Abstract
Meibomian gland dysfunction (MGD) is an umbrella term that encompasses several meibomian gland disorders, ranging from congenital to acquired. Disruption of meibomian gland function negatively impacts both the quality and quantity of meibum secreted, which in turn affects ocular surface health through changes in tear film composition. Increased tear evaporation, hyperosmolarity, inflammation, and ocular surface damage can subsequently occur. This may cause discomfort, visual disruption, and sensation of dry eye. This review article discusses the pathology, causes, and ocular surface impact of MGD, as well as its relationship to dry eye.
Introduction
The meibomian gland is a type of sebaceous gland with tubulo-acinar structure and holocrine function, located in the superior and inferior tarsal plates.1 Meibomian glands secrete meibum, a compound made up of polar lipids (phospholipids) and nonpolar lipids (cholesterol, wax esters, cholesterol esters).2 Meibum is delivered to the ocular surface where it coats the aqueous layer and provides tear film stability and protects against microbial agents and organic matter. Meibomian gland dysfunction (MGD) is a term used to describe a group of disorders, both congenital and acquired, linked by functional abnormalities of the meibomian glands. MGD can lead to altered tear film composition, ocular surface disease, ocular and eyelid discomfort, and evaporative dry eye.
Pathophysiology of MGD
MGD is traditionally classified by the rate of gland secretion.1 Low delivery states are defined as those with meibomian gland hyposecretion or obstruction (either cicatricial or non-cicatricial), whereas high delivery states are defined as those with meibomian gland hypersecretion. All of these entities are further split into primary and secondary causes; for example, mucus membrane pemphigoid is a secondary cause of obstructive, cicatricial MGD, and seborrheic dermatitis and acne rosacea are secondary causes of both obstructive non-cicatricial and hypersecretory MGD.1
Within these categories, the most common mechanism for MGD is a low delivery state characterized by gland obstruction. The underlying pathophysiology is reported to be epithelial hyperkeratinization which leads to duct obstruction, meibum stasis, cystic dilation, and eventual disuse acinar atrophy and gland dropout.3–5 Newer studies have added to this paradigm and describe meibocyte abnormalities as a contributing mechanism in MGD.6,7 Support for the role of the meibocyte in MGD comes from pathophysiologic studies evaluating intrinsic (e.g. aging) and extrinsic (e.g. environmental stress) MGD risk factors on meibocyte differentiation and renewal.
Aging
Aging is a known risk factor for MGD.8 With age, meibomian gland acinar epithelial cells atrophy, exhibiting decreased lipid production9 and altered meibum composition with changes in neutral and polar lipid profiles.10 Likely underlying these changes, aged meibomian glands exhibit decreased meibocyte differentiation, decreased meibocyte cell renewal, decreased meibomian gland size, and increased inflammatory cell infiltration, as studied in human and mouse models.6,11,12 These changes have been associated with decreased expression of peroxisome proliferator-activated receptor gamma (PPARγ). PPARγ is a nuclear receptor protein involved in regulating meibocyte differentiation and lipid biosynthesis, contributing to the formation and function of meibomian glands.13 Down regulation of PPARγ is thought to underlie the decreased meibocyte differentiation and lipid synthesis seen in aging, leading to gland atrophy and a hyposecretory state.6,11 Interestingly, hyperkeratinization and gland obstruction were not found to play a role in the development of MGD in this mouse model.6
Environmental Stress
Environmental stress also contributes to MGD.1,14 Specifically, desiccating stress delivered by low humidity in a mouse model resulted in several meibocyte associated abnormalities including a 3-fold increase in basal acinar cell proliferation, altered meibum protein/lipid ratio, irregular meibocyte differentiation and meibocyte stem cell depletion.6 These are likely interconnected; in normal conditions, protein to lipid ratios decrease as meibum travels from the acini to the central duct.6 However, this decrease was not observed in mice subjected to desiccating stress, suggesting that the increased cellular proliferation and turnover led to altered meibocyte function and the inability to remove protein from meibum.6 In the short term, increased proliferation can result in increased meibum production, with ductal dilation a possible consequence of this phase. In the long term, depletion in the number of functioning meibocytes can occur, with subsequent gland atrophy and hyposecretion.6 Additionally, a higher protein/lipid ratio increases the viscosity of meibum with a negative impact on tear film stability.15,16
Stem Cell Renewal
Taken together, these findings suggest that aging and environmental stress eventually lead to the depletion of meibocyte stem cells. Exhaustion of stem cells can then lead to the loss of acinar meibocytes and meibomian gland dropout seen in MGD. The location and description of meibomian gland stem cells has been debatable; some studies place their location along the central duct while others at the interface between the ductal and acinar basal cells.17,18 A recent paper examining several mouse models supported the latter location in the interface between ductal and acinar basal cells.6 Furthermore, different stem cell origins were found for different meibomian gland components; acini originated from a single stem cell while ducts originated from progenitor cells of various origins.
Interestingly, a similar correlate has been described in the cornea with aging and stress. Corneal epithelial thickness is in part a measure of the number and regenerative capabilities of limbal epithelial stem cells.19 Over the course of a lifetime, the eye undergoes many periods of stress, during which time stem cells proliferate and re-establish normal homeostasis. Similar to meibomian glands, with increasing age, the number and proliferative capacity of corneal epithelial stem cells diminishes, leading to an observed decreased epithelial thickness.20
Risk factors for MGD and their potential effect on meibocytes
With the evolving paradigm that meibocyte dysfunction underlies MGD, it becomes apparent that other factors previously associated with MGD can also affect meibocytes including hormones, systemic and topical medications, nutrition, and the ocular microbiome. Meibomian glands can also be altered through external factors such as contact lens wear and be decreased, absent, or replaced in a number of congenital disorders.
Hormonal aspects
Androgen and estrogen receptors are present within meibomian glands, and meibocytes contain the enzymes necessary for the intracrine synthesis and metabolism of sex steroids.9,21 Broadly, androgens stimulate meibum secretion and suppress inflammation, while estrogens increase inflammation.22 However, androgens regulate the expression of thousands of genes in human meibomian glands, including pathways involved in lipid dynamics and PPAR signaling.23 Clinically, MGD has been described in many androgen-depleted states including individuals on anti-androgen agents (treatment of benign prostatic hypertrophy, prostate cancer), individuals with complete androgen insensitivity syndrome, and Sjogren’s syndrome.9 In all these conditions, alterations in meibomian gland secretion and lipid profiles were observed.24–26
Systemic Medications
Administration of 13-cis-retinoid acid (Accutane, Roche Pharmaceuticals, Switzerland) is associated with severe atrophy of the meibomian glands.27 In hamster models, retinoids caused decreased acinar tissue, thickened ductal epithelium, and reduced mature lipid-laden acinar cells.28 Retinoic acid binds to nuclear receptors, causing changes in gene transcription, which in meibomian glands decreases the volume of acinar tissue and inhibits maturation of lipid-laden meibomian acinar cells.28,29 Clinically, these findings have been associated with meibum hyposecretion, effecting evaporation and tear osmolality, leading to dry eye symptoms.29
Topical Medications
Several topical medications have been found to alter meibomian gland function. The use of topical epinephrine caused hyperkeritinization of the duct epithelium, leading to meibomian gland plugging and dilation.30 Glaucoma medications (e.g. topical beta blockers, prostaglandin analogs, carbonic anhydrase inhibitors) are associated with changes in meibomian gland morphology, including decreased acinar area, acinar density, and homogenous acinar wall morphology.31
Dietary Intake
The use of oral fatty acids has been reported to improve dry eye symptom and signs, as well as the expressibility and quality of meibum in MGD.32,33 Specifically, the intake of omega-3 fatty acids is associated with alterations in the polar lipid profile and decreases in the saturated fatty acid content of meibomian gland secretions.34 Moreover, supplementation with omegas decreases ocular surface inflammation (as measured by HLA-DR) in patients with dry eye,35 and decreases inflammatory lipid mediator profile in tears.36 Examples of foods potentially rich in omega-3 fatty acids include flaxseed oil, fish oil, and olive oil.34 Moreover, as down regulation of the PPAR pathway is seen in MGD as described above, therapeutically targeting this pathway through agonists such as pharmaceuticals (thiazolidinediones: pioglitazone, troglitazone, and rosiglitazone) or dietary supplements (conjugated linoleic acid [vegetables, fruits, nuts, grains and seeds; linseed oil] or docosahexaenoic acid (DHA) may prove to be beneficial.37,38
Ocular Surface Microbiome
Cholesterol esters present in meibum may stimulate the proliferation of commensal organisms, such as Staphylococcus aureus, on the eyelid margin. Such commensal bacteria have lipolytic enzymes that break down neutral fats and esters, releasing glycerides and free fatty acids (polar lipids) into the tear film, altering the composition of meibum.21,39 This occurs through the release of bacterial products (e.g. toxins, lipases) in the absence of infection.40 The polar lipids can diffuse through the aqueous into the mucin layer, making it hydrophobic.22 This causes the tear film to become unstable. Moreover, the breakdown of triglycerides into free fatty acids can influence hyperkeratinization.9,41 Infestation with the Demodex mite has also been associated with MGD, with almost half (46.8%) of MGD patients having Demodex present in one study.42 However, as Demodex is also commonly found in the hair follicles of the general population,43 its contribution to MGD is not clear.
Contact Lens Wear
The use of contact lenses is associated with reduced meibomian gland morphology and function. Contact lens wearers have higher degrees of meibomian gland dropout, and the changes seem irreversible.44 Moreover, abnormal meibum quality and lid margin abnormalities are positively correlated with the duration of contact lens wear.45 While the driving pathophysiology is unknown hypotheses include meibomian gland disruption through mechanical trauma,46 plugging due to aggregation of desquamated epithelial cells at gland orifices,47 and/or chronic inflammation.48
Congenital Decrease
Meibomian glands can be decreased or absent from birth. Congenital absence of glands is seen in Turner syndrome, ectrodactyly with ectodermal dysplasia and cleft-lip and palate (ECC syndrome), and in anhidronic ectodermal dysplastic syndrome.49 Stub-like rudiments can present as yellow streaks on the tarsal conjunctival surface or may be largely absent. The remaining glands are often elongated and enlarged, with the proximal portion hooked back in a hairpin manner or folded into the horizontal meridian.50
Congenital Replacement
Dystichiasis is a congenital disorder in which the meibomian glands are each replaced by eyelashes. This causes meibum deficiency as well as ocular surface trauma due to eyelash misdirection. Dystichiasis can occur secondary to a metaplastic reaction in mucocutaneous disease of the lids or to the autosomal dominant disorder lymphoedema.2,51
Presentation and impact on the ocular surface
Meibomian glands provide key components to the tear film that help maintain a healthy ocular surface. When meibomian glands function correctly, the lipids secreted reduce ocular surface water evaporation and prevent dry eye. When these glands are reduced, absent, or dysfunctional, the impact on the ocular surface can be immense.
Presentation
Clinical signs of MGD are varied and include changes in eyelid morphology, altered secretions, and gland dropout.52 Morphological changes are assessed by slit lamp inspection and include meibomian orifice plugging (Figure 1), eyelid margin foaminess (Figure 2), hyperemia/telangiectasias (Figure 3), and changes in orifice position with respect to the muco-cutaneous junction. Meibomian gland secretions are assessed by applying pressure to the eyelid margins and rating the expressibility and texture of the meibum. Normal meibum is clear and easily expressed, while in MGD meibum takes on a more opaque and viscous like form that is difficult to express (Figure 4). Meibomian gland dropout is detected by transillumination through everted eyelids or more accurately, by infrared photography (Figure 5). Not all individuals have all clinical features and in fact, they are often disparate.21 For example, blacks rarely have visible eyelid margin telangiectasias but are just as likely as whites to have altered meibum consistency.53
Figure 1.
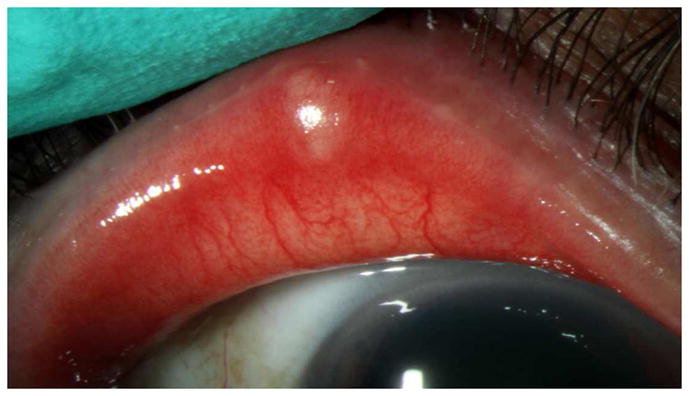
Slit lamp photograph of meibomian orifice plugging.
Figure 2.

Slit lamp photograph ofeyelid margin foaminess.
Figure 3.
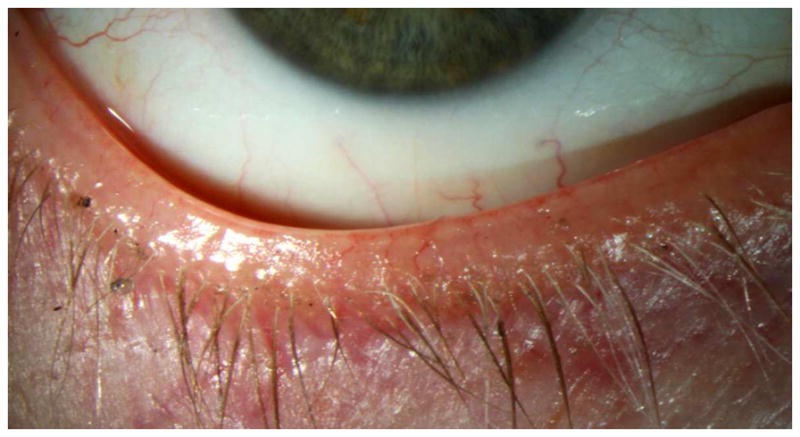
Slit lamp photograph of eyelid margin telangiectasias..
Figure 4.
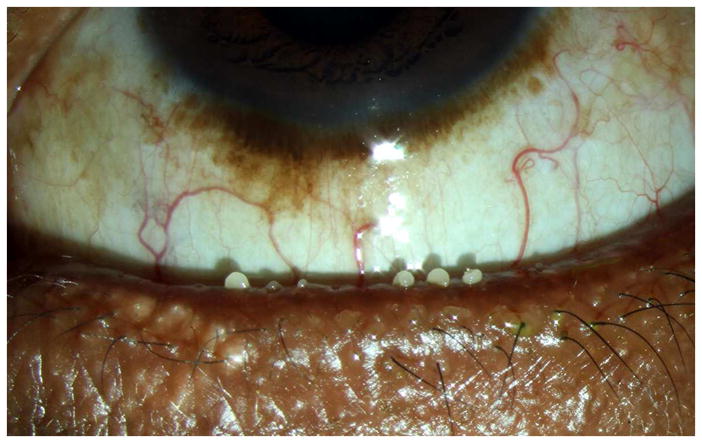
Slit lamp photograph of abnormal meibum which is opaque, viscous, and difficult to express.
Figure 5.
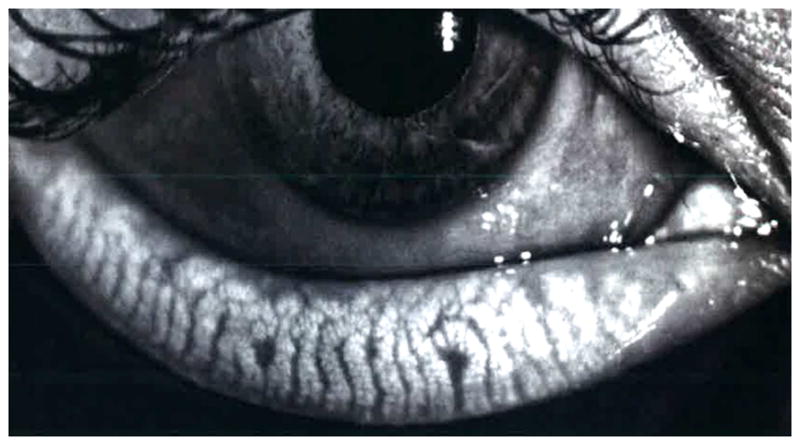
Infrared photography detailing meibomian gland architecture.
Ocular Surface Signs
Ocular surface damage results from a wide variety of interplaying factors, such as increased tear evaporation, hyperosmolarity, proinflammatory mediators in the tears, and decreased lubrication between the lids and globe.54 These may result in irritative symptoms of the ocular surface and eyelids. Many of these ocular signs and symptoms overlap with dry eye disease, and MGD is thought to be a key contributor to evaporative dry eye.55
Tear Evaporation
The tear film lipid layer is derived from meibomian glands and functions to prevent water evaporation from the optical surface.56 In a rabbit model, meibomian glands were completely occluded by squeezing out their contents and cauterizing the lid margin. A protective film layer subsequently did not form over the eye, and rapid evaporation resulted. Meibum was subsequently smeared over the ocular surface, and the lipid film reformed.57 In human studies, patients with obstructive MGD (defined as meibomian gland dropout, poor meibum expression, and lack of signs of inflammation) had increased tear evaporation rates compared to controls as measured by a high-sensitivity microbalance sensor.58 The rate of tear evaporation increased proportionally with the severity of MGD. These studies provide evidence that tear evaporation rates correlate with meibum quality and quantity on the ocular surface.
Tear Osmolarity
Tear hyperosmolarity occurs as a consequence of an increased rate of tear evaporation.2,54 In a rabbit model, meibomian gland orifices were closed using light cautery, inducing MGD in the setting of normal lacrimal function. These rabbits were observed over 20 weeks, and increased tear osmolarity, decreased corneal epithelial glycogen levels, and deceased conjunctival goblet cell density were noted.59 In a mouse model, hyperosmolar stress via instilment of hyperosmotic saline solution caused ocular surface inflammation via the expression and production of numerous inflammatory cytokines (e.g. TNF-alpha, MMP9, IL-1β).60 In humans, the association between MGD and tear osmolarity (measured via the TearLab instrument, San Diego, CA) is less clear. In 233 most male veterans living in South Florida, mean osmolarity values in the more severely affected eye were lower (300 mOsms/L standard deviation (SD) 17) in those with grade 2 or higher eyelid margin telangiectasias compared to those with normal eyelid vascularity (307 mOsms/L SD 18). Those with a meibum quality grade of 2 or higher had similar mean osmolarity levels (305 mosm/ml SD 16) compared to those with normal meibum quality (306 mosm/ml SD 20).53 These findings need to be interpreted with caution, however, as a single osmolarity measure is less informative than multiple measures, given the dynamic nature of the tear film. Moreover, in the setting of dry eye, intereye variability of osmolarity exists.61,62 In humans, large fluctuations in osmolarity values over a short time are a more robust indicator of tear film instability than one static measure.
Ocular Surface Damage
Taken together, abnormal meibum quality and quantity can lead to a decreased tear film lipid layer, tear hyperosmolarity, mechanical irritation through increased friction, and the onset of inflammatory cascades, all of which can lead to ocular surface damage. In humans, patients with MGD (defined as obstruction of meibomian orifices, absence of gland structure, or both) seen in a university-based referral practice in Japan were found to have higher staining with fluorescein and rose bengal compared to patients without MGD.63 In a similar manner, in a population of predominantly male South Florida veterans, those with abnormal meibum quality had higher fluorescein corneal staining scores (2.3 SD 2.3) compared to those with normal meibum quality (1.5 SD 2.1, p<0.05).53
Reflex Tearing
Increased tear evaporation causing hyperosmolar surface damage is hypothesized to cause a compensatory, reflex increase in aqueous tear secretion in evaporative dry eye.22 Interestingly, a study in humans demonstrated increased tear production (measured with Schirmer’s test) in patients with MGD. This is thought to be due to compensatory reflex tearing in the setting of ocular surface abnormalities and discomfort.63
Ocular Symptoms
In humans, as with other aspects of dry eye, signs of MGD often do not correlate with symptoms of discomfort and in fact, most patients with MGD are asymptomatic.64–66 In a study of 619 randomly selected individuals from a population based study in northern China, 21.9% were found to have asymptomatic MGD and 8.6% symptomatic MGD.65 This study showed asymptomatic MGD to be twice more common than symptomatic MGD, and that symptomatology did not correlate with the severity of damage to the ocular surface (tear break up time and staining).64 In another study of 263 men from the Miami Veterans Affairs eye clinic, no correlations between dry eye signs/symptoms and meibomian gland parameters (meibomian gland quality, orifice plugging, lid vascularity) were established.67
However, some patients with MGD do report dry eye symptoms and ocular pain (e.g. foreign-body sensation, photophobia, and conjunctival injection).53,63 In one clinic based study of 299 individuals with aqueous and evaporative dry eye, dry eye symptoms (e.g. pain, photophobia, blurry vision, problems with reading/driving/watching TV measured by OSDI) were higher in aqueous and/or evaporative dry eye patients compared to normal controls (normal controls: mean OSDI 5.5; aqueous dry eye: OSDI 26.7; evaporative dry eye: OSDI 39.8; mixed dry eye: OSDI 20.2).68
Inflammation likely drives symptoms in some individuals with MGD. Several downstream effects of MGD contribute to inflammation including hyperosmolarity and mechanical friction between the eyelids and globe.9 Hyperosmolarity, for example, activates mitogen-activated protein kinases and the nuclear factor-kappa B signaling pathway, leading to the generation of inflammatory cytokines and recruitment of inflammatory cells.2,69 A study examining the inflammatory cytokines IL-17 and IL-22 found that they were increased in patients with dry eye and positively correlated with dry eye questionnaire and keratopathy scores.70
In addition to ocular symptoms of discomfort/pain, visual acuity and bulbar hyperemia can be manifestations of MGD,2 and can occur in the absence or presence of tear abnormalities.22 As the tear film lipid layer provides a smooth optical surface for the cornea,56 it is not surprising that alterations in this layer can have implications for visual quality.9
The above information is as an overall summary of alterations in lipid composition and behavior in MGD. For a complete discussion, we refer you to the International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Tear Film Lipids and Lipid–Protein Interactions in Health and Disease.71
Summary
Meibomian glands are important contributors to the maintenance of a healthy ocular surface. MGD is a broad term referring to any functional abnormality of the meibomian glands. Many pathologies can disrupt their function ranging from congenital to acquired causes. Once gland disruption occurs, the quality and quantity of meibum is altered with a negative impact on the ocular surface. Increased tear evaporation, tear hyperosmolarity, increased ocular surface staining, and increased inflammation have all been observed. Symptomatic irritation of the eyelid and globes as well as decreased visual acuity has been reported in some cases.
Highlights.
Meibomian gland obstruction and meibocyte depletion are important components of meibomian gland dysfunction. Many risk factors for MGD influence meibocyte function including aging, desiccating stress, androgen depletion, diet, microbiome alterations, and contact lens wear. MGD leads to changes in meibum quality and quantity that can cause evaporative dry eye and ocular surface disruption, leading to dry eye symptoms in some individuals.
Acknowledgments
Funding: Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (Dr. Galor), R01EY026174 (Dr. Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant.
Footnotes
Conflicts of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Investigative Ophthalmology & Visual Science. 2011;52(4):1922–1929. doi: 10.1167/iovs.10-6997a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foulks GN, Bron AJ. Meibomian gland dysfunction: a clinical scheme for description, diagnosis, classification, and grading. The Ocular Surface. 2003;1(3):107–126. doi: 10.1016/s1542-0124(12)70139-8. [DOI] [PubMed] [Google Scholar]
- 3.Korb DR, Henriquez AS. Meibomian gland dysfunction and contact lens intolerance. Journal of the American Optometric Association. 1980;51(3):243–251. [PubMed] [Google Scholar]
- 4.Gutgesell VJ, Stern GA, Hood CI. Histopathology of meibomian gland dysfunction. American Journal of Ophthalmology. 1982;94(3):383–387. doi: 10.1016/0002-9394(82)90365-8. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Richards SM, Lo K, et al. Changes in gene expression in human meibomian gland dysfunction. Investigative Ophthalmology & Visual Science. 2011;52(5):2727–2740. doi: 10.1167/iovs.10-6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang HS, Parfitt GJ, Brown DJ, et al. Meibocyte differentiation and renewal: Insights into novel mechanisms of meibomian gland dysfunction (MGD) Experimental Eye Research. 2017 Feb 17; doi: 10.1016/j.exer.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jester JV, Parfitt GJ, Brown DJ. Meibomian gland dysfunction: hyperkeratinization or atrophy? BMC Ophthalmology. 2015;15(Suppl 1):156. doi: 10.1186/s12886-015-0132-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schaumberg DA, Nichols JJ, Papas EB, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on the epidemiology of, and associated risk factors for, MGD. Investigative Ophthalmology & Visual Science. 2011;52(4):1994–2005. doi: 10.1167/iovs.10-6997e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knop E, Knop N, Millar T, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Investigative Ophthalmology & Visual Science. 2011;52(4):1938–1978. doi: 10.1167/iovs.10-6997c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan BD, Evans JE, Dana MR, et al. Influence of aging on the polar and neutral lipid profiles in human meibomian gland secretions. Archives of Ophthalmology. 2006;124(9):1286–1292. doi: 10.1001/archopht.124.9.1286. [DOI] [PubMed] [Google Scholar]
- 11.Jester JV, Brown DJ. Wakayama Symposium: Peroxisome proliferator-activated receptor-gamma (PPARgamma) and meibomian gland dysfunction. The Ocular Surface. 2012;10(4):224–229. doi: 10.1016/j.jtos.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nien CJ, Massei S, Lin G, et al. Effects of age and dysfunction on human meibomian glands. Archives of Ophthalmology. 2011;129(4):462–469. doi: 10.1001/archophthalmol.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Call M, Fischesser K, Lunn M, et al. Notch regulation of PPAR-gamma and development of meibomian gland dysfunction. Investigative Ophthalmology & Visual Science. 2013;54:924–924. [Google Scholar]
- 14.Suhalim JL, Parfitt GJ, Xie Y, et al. Effect of desiccating stress on mouse meibomian gland function. The Ocular Surface. 2014;12(1):59–68. doi: 10.1016/j.jtos.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palaniappan CK, Schutt BS, Brauer L, et al. Effects of keratin and lung surfactant proteins on the surface activity of meibomian lipids. Investigative Ophthalmology & Visual Science. 2013;54(4):2571–2581. doi: 10.1167/iovs.12-11084. [DOI] [PubMed] [Google Scholar]
- 16.Ong BL, Hodson SA, Wigham T, et al. Evidence for keratin proteins in normal and abnormal human meibomian fluids. Current Eye Research. 1991;10(12):1113–1119. doi: 10.3109/02713689109024128. [DOI] [PubMed] [Google Scholar]
- 17.Lavker RM. Label-retaining Cells (LRCs) Are Preferentially Located in the Ductal Epithelium of the Meibomian Gland: Implications on the Mucocutaneous Junctional (MCJ) Epithelium of the Eyelid. Investigative Ophthalmology & Visual Science. 2003;44:3781. [Google Scholar]
- 18.Olami Y, Zajicek G, Cogan M, et al. Turnover and migration of meibomian gland cells in rats’ eyelids. Ophthalmic Research. 2001;33(3):170–175. doi: 10.1159/000055665. [DOI] [PubMed] [Google Scholar]
- 19.Mort RL, Ramaesh T, Kleinjan DA, et al. Mosaic analysis of stem cell function and wound healing in the mouse corneal epithelium. BMC Developmental Biology. 2009;9:4. doi: 10.1186/1471-213X-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim BJ, Ryu IH, Kim SW. Age-related differences in corneal epithelial thickness measurements with anterior segment optical coherence tomography. Japanese Journal of Ophthalmology. 2016;60(5):357–364. doi: 10.1007/s10384-016-0457-x. [DOI] [PubMed] [Google Scholar]
- 21.Galor A. MGD: Definition Versus Dry Eye Disease, Risk Factors. Current Ophthalmology Reports. 2014;2(2):58–64. [Google Scholar]
- 22.Bron AJ, Tiffany JM. The contribution of meibomian disease to dry eye. The Ocular Surface. 2004;2(2):149–165. doi: 10.1016/s1542-0124(12)70150-7. [DOI] [PubMed] [Google Scholar]
- 23.Khandelwal P, Liu S, Sullivan DA. Androgen regulation of gene expression in human meibomian gland and conjunctival epithelial cells. Molecular Vision. 2012;18:1055–1067. [PMC free article] [PubMed] [Google Scholar]
- 24.Sullivan DA, Sullivan BD, Evans JE, et al. Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Annals of the New York Academy of Sciences. 2002;966:211–222. doi: 10.1111/j.1749-6632.2002.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 25.Cermak JM, Krenzer KL, Sullivan RM, et al. Is complete androgen insensitivity syndrome associated with alterations in the meibomian gland and ocular surface? Cornea. 2003;22(6):516–521. doi: 10.1097/00003226-200308000-00006. [DOI] [PubMed] [Google Scholar]
- 26.Krenzer KL, Dana MR, Ullman MD, et al. Effect of androgen deficiency on the human meibomian gland and ocular surface. The Journal of Clinical Endocrinology and Metabolism. 2000;85(12):4874–4882. doi: 10.1210/jcem.85.12.7072. [DOI] [PubMed] [Google Scholar]
- 27.Mathers WD, Shields WJ, Sachdev MS, et al. Meibomian gland morphology and tear osmolarity: changes with Accutane therapy. Cornea. 1991;10(4):286–290. doi: 10.1097/00003226-199107000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Lambert RW, Smith RE. Effects of 13-cis-retinoic acid on the hamster meibomian gland. The Journal of Investigative Dermatology. 1989;92(3):321–325. doi: 10.1111/1523-1747.ep12277122. [DOI] [PubMed] [Google Scholar]
- 29.Samarawickrama C, Chew S, Watson S. Retinoic acid and the ocular surface. Survey of Ophthalmology. 2015;60(3):183–195. doi: 10.1016/j.survophthal.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Jester JV, Nicolaides N, Kiss-Palvolgyi I, et al. Meibomian gland dysfunction. II. The role of keratinization in a rabbit model of MGD. Investigative Ophthalmology & Visual Science. 1989;30(5):936–945. [PubMed] [Google Scholar]
- 31.Agnifili L, Fasanella V, Costagliola C, et al. In vivo confocal microscopy of meibomian glands in glaucoma. The British Journal of Ophthalmology. 2013;97(3):343–349. doi: 10.1136/bjophthalmol-2012-302597. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Kam WR, Sullivan DA. Influence of Omega 3 and 6 Fatty Acids on Human Meibomian Gland Epithelial Cells. Cornea. 2016;35(8):1122–1126. doi: 10.1097/ICO.0000000000000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korb DR, Blackie CA, Finnemore VM, et al. Effect of using a combination of lid wipes, eye drops, and omega-3 supplements on meibomian gland functionality in patients with lipid deficient/evaporative dry eye. Cornea. 2015;34(4):407–412. doi: 10.1097/ICO.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 34.Macsai MS. The role of omega-3 dietary supplementation in blepharitis and meibomian gland dysfunction (an AOS thesis) Transactions of the American Ophthalmological Society. 2008;106:336–356. [PMC free article] [PubMed] [Google Scholar]
- 35.Brignole-Baudouin F, Baudouin C, Aragona P, et al. A multicentre, double-masked, randomized, controlled trial assessing the effect of oral supplementation of omega-3 and omega-6 fatty acids on a conjunctival inflammatory marker in dry eye patients. Acta Ophthalmologica. 2011;89(7):e591–597. doi: 10.1111/j.1755-3768.2011.02196.x. [DOI] [PubMed] [Google Scholar]
- 36.Walter SD, Gronert K, McClellan AL, et al. omega-3 Tear Film Lipids Correlate With Clinical Measures of Dry Eye. Investigative Ophthalmology & Visual Science. 2016;57(6):2472–2478. doi: 10.1167/iovs.16-19131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin H. Role of PPAR-gamma in inflammation. Prospects for therapeutic intervention by food components. Mutation Research. 2010;690(1–2):57–63. doi: 10.1016/j.mrfmmm.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 38.Zhang S, Gu H, Hu N. Role of Peroxisome Proliferator-Activated Receptor gamma in Ocular Diseases. Journal of Ophthalmology. 2015;2015:275435. doi: 10.1155/2015/275435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson JD, Shimazaki J, Benitez-del-Castillo JM, et al. The international workshop on meibomian gland dysfunction: report of the definition and classification subcommittee. Investigative Ophthalmology & Visual Science. 2011;52(4):1930–1937. doi: 10.1167/iovs.10-6997b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Driver PJ, Lemp MA. Meibomian gland dysfunction. Survey of Ophthalmology. 1996;40(5):343–367. doi: 10.1016/s0039-6257(96)80064-6. [DOI] [PubMed] [Google Scholar]
- 41.Dougherty JM, McCulley JP. Bacterial lipases and chronic blepharitis. Investigative Ophthalmology & Visual Science. 1986;27(4):486–491. [PubMed] [Google Scholar]
- 42.Schachter SE, Schachter A, Hom MM, et al. Prevalence of MGD, blepharitis, and demodex in an optometric practice. Investigative Ophthalmology & Visual Science. 2014;55(13):49–49. [Google Scholar]
- 43.Andrews JR. The prevalence of hair follicle mites in caucasian New Zealanders. The New Zealand Medical Journal. 1982;95(711):451–453. [PubMed] [Google Scholar]
- 44.Alghamdi WM, Markoulli M, Holden BA, et al. Impact of duration of contact lens wear on the structure and function of the meibomian glands. Ophthalmic & Physiological Optics: The Journal of the British College of Ophthalmic Opticians. 2016;36(2):120–131. doi: 10.1111/opo.12278. [DOI] [PubMed] [Google Scholar]
- 45.Machalinska A, Zakrzewska A, Adamek B, et al. Comparison of Morphological and Functional Meibomian Gland Characteristics Between Daily Contact Lens Wearers and Nonwearers. Cornea. 2015;34(9):1098–1104. doi: 10.1097/ICO.0000000000000511. [DOI] [PubMed] [Google Scholar]
- 46.Larke JR. The eye in contact lens wear. London: Butterworth; 1985. pp. 5–6. [Google Scholar]
- 47.Henriquez AS, Korb DR. Meibomian glands and contact lens wear. The British Journal of Ophthalmology. 1981;65(2):108–111. doi: 10.1136/bjo.65.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arita R, Itoh K, Inoue K, et al. Contact lens wear is associated with decrease of meibomian glands. Ophthalmology. 2009;116(3):379–384. doi: 10.1016/j.ophtha.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Baum JL, Bull MJ. Ocular manifestations of the ectrodactyly, ectodermal dysplasia, cleft lip-palate syndrome. American Journal of Ophthalmology. 1974;78(2):211–216. doi: 10.1016/0002-9394(74)90078-6. [DOI] [PubMed] [Google Scholar]
- 50.Bron AJ, Benjamin L, Snibson GR. Meibomian gland disease. Classification and grading of lid changes. Eye. 1991;5(Pt 4):395–411. doi: 10.1038/eye.1991.65. [DOI] [PubMed] [Google Scholar]
- 51.Erickson RP, Dagenais SL, Caulder MS, et al. Clinical heterogeneity in lymphoedema-distichiasis with FOXC2 truncating mutations. Journal of Medical Genetics. 2001;38(11):761–766. doi: 10.1136/jmg.38.11.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Investigative Ophthalmology & Visual Science. 2011;52(4):2006–2049. doi: 10.1167/iovs.10-6997f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alghamdi YA, Mercado C, McClellan AL, et al. Epidemiology of Meibomian Gland Dysfunction in an Elderly Population. Cornea. 2016;35(6):731–735. doi: 10.1097/ICO.0000000000000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bron AJ, Tiffany JM, Yokoi N, et al. Using osmolarity to diagnose dry eye: a compartmental hypothesis and review of our assumptions. Advances in Experimental Medicine and Biology. 2002;506(Pt B):1087–1095. doi: 10.1007/978-1-4615-0717-8_153. [DOI] [PubMed] [Google Scholar]
- 55.Lemp MA. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. The CLAO Journal: Official Publication of the Contact Lens Association of Ophthalmologists, Inc. 1995;21(4):221–232. [PubMed] [Google Scholar]
- 56.Bron AJ, Tiffany JM, Gouveia SM, et al. Functional aspects of the tear film lipid layer. Experimental Eye Research. 2004;78(3):347–360. doi: 10.1016/j.exer.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 57.Mishima S, Maurice DM. The oily layer of the tear film and evaporation from the corneal surface. Experimental Eye Research. 1961;1:39–45. doi: 10.1016/s0014-4835(61)80006-7. [DOI] [PubMed] [Google Scholar]
- 58.Goto E, Endo K, Suzuki A, et al. Tear evaporation dynamics in normal subjects and subjects with obstructive meibomian gland dysfunction. Investigative Ophthalmology & Visual Science. 2003;44(2):533–539. doi: 10.1167/iovs.02-0170. [DOI] [PubMed] [Google Scholar]
- 59.Gilbard JP, Rossi SR, Heyda KG. Tear film and ocular surface changes after closure of the meibomian gland orifices in the rabbit. Ophthalmology. 1989;96(8):1180–1186. doi: 10.1016/s0161-6420(89)32753-9. [DOI] [PubMed] [Google Scholar]
- 60.Luo L, Li DQ, Corrales RM, et al. Hyperosmolar saline is a proinflammatory stress on the mouse ocular surface. Eye & Contact Lens. 2005;31(5):186–193. doi: 10.1097/01.icl.0000162759.79740.46. [DOI] [PubMed] [Google Scholar]
- 61.Lemp MA, Bron AJ, Baudouin C, et al. Tear osmolarity in the diagnosis and management of dry eye disease. American Journal of Ophthalmology. 2011;151(5):792–798. doi: 10.1016/j.ajo.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 62.Bron AJ, Tomlinson A, Foulks GN, et al. Rethinking dry eye disease: a perspective on clinical implications. The Ocular Surface. 2014;12(2 Suppl):S1–31. doi: 10.1016/j.jtos.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 63.Shimazaki J, Sakata M, Tsubota K. Ocular surface changes and discomfort in patients with meibomian gland dysfunction. Archives of Ophthalmology. 1995;113(10):1266–1270. doi: 10.1001/archopht.1995.01100100054027. [DOI] [PubMed] [Google Scholar]
- 64.Viso E, Rodriguez-Ares MT, Abelenda D, et al. Prevalence of asymptomatic and symptomatic meibomian gland dysfunction in the general population of Spain. Investigative Ophthalmology & Visual Science. 2012;53(6):2601–2606. doi: 10.1167/iovs.11-9228. [DOI] [PubMed] [Google Scholar]
- 65.Jie Y, Xu L, Wu YY, et al. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye. 2009;23(3):688–693. doi: 10.1038/sj.eye.6703101. [DOI] [PubMed] [Google Scholar]
- 66.Lekhanont K, Rojanaporn D, Chuck RS, et al. Prevalence of dry eye in Bangkok, Thailand. Cornea. 2006;25(10):1162–1167. doi: 10.1097/01.ico.0000244875.92879.1a. [DOI] [PubMed] [Google Scholar]
- 67.Galor A, Feuer W, Lee DJ, et al. Ocular surface parameters in older male veterans. Investigative Ophthalmology & Visual Science. 2013;54(2):1426–1433. doi: 10.1167/iovs.12-10819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lemp MA, Crews LA, Bron AJ, et al. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi: 10.1097/ICO.0b013e318225415a. [DOI] [PubMed] [Google Scholar]
- 69.Baudouin C. The pathology of dry eye. Survey of Ophthalmology. 2001;45(Suppl 2):S211–220. doi: 10.1016/s0039-6257(00)00200-9. [DOI] [PubMed] [Google Scholar]
- 70.Tan X, Sun S, Liu Y, et al. Analysis of Th17-associated cytokines in tears of patients with dry eye syndrome. Eye. 2014;28(5):608–613. doi: 10.1038/eye.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Green-Church KB, Butovich I, Willcox M, et al. The international workshop on meibomian gland dysfunction: report of the subcommittee on tear film lipids and lipid-protein interactions in health and disease. Investigative Ophthalmology & Visual Science. 2011;52(4):1979–1993. doi: 10.1167/iovs.10-6997d. [DOI] [PMC free article] [PubMed] [Google Scholar]


