Abstract
Background
Although dofetilide labeling states the drug must be initiated or re-initiated with continuous electrocardiographic monitoring and in the presence of trained personnel, the risks of dofetilide reloading justifying repeat hospitalization have not been investigated.
Methods and Results
Patients admitted for dofetilide reloading for atrial arrhythmias were retrospectively reviewed. The need for dose adjustment and incidence of torsades de pointes (TdP) were identified. The incidence of TdP in dofetilide reloading was compared with patients admitted for dofetilide initial loading. Of 138 patients admitted for dofetilide reloading for atrial arrhythmias, 102 were reloaded at a previously tolerated dose, 30 with a higher dose from a previously tolerated dose and 2 at a lower dose; prior dosage was unknown in 4 patients. Dose adjustment or discontinuation was required in 44 patients (31.9%). No TdP occurred in the same-dose reloading group, but TdP occurred in 2 patients admitted to increase dofetilide dosage (0 vs. 6.7%, p=0.050). Dofetilide dose adjustment or discontinuation was required in 30/102 patients (29.4%) reloaded at a previously tolerated dose and in 11/30 patients (36.7%) admitted for an increase in dose.
Conclusions
Although no TdP occurred in patients admitted to reload dofetilide at the same dose as previously tolerated, dosage adjustments or discontinuation were frequent and support the need for hospitalization for dofetilide reloading. Patients admitted for reloading with a higher dose tended to be at higher risk for TdP than patients reloaded at a prior tolerated dose.
Keywords: atrial fibrillation, torsade de pointes, hospitalization, antiarrhythmic drug, Dofetilide, rehospitalization, reloading
INTRODUCTION
Dofetilide is a Vaughan Williams Class III anti-arrhythmic agent which blocks the rapid component of the delayed rectifier potassium current.1 It has been used for atrial fibrillation and flutter to terminate the arrhythmia and to maintain sinus rhythm after pharmacologic or electrical conversion. Because of the risk of life-threatening torsades de pointes (TdP) associated with excessive QT prolongation, dofetilide was approved by the United States Food and Drug Administration (FDA) requiring a strict protocol of initiation and hospitalization under personnel specifically trained in dofetilide use. According to dofetilide labeling, therapy with dofetilide must be initiated or re-initiated "in a setting that provides continuous electrocardiographic (ECG) monitoring and in the presence of personnel trained in the management of serious ventricular arrhythmias." It is required that patients should be monitored in this way for a minimum of 3 days to monitor the corrected QT (QTc) interval, since TdP occurs most frequently within the first 3 days.2
The incidence of TdP during the initiation of dofetilide has been reported in several studies. The Danish Investigations of Arrhythmia and Mortality on Dofetilide in Congestive Heart Failure (DIAMOND-CHF) trial reported that 25 patients (3.3%) developed TdP out of 762 patients with left ventricular ejection fraction (LVEF) ≤ 35%.3 The Dofetilide in Myocardial Infarction (DIAMOND-MI) trial reported that TdP occurred in 7 of 749 (0.9%) patients who had recent myocardial infarction (within 7 days) and LVEF ≤ 35%.4 In the Symptomatic Atrial Fibrillation Investigative Research on Dofetilide (SAFIRE-D) study, 2 of 241 (0.8%) patients developed TdP during dofetilide initiation.5
In contrast to the initial loading of dofetilide, the risks of dofetilide reloading after discontinuation of a previously tolerated dose have not been previously investigated, although this need occurs commonly in clinical practice. We retrospectively reviewed medical records of patients who were hospitalized for the reloading of dofetilide for the treatment of atrial arrhythmias to assess the incidence of life-threatening arrhythmias and requirement for therapy modifications during hospitalization for re-initiation of dofetilide, aiming to identify potential subgroups which might not need re-hospitalization for re-initiation. We compared these results to those from initial hospitalizations for dofetilide initiation to determine if hospitalization for reloading of dofetilide is justified.
METHODS
Patient Selection
The cohort was established through a pharmacy query based on inpatient pharmacy dofetilide records for patients who were admitted at the Cleveland Clinic from 2008 to 2012. Patients were included in this study if they had been on dofetilide previously and were using dofetilide for treatment of atrial fibrillation or flutter. We retrospectively reviewed medical records of these patients to analyze the need for dose adjustment and the incidence of TdP. The cohort admitted for initial dofetilide loading has been reported previously and was used as a comparison group.6 The study was approved by The Cleveland Clinic Institutional Review Board for retrospective medical records review and performed in accordance with institutional guidelines.
Dofetilide Loading Protocol
Patients were admitted for a minimum of 3 days for dofetilide re-initiation with an estimate of creatinine clearance (CrCl) calculated based on the Cockcroft-Gault equation, continuous electrocardiographic monitoring, ECG QTc (Bazett formula) assessments 2 hours after each dose and dosage adjustments according to the FDA-approved labeling and prescribing information.2 For patients undergoing initial loading, initial dosing was determined according to the CrCl as per the dofetilide labeling. For patients undergoing reloading, we divided subjects into subgroups based upon whether dofetilide reloading dose was the same (Same Dose), higher (Increased Dose), or lower (Decreased Dose) than their prior dofetilide dose. Patients in whom the prior dose was unknown (Unknown previous dose) were also included. The dofetilide package insert recommends that dofetilide should not be started for baseline QTc > 440 ms (>500 ms with ventricular conduction abnormality). There were exceptions and variability in our clinical practice, however; for example in the setting of an ICD or with marked QRS widening, some clinicians estimate a correction for marked QRS widening by subtracting QRS duration over 100 ms from the QTc. Other medications that prolong QT intervals or interfere with dofetilide metabolism were stopped before dofetilide reloading. We monitor patients for 3 days (6 doses) with ECG QTc assessment 2 hours after each dose and dosage adjustment. QTc prolongation was defined according to the FDA-approved package insert. After the first dose, if QTc increases > 15 % or > 500 ms (> 550 ms with ventricular conduction abnormality), the second dose is reduced by 50%. After subsequent doses, dofetilide was generally stopped or reduced for QTc > 500 ms (> 550 ms with ventricular conduction abnormality). If the patient is in persistent atrial fibrillation and does not pharmacologically convert, we typically DC cardiovert on day 2 after the fourth dose and monitor overnight, discharging after the sixth dose. If DC cardioversion is unsuccessful, we may try another DC cardioversion after the sixth dose the following day, but would using monitor again overnight and discharge the following day.
Torsades de Pointes
Medical records were reviewed to identify the incidence of TdP. TdP was defined as more than 10 beats of polymorphic ventricular tachycardia with a twisting QRS axis and long QTc interval.
Statistical Analysis
SPSS V. 18 and 19 were used to perform the statistical analysis. QTc intervals were calculated using the Bazett formula. "Adjusted QTc" was defined as QTc minus the QRS duration over 100 ms. "Calculated JTc" interval was defined as the difference between QTc and QRS duration. Baseline patient characteristics are presented as numbers and percentages for categorical variables and means ± standard deviation (SD) for continuous variables. The Fisher’s exact test was used to compare the rate of TdP between groups and independent t tests to compare means. Results were considered significant at a p value of <0.05. Multivariable logistic regression modeling was performed to identify characteristics predictive of QTc prolongation and/or developing TdP. Candidate variables considered for inclusion in the model were limited to baseline characteristics using p<0.05 for selection and stay criteria.
RESULTS
Between 2008 and 2012, a total of 1552 patients received dofetilide during hospitalization. Of these, 138 patients were identified to have been on dofetilide previously, and were admitted to re-initiate dofetilide at a previously tolerated dose or to increase/decrease the dose for atrial arrhythmias. A total of 102 patients were reloaded with the same previously tolerated dose (Same Dose group) and 30 patients with an increased dose (Increased Dose group), typically based on a clinical assessment that there was adequate QTc margin on a lower dose to support an increase in dosage. There were 2 patients who were in the Decreased dose group and 4 patients in the Unknown previous dose group. The cohort undergoing initial dofetilide loading has been reported separately and consisted of 1404 patients.6
Baseline Patient Characteristics
The baseline characteristics of the 138 patients previously on dofetilide are summarized in Table 1. In the total cohort mean age was 64.6 ± 11.7 years, 72.5% were males, 84.8% had hypertension, 21.7% had diabetes mellitus, 14.5% had chronic kidney disease, 26.8% had coronary artery disease, 12.3% had an implantable cardioverter defibrillator, mean left ventricular ejection fraction was 48.9%, mean CrCl was 107.4 ± 40.4 ml/min, and mean QTc prior to re-loading was 452.9 ± 36.2 ms. Compared with the Same dose group, patients in the Increased dose group were older and had a longer QTc prior to reloading (464.1 ± 39.9 vs 449.0 ± 32.9 ms, p=0.038). The longest baseline QTc interval was 558 ms in a patient with a QRS duration of 178 ms and an ICD; dofetilide was stopped in this patient due to QTc prolongation related to acute kidney injury.
Table 1.
Baseline patient characteristics.
| All patients admitted for dofetilide reloading N=138 (%) |
Reloading at same dose N = 102 (%) |
Reloading at higher dose N= 30 (%) |
Same dose vs. higher dose reloading P Value |
|
|---|---|---|---|---|
| Age, years | 64.6 ± 11.7 | 62.5 ± 11.4 | 70.5 ± 10.9 | 0.001 |
| Male sex | 100 (72.5) | 75 (73.5) | 21 (70.0) | 0.70 |
| Hypertension | 117 (84.8) | 85 (83.3) | 26 (86.7) | 0.78 |
| Diabetes mellitus | 30 (21.7) | 24 (23.5) | 4 (13.3) | 0.31 |
| Hyperlipidemia | 77 (55.8) | 61 (59.8) | 14 (46.7) | 0.20 |
| Chronic kidney disease | 20 (14.5) | 15 (14.7) | 4 (13.3) | 1.00 |
| Coronary artery disease | 37 (26.8) | 25 (24.5) | 10 (33.3) | 0.34 |
| Congestive heart failure | 66 (47.8) | 51 (50.0) | 13 (43.3) | 0.52 |
| Left ventricular ejection fraction, % | 48.9 ± 12.1 | 48.3 ± 12.5 | 49.3 ± 11.7 | 0.71 |
| Creatinine clearance, ml/min | 107.4 ± 40.4 | 111.3 ± 39.6 | 97.9 ± 41.3 | 0.11 |
| Pacemaker | 21 (15.2) | 15 (14.7) | 4 (13.3) | 1.00 |
| Implantable cardioverter defibrillator | 17 (12.3) | 13 (12.7) | 4 (13.3) | 1.00 |
| ECG intervals before reloading, ms | ||||
| QRS | 105.7 ± 25.4 | 104.1 ± 22.7 | 112.8 ± 32.6 | 0.18 |
| QT | 398.4 ± 51.9 | 395.8 ± 45.0 | 408.0 ± 67.0 | 0.36 |
| QTc | 452.9 ± 36.2 | 450.8 ± 35.1 | 460.9 ± 41.1 | 0.19 |
| Adjusted QTc | 441.4 ±32.1 | 441.1 ±32.4 | 442.7 ± 34.1 | 0.82 |
| Calculated JTc | 347.2 ± 32.7 | 346.7 ± 32.8 | 348.1 ± 35.5 | 0.84 |
Of the 138 patients admitted for dofetilide reloading, we were able to estimate the last date of prior dofetilide intake in 75 patients (Table 2). The average number of days off dofetilide was approximately 660 ± 701 days (median 114 days). The most common reasons for discontinuation were ineffectiveness, successful cardioversion, stopped by another provider and QTc prolongation.
Table 2.
Reasons for previous dofetilide discontinuation
| Number (%) | |
|---|---|
| Total patients | 138 (100 %) |
| Patients with known discontinuation data | 75 (54.3 %) |
| Average days of dofetilide discontinuation | 660 days |
| Reasons for previous dofetilide discontinuation* | |
| Ineffectiveness | 50 (66.7 %) |
| Successful cardioversion | 24 (32.0 %) |
| Another provider discontinued | 13 (17.3 %) |
| QTc prolongation | 10 (13.3 %) |
| System error/Forgotten by provider | 5 (6.7 %) |
| Financial burden on patient | 5 (6.7 %) |
| Presence of infection | 4 (5.3 %) |
| Pulmonary vein isolation | 3 (4.0 %) |
| Initial loading failed | 2 (2.7 %) |
| Acute kidney injury | 2 (2.7 %) |
| Need for surgery | 1 (1.3 %) |
| Left atrial appendage clot | 1 (1.3 %) |
| Failed absorption | 1 (1.3 %) |
| Bradycardia | 1 (1.3 %) |
| Patient compliance concerns | 1 (1.3 %) |
Patients may have more than one reason for discontinuation.
Dofetilide Dosage
Dofetilide re-initiation is summarized in Figure 1. A total of 138 patients were admitted for dofetilide re-initiation for atrial arrhythmias from 2008 to 2012 at the Cleveland Clinic. Based on CrCl dosage recommendations, 82 (59.4%) were re-loaded on CrCl predicted recommended dosages, 52 (37.7%) on lower than CrCl predicted dosages, and 4 (2.9%) on higher than CrCl predicted dosages. Of the 4 patients reloaded with a dose above the predicted dose by CrCl recommendations, 1 was in the Same Dose group and 3 were in the Increased Dose group. All 4 were discharged without need for modification of dosage.
Figure 1. Dofetilide reloading summary.
Same Dose Reloading
A total of 102 patients were reloaded with the same previously tolerated dose. Eight patients were started with dofetilide 125 mcg bid, 30 patients with 250 mcg bid, 2 patients with 375 mcg bid and 62 patients with 500 mcg bid. During the reloading, dofetilide was discontinued in 7 patients (6.9%), including 4 patients due to ineffectiveness and 3 patients due to excessive QTc prolongation. In the first patient, a 55-year-old male, stopped for QTc prolongation, CrCl was 116.4 ml/min, but dofetilide 250 mcg bid was associated with marked increases in QTc from 480 to >600 ms; dofetilide was reduced to 125 mcg bid, but QTc remained excessively prolonged, and the drug was stopped. In the second patient, a 79-year-old male who had right bundle branch block, CrCl was 72.8 ml/min and QTc increased from 495 to 519 ms on dofetilide 250 mcg bid; the drug was stopped due to QTc prolongation associated with increased premature ventricular complexes and an increase in creatinine due to contrast loads and ICD infection. The third patient was a 39-year-old male with CrCl 161 ml/min begun on 500 mcg bid, but with QTc increasing from 460 to 504 and remaining prolonged on 250 mcg bid with a 3-beat run of non-sustained ventricular tachycardia, leading to dofetilide discontinuation and a change to flecainide. Out of the remaining 95 patients, 68 patients (66.7%) were initiated on the same previously tolerated dose, but required dose reduction in 27 patients (26.5%) due to QTc prolongation. TdP was not observed. In total, 30 of 102 patients (29.4%) needed dose adjustment or discontinuation.
Increased Dose Reloading
A total of 30 patients were admitted to increase their dose of dofetilide. One patient was increased to 125 mcg bid from 125 mcg qd, 5 patients with 250 mcg bid from 125 mcg bid, 1 patient with 500 mcg bid from 125 mcg bid, 10 patients with 375 mcg bid from 250 mcg bid, 11 patients with 500 mcg bid from 250 mcg bid and 2 patients with 500 mcg bid from 375 mcg bid. Two patients (6.7%) had TdP. One patient had TdP with shortness of breath and spontaneous termination to sinus rhythm without intervention. This male patient had been increased to 375 mcg bid from 250 mcg bid for persistent AF. The QTc before reloading was 436–484 ms in atrial fibrillation and CrCl was 82.2 ml/min. The AF spontaneously converted to sinus rhythm overnight after the third dose of dofetilide, but the QTc in AF prior to conversion 2 hours after the third dose had increased to 530 ms, and after conversion QTc was 634 ms. He had non-sustained TdP, and the dofetilide dose was reduced back to 250 mcg bid with QTc on discharge of 462 ms. A second patient who had TdP was female and had a cardiac arrest requiring defibrillation. Dofetilide dosage had been increased to 500 mcg bid from a previously tolerated dose of 250 mcg bid with a QTc before reloading of 389 ms and CrCl of 104 ml/min. The patient was in persistent AF and underwent DC cardioversion during the re-load. QTc that evening 2 hours after her fifth dose was 445 ms in sinus rhythm. However, the next morning she began having premature ventricular complexes, an ECG (prior to dosing) showed the QTc had increased to 572 ms, and she shortly thereafter arrested with TdP, requiring chest compressions and defibrillation. She recovered, and dofetilide was stopped. Three patients (10.0%) discontinued dofetilide due to QTc prolongation in 1 patient, acute kidney injury with QTc prolongation in 1 patient, and the episode of TdP with cardiac arrest requiring defibrillation in 1 patient. The patient who had QTc prolongation had a CrCl of 54.5 ml/min, but was increased from 125 to 250 mcg bid, the CrCl predicted recommended dose. QTc increased from 446 to 511 ms, and it was elected to stop rather than reduce the dosage. Out of 27 patients (90.0%) who were discharged on dofetilide, 19 patients (63.3%) did not need any dose adjustment, and 8 patients (26.6%) needed the dose to be decreased due to QTc prolongation or TdP. In total, 11 of 30 patients (36.7%) required dose adjustment or discontinuation in this group.
Decreased Dose Reloading
Two patients were re-initiated with a lower dose. One patient was re-initiated with 125 mcg bid from 250 mcg bid and the other patient with 250 mcg bid from 500 mcg bid. The CrCl of both of these patients were lower than when they were previously on dofetilide (27.2 and 52.6 ml/min, respectively). These patients did not require any dose adjustment and were discharged in stable condition.
Unknown Previous Dose Reloading
A total of 4 patients were re-initiated with 500 mcg bid but their previous dose was unknown. One patient (25%) did not require any dose adjustment, but 3 patients (75%) required dose reduction due to QTc prolongation.
Predictors of QTc prolongation or TdP
In the total cohort 44 (31.9%) had QTc prolongation or torsades de pointes requiring dose modification or discontinuation of drug. The only baseline characteristics associated with QTc prolongation or TdP (Table 3) were longer adjusted QTc (451.8 ± 30.7 vs. 436.6 ±31.8 ms, p=0.009) and calculated JTc (359.5 ± 30.3 vs. 341.5 ± 32.4 ms, p=0.002) with trends toward higher risk in patients with hypertension (p=0.076) or diabetes (p=0.05). Group assignment by dose change was not significantly associated with QTc prolongation or TdP. Only up titration of dose was significantly associated with the 2 occurrences of torsades de pointes, both instances of which occurred in the Increased dose group (2/30 [6.7%] TdP in the Increased Dose group vs 0/104 [0%] in the Same Dose or Decreased Dose groups, p=0.049). Multivariable regression analyses (Table 4) identified longer pre-reload JTc (odds ratio 1.057, 95% confidence interval 1.008, 1.109, p=0.023) and diabetes mellitus (odds ratio 2.626, 95% confidence interval 1.093, 6.313, p=0.031) as significant independent predictors of QTc prolongation or TdP. In the Same Dose group, the significant baseline characteristics associated with QTc prolongation or TdP requiring dose change were pre-reload shorter QRS duration (96.9 ± 21.3 vs. 107.1±22.8 ms, p=0.039, R2 0.118), longer adjusted QTc (455.6 ± 31.6 vs. 435.1 ± 30.9 ms, p = 0.003) and longer calculated JTc (364.0 ± 30.4 vs. 339.4 ± 31.1 ms, p<0.001). In this subgroup multivariable regression analyses identified longer pre-reload JTc (odds ratio 1.104, 95% confidence interval 1.009, 1.207, p=0.032) as the only significant independent predictor of QTc prolongation or TdP (Table 4).
Table 3.
Baseline characteristics by QTc prolongation or TdP requiring dose change.
| Total Cohort | Same dose reloading | |||||
|---|---|---|---|---|---|---|
| QTc prolongation or TdP | P Value | QTc prolongation or TdP | P Value | |||
| No N=94 (68.1%) |
Yes N=44 (31.9%) |
No N=72 (70.6%) |
Yes N=30 (29.4%) |
|||
| Age, years | 64.8 ± 11.9 | 64.2 ± 11.3 | 0.77 | 62.4 ± 11.5 | 62.5 ± 11.4 | 0.96 |
| Male sex | 70 (74.5) | 30 (68.2) | 0.44 | 54 (75.0) | 21 (70.0) | 0.60 |
| Hypertension | 76 (80.9) | 41 (93.2) | 0.076 | 57 (79.2) | 28 (93.3) | 0.14 |
| Diabetes mellitus | 16 (17.0) | 14 (31.8) | 0.05 | 15 (20.8) | 9 (30.0) | 0.32 |
| Hyperlipidemia | 52 (55.3) | 25 (56.8) | 0.87 | 42 (58.3) | 19 (63.3) | 0.64 |
| Chronic kidney disease | 13 (13.8) | 7 (15.9) | 0.75 | 12 (16.7) | 3 (10.0) | 0.54 |
| Coronary artery disease | 24 (25.5) | 13 (29.5) | 0.62 | 19 (26.4) | 6 (20.0) | 0.49 |
| Congestive heart failure | 48 (51.1) | 18 (40.9) | 0.27 | 39 (54.2) | 12 (40.0) | 0.19 |
| Left ventricular ejection fraction, % | 48.4 ± 12.2 | 50.1 ± 12.2 | 0.48 | 47.8 ± 12.5 | 49.7 ± 12.6 | 0.51 |
| Creatinine clearance, ml/min | 108.2 ± 42.5 | 105.8 ± 36.0 | 0.75 | 112.8 ± 40.3 | 107.5 ± 38.1 | 0.54 |
| Pacemaker | 17 (18.1) | 4 (9.1) | 0.21 | 12 (16.7) | 3 (10.0) | 0.54 |
| Implantable cardioverter defibrillator | 13 (13.8) | 4 (9.1) | 0.58 | 9 (12.5) | 4 (13.3) | 1.00 |
| Dose higher than recommended by CrCl | 4 (4.3%) | 0 (0%) | 0.31 | 1 (1.4%) | 0 (0%) | 1.00 |
| Dofetilide dose increase | 19 (20.4) | 11 (26.8) | 0.41 | Not applicable | Not applicable | Not applicable |
| ECG Intervals before Reload, ms | ||||||
| QRS duration | 108.1 ± 25.9 | 100.6 ± 23.8 | 0.11 | 107.1 ± 22.8 | 96.9 ± 21.3 | 0.039 |
| QT | 396.6 ± 51.6 | 402.4 ± 53.0 | 0.54 | 394.9 ± 43.6 | 398.0 ± 48.9 | 0.75 |
| QTc | 449.6 ± 37.4 | 460.1 ± 32.8 | 0.11 | 446.5 ± 36.1 | 461.0 ± 30.8 | 0.058 |
| Adjusted QTc | 436.6 ± 31.8 | 451.8 ± 30.7 | 0.009 | 435.1 ± 30.9 | 455.6 ± 31.6 | 0.003 |
| Calculated JTc | 341.5 ± 32.4 | 359.5 ± 30.3 | 0.002 | 339.4 ± 31.1 | 364.0 ± 30.4 | <0.001 |
Table 4.
Multivariable predictors of QTc prolongation or TdP requiring dose change.
| Odds ratio | 95% confidence interval |
p value | |
|---|---|---|---|
| Total Cohort | |||
| JTc, ms | 1.057 | 1.008, 1.109 | 0.023 |
| Adjusted QTc | 0.963 | 0.918, 1.010 | 0.120 |
| Diabetes mellitus | 2.626 | 1.093, 6.313 | 0.031 |
| Same Dose Cohort | |||
| JTc | 1.104 | 1.009, 1.207 | 0.032 |
| QRS duration, ms | 1.001 | 0.970, 1.032 | 0.966 |
| Adjusted QTc | 0.930 | 0.853, 1.013 | 0.097 |
In an attempt to identify a truly low-risk population, we examined characteristics associated with QTc prolongation and TdP leading to dose reductions or discontinuation, but the small sample size did not allow for a robust assessment. In particular we examined baseline QTc and JTc intervals. Among the Same Dose reloading group who developed QTc prolongation requiring dose modification, the minimum baseline QTc interval was 409 ms and the minimum JTc interval 309 ms. Of 102 patients in the Same Dose reloading group, 9 (8.8%) had QTc <409 ms and 13 (12.7%) had JTc <309 ms. In the total cohort, among the patients who developed TdP or QTc prolongation requiring dose modification, the shortest baseline QTc was 398 ms in one of the patients who developed TdP and the shortest JTc was 288 ms. There did not appear to be a useful QTc or JTc cutoff in which no patient developed excessive QTc prolongation or TdP, as the shortest baseline QTc and JTc in the whole cohort was 365 ms and 269, respectively, and only 5 subjects (3.6%) had baseline QTc <398 ms and 5(3.6%) had JTc <288 ms.
Comparison Between Groups and with Initial Loading
Comparisons between groups and with previously published studies are shown in Table 5. Comparing the incidence of TdP in Same Dose vs. Increased Dose reloading groups, there was a strong trend toward higher incidence in the Increased Dose group (0 vs. 6.7%, p=0.050). Through the same inpatient pharmacy dofetilide discharge query, a total of 1404 patients were identified to be admitted for dofetilide initial loading. The incidence of TdP was previously reported to be 17 of 1404 patients (1.2%).6 The rate of TdP in Same Dose dofetilide reload patients was not statistically different from the incidence observed during dofetilide initial loading (1.2%), p=0.62. However, there was again a trend toward higher incidence in the Increased Dose reload group (p=0.058). Compared to prior studies, DIAMOND-CHF3 reported a TdP rate of 3.3%, which was not significantly different from the higher dose reload group, but tended to be higher compared to the Same Dose reload group (p=0.062). There were no significant differences in the Same Dose or Increased Dose reload groups compared to the DIAMOND-AF group,7 but a trend toward higher TdP in the Increased Dose reload group when compared to SAFIRE-D group,5 which had a TdP incidence of 0.8% (p=0.062), and a significantly higher incidence compared to DIAMOND-MI patients,4 who had a TdP incidence of 0.9% (p=0.044).
Table 5.
Comparison between dofetilide reloading groups and previously published studies.
| Studies | Incidence of TdP (%) | Comparison to Same dose reloading P Value |
Comparison to Higher dose reloading P Value |
|---|---|---|---|
| Dofetilide same-dose reloading | 0/102 (0%) | -- | 0.050 |
| Dofetilide higher-dose reloading | 2/30 (6.7%) | 0.050 | -- |
| Cleveland Clinic dofetilide initial loading6 | 17/1404 (1.2%) | 0.62 | 0.058 |
| DIAMOND-CHF3 | 25/762 (3.3%) | 0.062 | 0.27 |
| DIAMOND-MI4 | 7/749 (0.9%) | 1.0 | 0.044 |
| DIAMOND-AF7 | 4/249 (1.6%) | 0.33 | 0.13 |
| SAFIRE-D5 | 2/241 (0.8%) | 1.0 | 0.062 |
P values are from Fisher's exact tests.
DISCUSSION
Although the FDA recommends hospitalization for initiation or re-initiation of dofetilide, the evidence justifying the need for hospitalization for dofetilide reloading has been lacking. We performed a retrospective medical record review of 138 patients who were admitted for dofetilide reloading for atrial arrhythmias. Of these 31.9% had QTc prolongation or torsades de pointes requiring dose modification or discontinuation of drug. While there was no incidence of TdP among patients admitted to reload the same previously tolerated dofetilide dose, dofetilide dose adjustment or discontinuation rate was required in 29.4%. In contrast, the smaller number of subjects admitted to reload dofetilide at a higher dose experienced a higher rate of TdP (6.7%), which showed a strong trend toward being significantly higher than in the same-dose reloading group (p=0.050) and in several previously reported studies, reaching statistical significance when compared to the DIAMOND-MI study.3–7 Our study strengthens the evidence that hospitalization for dofetilide reloading is necessary and disproves the misconception that dofetilide can be re-initiated as an outpatient for patients who are being re-started on a previously well tolerated dose
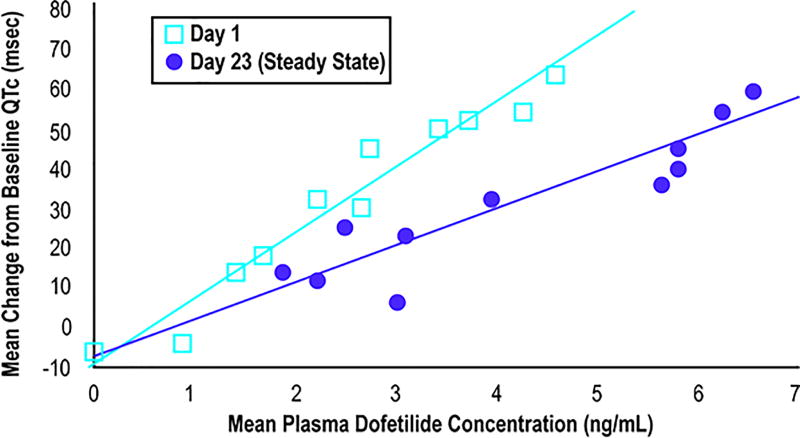
Although no TdP occurred in the group admitted to reload with the same dose previously tolerated, the requirement for dosage adjustments in 29.4% of patients supports the need for hospitalization to monitor QTc and proarrhythmia. The need for admission for re-initiation despite use of the same previous dose has been rationalized based on the initial loading pharmacokinetics of dofetilide. At a given plasma dofetilide concentration, the mean QTc change from baseline is reported to be higher on day 1 compared to day 23 steady state levels (Figure 2). We can speculate that the patients will have less prolonged QTc 3 weeks later due to the attenuated QTc response to dofetilide. However, the higher QTc response with initial loading may explain why dose adjustments were required even in patients admitted to reload at a dose that had been tolerated previously, and perhaps why there was such a significant rate of TdP in the higher dose reload subjects. Also of note, both cases of TdP occurred after electrical or pharmacologic conversion from persistent AF, supporting the need to monitor patients at least overnight after spontaneous or electrical conversion from AF to sinus rhythm.
Figure 2. Dofetilide pharmacokinetics on day 1 and day 23.
Modified from TIKOSYN® (dofetilide) Capsules package insert. Figure used courtesy of Pfizer, Inc.
Whether patients who miss only one or two doses of dofetilide need to be hospitalized cannot be extrapolated from these results. It is recommended that patients missing more than 2 doses should be readmitted to reload. The elimination half-life of dofetilide is approximately 8 to 10 hours.8 Theoretically, after missing 4–5 doses or 2 days, re-initiation of dofetilide might be viewed as reloading. It appears prudent to urge caution and re-admission or cessation of dofetilide use altogether for patients who are non-compliant with consistent use of dofetilide.
The relatively high (29.4%) rate of dosage adjustment in the Same Dose reloading group might also have reflected a different clinical state of the patient after longer periods of being off the drug. We cannot exclude that differences in renal function or other intervening clinical events, such as surgical procedures or hospitalizations, might have increased the risk of reloading in our cohort. However, all except 4 patients were reloaded with doses that were at or lower than the predicted dose by creatinine clearance recommendations. Thus, baseline CrCl was not a predictor of excessive QTc prolongation. The only significant predictors of QTc prolongation requiring dose changes in this group was baseline pre-reloading JTc. However, only 13 patients in the Same Dose re-loading group had JTc <309 ms, the lowest JTc that was associated with QTc prolongation, and only 5 patients in the total cohort had JTc <288 ms, the lowest JTc associated with any QTc prolongation or TdP requiring dose reduction. Thus, as QTc and JTc lacked adequate discrimination between patients who did and did not require dose adjustments for QTc prolongation, hospital admission for reloading even at a same dose as previously tolerated seems prudent.
Limitations
Our study has several limitations. First, the relatively small number of Same Dose reloads (102 patients) has limited power to detect significant differences between reloading and initiation patients. Nevertheless, the dofetilide admissions at our institution represent one of the largest post-marketing clinical cohorts, and the absolute yield of hospitalization with over a quarter to a third of patients requiring dosage adjustments has served to reaffirm our need to continue re-admitting for reloading. Secondly, this was a retrospective study. Due to the safety concerns, we were unable to design a prospective study randomizing inpatient vs. outpatient reloading. Moreover, such a study would need an impractically large sample size to detect a difference in TdP, and use of dose adjustment as an endpoint might be at least as inherently observationally biased as a retrospective study. We were also unable to derive conclusions within the Decreased dose or Unknown dose group due to low sample size.
CONCLUSIONS
Although TdP did not occur in patients admitted to reload dofetilide at the same dose as previously tolerated, requirements for downward dosage adjustments or discontinuation were common. Patients admitted for reloading with a higher dose tended to be at higher risk for TdP than patients reloaded at a prior tolerated dose or patients admitted for initial loading. Study results did not identify a low risk cohort in which admission for dofetilide reloading could be avoided. These results support the need for hospitalization for dofetilide reloading, not only for higher dose titration but for patients re-initiating dofetilide at doses previously tolerated.
Supplementary Material
What is Known
Initiation of dofetilide for treatment of atrial arrhythmias requires hospitalization to adjust the dose in order to reduce the risk of torsades de pointes (TdP).
If dofetilide is stopped or missed, re-admission for dofetilide reloading is recommended, although there have been no clinical studies justifying this recommendation.
What the Study Adds
TdP occurred in 6.7% of patients hospitalized for reloading with a higher dose of dofetilide, compared to 0% in patients admitted to reload at the same dose previously tolerated (p=0.05), and dosage adjustments were required in 36.7%, justifying the need for repeat hospitalization in patients admitted to increase their dose of dofetilide.
Although no TdP occurred in patients admitted to reload dofetilide at the same dose previously tolerated, the need for dosage adjustment for excessive QTc prolongation was frequent (29.4%), supporting the need for re-hospitalization.
Longer baseline JTc was associated with a higher risk of excessive QTc prolongation, but baseline JTc and QTc lacked adequate discrimination. As a low risk population could not be identified, hospital admission for reloading even at a same dose as previously tolerated seems prudent.
Acknowledgments
SOURCES OF FUNDING: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. MKC is supported by National Institutes of Health grant R01 HL111314.
Footnotes
DISCLOSURES: None
References
- 1.Tande PM, Bjornstad H, Yang T, Refsum H. Rate-dependent class III antiarrhythmic action, negative chronotropy, and positive inotropy of a novel Ik blocking drug, UK-68,798: potent in guinea pig but no effect in rat myocardium. J Cardiovasc Pharmacol. 1990;16:401–10. doi: 10.1097/00005344-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 2.TIKOSYN® (dofetilide) Capsules package insert. Pfizer, Inc.; [Google Scholar]
- 3.Torp-Pedersen C, Moller M, Bloch-Thomsen PE, Kober L, Sandoe E, Egstrup K, Agner E, Carlsen J, Videbaek J, Marchant B, Camm AJ. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999;341:857–65. doi: 10.1056/NEJM199909163411201. [DOI] [PubMed] [Google Scholar]
- 4.Kober L, Bloch Thomsen PE, Moller M, Torp-Pedersen C, Carlsen J, Sandoe E, Egstrup K, Agner E, Videbaek J, Marchant B, Camm AJ Danish Investigations of A and Mortality on Dofetilide Study G. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: a randomised trial. Lancet. 2000;356:2052–8. doi: 10.1016/s0140-6736(00)03402-4. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Zoble RG, Yellen L, Brodsky MA, Feld GK, Berk M, Billing CB., Jr Efficacy and safety of oral dofetilide in converting to and maintaining sinus rhythm in patients with chronic atrial fibrillation or atrial flutter: the symptomatic atrial fibrillation investigative research on dofetilide (SAFIRE-D) study. Circulation. 2000;102:2385–90. doi: 10.1161/01.cir.102.19.2385. [DOI] [PubMed] [Google Scholar]
- 6.Abraham JM, Saliba WI, Vekstein C, Lawrence D, Bhargava M, Bassiouny M, Janiszewski D, Lindsay B, Militello M, Nissen SE, Poe S, Tanaka-Esposito C, Wolski K, Wilkoff BL. Safety of Oral Dofetilide for Rhythm Control of Atrial Fibrillation and Atrial Flutter. Circ Arrhythm Electrophysiol. 2015;8:772–6. doi: 10.1161/CIRCEP.114.002339. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen OD, Bagger H, Keller N, Marchant B, Kober L, Torp-Pedersen C. Efficacy of dofetilide in the treatment of atrial fibrillation-flutter in patients with reduced left ventricular function: a Danish investigations of arrhythmia and mortality on dofetilide (diamond) substudy. Circulation. 2001;104:292–6. doi: 10.1161/01.cir.104.3.292. [DOI] [PubMed] [Google Scholar]
- 8.Mounsey JP, DiMarco JP. Cardiovascular drugs. Dofetilide. Circulation. 2000;102:2665–70. doi: 10.1161/01.cir.102.21.2665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.