jamapsychiatry.2015.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh-Manoux A, Czernichow S, Elbaz A, Dugravot A, Sabia S, Hagger-Johnson G, Kaffashian S, Zins M, Brunner EJ, Nabi H, Kivimaki M. Obesity phenotypes in midlife and cognition in early old age: the Whitehall II cohort study. Neurology. 2012;79(8):755–762. doi: 10.1212/WNL.0b013e3182661f63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64(2):277–281. doi: 10.1212/01.WNL.0000149519.47454.F2. [DOI] [PubMed] [Google Scholar]
- 40.Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65(4):545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. Jama. 2003;289(11):1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- 42.Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN, 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289(20):2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 43.Rothman KJ, Greenland S contributors w. Modern Epidemiology. 2. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 44.Kleinbaum DG, Kupper LL, Morgenstern H. Confounding. In: Kleinbaum DG, Kupper LL, Morgenstern H, editors. Epidemiologic Research: Principles and Quantitative Methods. Belmont, CA: Lifetime Learning Publications; 1982. pp. 242–265. [Google Scholar]
- 45.Amieva H, Jacqmin-Gadda H, Orgogozo JM, Le CN, Helmer C, Letenneur L, Barberger-Gateau P, Fabrigoule C, Dartigues JF. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain. 2005;128(Pt 5):1093–1101. doi: 10.1093/brain/awh451. [DOI] [PubMed] [Google Scholar]
- 46.Driscoll I, Espeland MA, Wassertheil-Smoller S, Gaussoin SA, Ding J, Granek IA, Ockene JK, Phillips LS, Yaffe K, Resnick SM. Weight Change and Cognitive Function: Findings From the Women’s Health Initiative Study of Cognitive Aging. Obesity (Silver Spring) 2011;19(8):1595–1600. doi: 10.1038/oby.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arnold AM, Newman AB, Cushman M, Ding J, Kritchevsky S. Body weight dynamics and their association with physical function and mortality in older adults: the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci. 2010;65(1):63–70. doi: 10.1093/gerona/glp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taing KY, Ardern CI, Kuk JL. Effect of the Timing of Weight Cycling During Adulthood on Mortality Risk in Overweight and Obese Postmenopausal Women. Obesity (Silver Spring) 2011 doi: 10.1038/oby.2011.207. [DOI] [PubMed] [Google Scholar]
- 49.Field AE, Malspeis S, Willett WC. Weight cycling and mortality among middle-aged or older women. Arch Intern Med. 2009;169(9):881–886. doi: 10.1001/archinternmed.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Inelmen EM, Sergi G, Coin A, Girardi A, Manzato E. An open-ended question: Alzheimer’s disease and involuntary weight loss: which comes first? Aging Clin Exp Res. 2010;22(3):192–197. doi: 10.1007/BF03324796. [DOI] [PubMed] [Google Scholar]
- 51.Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57(25):2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 52.Van de Voorde J, Pauwels B, Boydens C, Decaluwe K. Adipocytokines in relation to cardiovascular disease. Metabolism. 2013;62(1):513–521. doi: 10.1016/j.metabol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 53.Arnoldussen IA, Kiliaan AJ, Gustafson DR. Obesity and dementia: adipokines interact with the brain. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2014;24(12):1982–1999. doi: 10.1016/j.euroneuro.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? The Lancet. Neurology. 2014;13(9):913–923. doi: 10.1016/S1474-4422(14)70085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly: Findings from the Health ABC Study. Neurobiol Aging. 2009;30(9):1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeki Al Hazzouri A, Stone KL, Haan MN, Yaffe K. Leptin, mild cognitive impairment, and dementia among elderly women. J Gerontol A Biol Sci Med Sci. 2013;68(2):175–180. doi: 10.1093/gerona/gls155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S. Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging. Jama. 2009;302(23):2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fewlass DC, Noboa K, Pi-Sunyer FX, Johnston JM, Yan SD, Tezapsidis N. Obesity-related leptin regulates Alzheimer’s Abeta. Faseb j. 2004;18(15):1870–1878. doi: 10.1096/fj.04-2572com. [DOI] [PubMed] [Google Scholar]
- 59.Grundman M, Corey-Bloom J, Jernigan T, Archibald S, Thal LJ. Low body weight in Alzheimer’s disease is associated with mesial temporal cortex atrophy. Neurology. 1996;46(6):1585–1591. doi: 10.1212/wnl.46.6.1585. [DOI] [PubMed] [Google Scholar]
- 60.Ehrlich S, Burghardt R, Weiss D, Salbach-Andrae H, Craciun EM, Goldhahn K, Klapp BF, Lehmkuhl U. Glial and neuronal damage markers in patients with anorexia nervosa. Journal of neural transmission (Vienna, Austria : 1996) 2008;115(6):921–927. doi: 10.1007/s00702-008-0033-8. [DOI] [PubMed] [Google Scholar]
- 61.Katzman DK, Lambe EK, Mikulis DJ, Ridgley JN, Goldbloom DS, Zipursky RB. Cerebral gray matter and white matter volume deficits in adolescent girls with anorexia nervosa. J Pediatr. 1996;129(6):794–803. doi: 10.1016/s0022-3476(96)70021-5. [DOI] [PubMed] [Google Scholar]
- 62.Lambe EK, Katzman DK, Mikulis DJ, Kennedy SH, Zipursky RB. Cerebral gray matter volume deficits after weight recovery from anorexia nervosa. Arch Gen Psychiatry. 1997;54(6):537–542. doi: 10.1001/archpsyc.1997.01830180055006. [DOI] [PubMed] [Google Scholar]
- 63.Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF., 3rd Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202(5):329–335. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fratiglioni L, Paillard-Borg S, Winblad B. An active and socially integrated lifestyle in late life might protect against dementia. The Lancet. Neurology. 2004;3(6):343–353. doi: 10.1016/S1474-4422(04)00767-7. [DOI] [PubMed] [Google Scholar]
- 65.Church TS, Thomas DM, Tudor-Locke C, Katzmarzyk PT, Earnest CP, Rodarte RQ, Martin CK, Blair SN, Bouchard C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS One. 2011;6(5):e19657. doi: 10.1371/journal.pone.0019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Wit LM, van Straten A, van Herten M, Penninx BW, Cuijpers P. Depression and body mass index, a u-shaped association. BMC Public Health. 2009;9:14. doi: 10.1186/1471-2458-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suemoto CK, Gilsanz P, Mayeda ER, Glymour MM. Body mass index and cognitive function: the potential for reverse causation. Int J Obes (Lond) 2015;39(9):1383–1389. doi: 10.1038/ijo.2015.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tolppanen AM, Ngandu T, Kareholt I, Laatikainen T, Rusanen M, Soininen H, Kivipelto M. Midlife and late-life body mass index and late-life dementia: results from a prospective population-based cohort. Journal of Alzheimer’s disease : JAD. 2014;38(1):201–209. doi: 10.3233/JAD-130698. [DOI] [PubMed] [Google Scholar]
- 69.Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. 2002;59(11):1737–1746. doi: 10.1001/archneur.59.11.1737. [DOI] [PubMed] [Google Scholar]


