Abstract
Extracellular fluid volume expansion is nearly universal in patients with chronic kidney disease. Such volume expansion often overlaps with the syndrome of heart failure with preserved ejection fraction, which can not only lead to symptoms, but can also lead to further organ damage. Unique treatment challenges are present in this patient population, including low glomerular filtration, which limits sodium chloride filtration, intrinsic tubule predisposition to sodium chloride retention, and proteinuria. Additionally, pharmacokinetic considerations alter the disposition of diuretics in patients with chronic kidney disease and nephrotic syndrome. Maintaining extracellular fluid volume near to normal is often necessary for hypertension treatment in this population, but it may also help prevent progressive cardiovascular and renal damage. Although powerful diuretics can often accomplish this goal, this often comes at a cost of competing side effects. An approach to reduce extracellular fluid volume while avoiding side effects, therefore, requires a nuanced yet aggressive therapeutic approach.
Keywords: Diuretics, salt-sensitive hypertension, nephrotic syndrome, extracellular fluid volume expansion
Introduction
Disordered extracellular fluid (ECF) volume is nearly universal in chronic kidney disease (CKD), and typically presents with one of three common patterns. The most common includes mild ECF volume expansion, with salt-sensitive hypertension and left ventricular hypertrophy as predominant signs, but CKD can also present with more severe ECF volume expansion, typically together with the nephrotic syndrome. Less well recognized, at least today, is that CKD may also have components of salt wasting syndrome, sometimes severe enough to cause ECF volume contraction. The pathogenesis of these disorders will be reviewed, followed by a discussion of treatment.
Phenomenology of Salt Homeostasis in CKD
The rate at which kidneys excrete NaCl is related to the ECF volume and the blood pressure, which are therefore also related to each other. Although the nature of the relation between ECF volume and urinary NaCl excretion has been debated, Walser’s summary of the literature (1) suggested that human urinary NaCl excretion, at steady state, is normally a linear function of the ECF volume in excess of a critical value. The relation between NaCl excretion and ECF volume, therefore, describes a ‘renal function curve’ as shown in Figure 1A. The supporting human experiments were often conducted during several days to weeks, so that the tested persons were at steady state, with NaCl excretion equal to the NaCl intake (minus minor extrarenal losses). Recent work by Titze and colleagues has added nuance to these precepts, showing that sodium chloride excretion is more variable than previously appreciated, when measured on a daily basis,1 and that there is more sodium storage outside of ECF than previously understood.2 Sodium storage in the skin associates with left ventricular hypertrophy in CKD.3 Furthermore, in longer studies, it appears that some of the initial gain in ECF volume may dissipate over time.4 Nevertheless, all agree that, in normal humans, ‘on a long-term basis, indeed what goes in also comes out’5, satisfying the law of mass balance. Further, even in the studies by Titze and colleagues, markers of ECF volume expansion remained suppressed when dietary salt intake is high,4 suggesting that dietary NaCl loading does expand the ECF volume chronically; as noted below, this relationship is exaggerated in CKD.
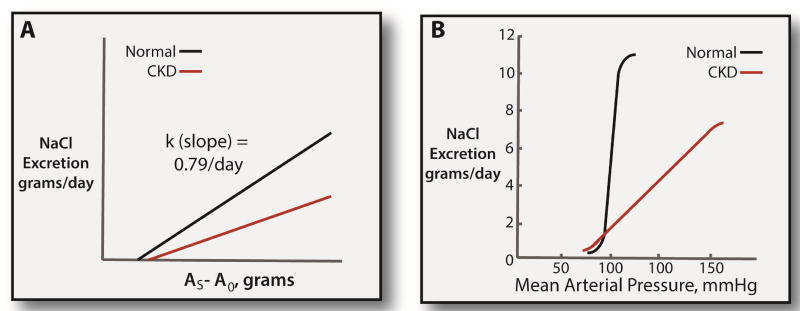
Figure 1. Renal function curves in normal individuals and CKD.
Panel A: relationship between NaCl excretion and body sodium chloride content (AS) above a basal value (A0). This analysis is based on Walser.69 The slope of the normal relationship (k, which is a time constant) is taken from Walser’s review of the literature. The slope appears to be reduced by CKD. Panel B: Classic renal function curve, as drawn by Guyton and colleagues.6 As argued by Guyton, CKD shifts the renal function curve downward and to the right, describing the increased salt-sensitivity in this population.
Guyton and colleagues demonstrated that renal salt excretion plays a central role in setting the mean arterial pressure.6 According to their analysis, which is related to, but distinct from Walser’s, the relation between mean arterial pressure and urinary NaCl excretion at steady state is also nearly linear through a wide range of dietary salt intake; in fact, only very small changes in mean arterial pressure are required to produce substantial natriuresis (see Figure 1B). The relationship between mean arterial pressure and sodium excretion defines a different, but closely related, ‘renal function curve’,6 and the effect of arterial pressure on urinary NaCl excretion has been called the pressure natriuresis.
These models are essentially phenomenological and do not provide specific insight into physiological control mechanisms. Both models, however, have been corroborated by experimental data and accurately describe renal salt homeostasis under many conditions. They also have interesting implications for understanding renal salt retention and renal salt-wasting disorders. For example, the slope of the relation between ECF volume and renal salt excretion (the ‘time constant’, Figure 1A) determines the speed with which an individual can adapt to a change in dietary intake. The slope appears to be reduced by aging and CKD (Figure 1A).7 This means that it takes longer for the kidney to adapt to a change in dietary NaCl intake when renal function is compromised or an individual is aged (see Figure 2). Thus, if dietary salt intake is reduced suddenly, ECF volume will decline more in older individuals and in individuals with CKD than in younger individuals with normal renal function. Surprisingly, a reduced slope of the relation between ECF volume and dietary NaCl intake also predicts that the ECF volume will be elevated when the dietary NaCl intake is normal or high in such patients.8 This is the reason that individuals with CKD so often have ECF volume expansion and respond to dietary NaCl restriction with a marked decline in blood pressure. On the typical NaCl-rich ‘Western’ diet, the kidneys’ slow responses shift the renal function curve downward and to the right (Figure 1B). If dietary salt intake is reduced suddenly, however, salt-wasting may occur.
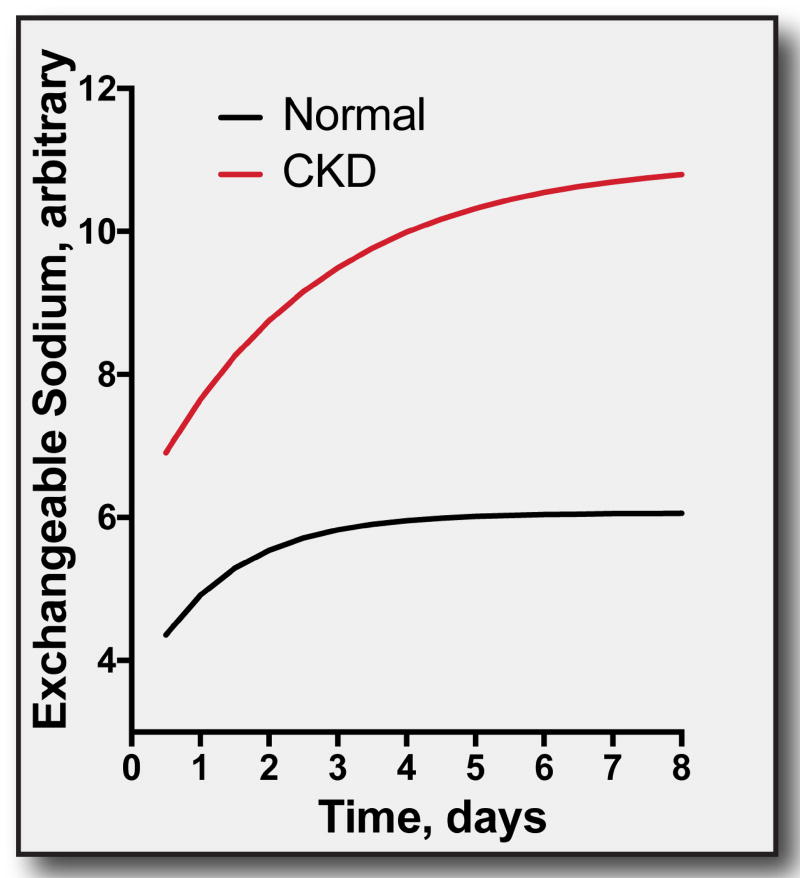
Figure 2. Effects of an increase in exchangeable sodium in normal individuals and CKD.
This analysis is based on a summary of the literature,69 and the equation, described therein. At: body sodium at time t, A0: body sodium at the value that sodium excretion ceases, I2: intake of sodium at time 2, I1: intake of sodium at time 1, k is the time constant, defined as above. When dietary salt intake is increased, total body salt content rises approaching a new steady state. When kidney function is reduced, k is reduced, and the effect of a change is slowed and magnified. Note that longer observations suggest that the initial change in exchangeable sodium regresses toward baseline,4,5 as described by Guyton,6, and likely resulting from pressure natriuresis. In CKD, however, easily detectable ECF volume expansion at steady state is still clear.34
Another important implication of the relation between ECF volume (or mean arterial pressure) and renal salt excretion is that salt-wasting may be present despite a preserved ability to reduce urinary salt excretion to negligible levels.9 Clinical and experimental examples of salt wasting disorders in which urinary NaCl excretion can be very low include the Mendelian disease, Gitelman syndrome, which is caused by loss of function of the thiazide-sensitive NaCl cotransporter. This observation indicates that the diagnosis of salt wasting relies on the ability to estimate the extracellular fluid volume precisely. Because such determinations are nearly always imprecise clinically, the diagnosis of subtle renal salt wasting may be difficult.
Diuretics in CKD
Loop diuretics are typically drugs of first choice for treating ECF volume expansion in CKD, as discussed below. As shown in Figure 3, CKD alters the effectiveness of these diuretics in several ways. First, loop (and thiazide) diuretics are organic anions that reach their sites of action in the lumen of the thick ascending limb via secretion along the proximal tubules. The primary transport proteins involved, at the basolateral membrane, are organic anion transporters. Deletion of these proteins in mice produces diuretic resistance by inhibiting diuretic secretion into the tubule lumen.10 These transport processes are relatively nonspecific, and a single transporter type can facilitate the movement of a variety of similarly charged molecules into the tubular lumen. Accordingly, any exogenous or endogenous substance that competes with a diuretic for one of these transport processes can potentially limit the efficient arrival of that diuretic to its site of action. Uremic anions are examples of endogenous substances that compete with loop and thiazide diuretics for tubular secretion and the dose response curve of these diuretics in CKD is shifted to the right (Effect A, in Figure 2).11 This means that higher doses are required to present the same diuretic concentration to its active site.
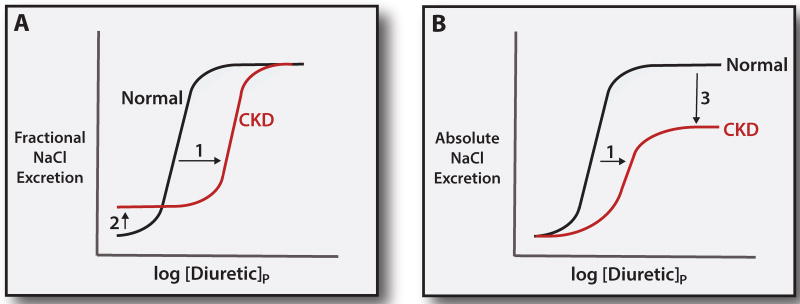
Figure 3. Mechanisms of diuretic resistance in CKD.
Panel A: relationship between the log of the plasma diuretic concentration ([diuretic]p) and fractional NaCl excretion. Note that CKD shifts the dose response curve to the right, owing to the impairment in diuretic secretion by the proximal tubule (Effect 1). At baseline, the fractional NaCl excretion is elevated in CKD, to preserve normal NaCl excretion (Effect 2). The ceiling effect, however, is preserved. Panel B: relationship between plasma diuretic concentration and NaCl excretion, expressed in absolute terms. Note that the dose response shift is also apparent in this analysis (Effect 1). In this case, however, the maximal (ceiling) effect is strikingly reduced (Effect 3).
A second, perhaps more important, effect of CKD, however, is related to the loss of NaCl filtration. Even though enhanced NaCl reabsorption by tubules is typically the primary cause of ECF volume expansion, as GFR declines, the amount of sodium chloride reabsorbed by each nephron must also decline, to maintain sodium chloride excretion equal to intake. This decline limits the effects of blocking sodium chloride reabsorption with diuretics. Viewed another way, the basal fractional sodium excretion increases as CKD progresses (Effect B in Figure 3A) to maintain sodium chloride balance. This means that, although the maximal fractional rates of NaCl excretion are preserved in CKD (Figure 3A), maximal absolute rates, those rates that actually determine ECF volume control, are decreased substantially (Effect C in Figure 3B). This means that more aggressive approaches, such as adding a thiazide or thiazide-like drug, are often necessary.
It should also be emphasized that, although there exists a ‘ceiling’ above which NaCl excretion does not increase, diuretic doses that exceed this ceiling can maintain drug levels in the natriuretic range for a longer period of time, making it appear that such a ceiling does not exist. This may be the reason that higher doses of loop diuretics may be more effective than lower ones in heart failure trials,12 even when they exceed a theoretical ‘ceiling’.
Hypertension in CKD
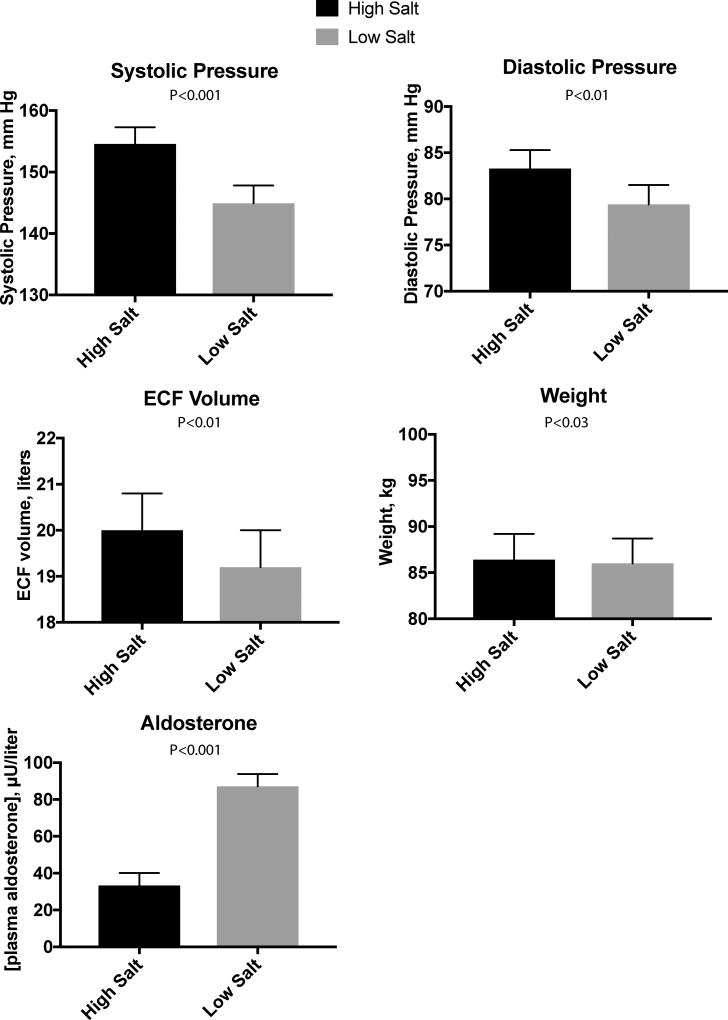
Most patients with CKD are salt-sensitive and have mild expansion of the ECF volume (described above). Yet this is often difficult to detect clinically, as homeostatic processes maintain the ECF volume close to normal until the glomerular filtration rate declines below 10 to 15 mL/min/1.73 M2. As described by the Guyton model, ECF and plasma volume expansion occurs with minimal edema because the accompanying hypertension causes pressure natriuresis, preventing further ECF volume overload, which would otherwise lead to edema (see below). Scribner and colleagues13 found that the exchangeable sodium content of the body was highly correlated with lean body mass in normal individuals (r=0.993) and was higher in patients with stage 4–5 CKD than in age-matched controls (62.0 versus 59.5 mEq/kg lean body mass) on their typical diets. When the individuals with CKD were switched to a salt restricted diet (0.5–2 g sodium), the exchangeable sodium content fell into the normal range, and the blood pressure declined by 53/22 mm Hg. Much more recently, Campbell and colleagues14 performed a double blind placebo controlled trial of salt restriction in CKD. Although they initially evaluated 538 patients, only 25 qualified for randomization, and 20 finished the protocol. High sodium, 60–80 plus 120 mEq/d given as a slow release pill, was compared with a low salt, 60–80 mEq/day, for 2 weeks. The low salt intake led to a drop in systolic pressure of 9.7 ± 10.3 SD mm Hg (by ambulatory recording, see Figure 4), and to a decline in ECF volume of 800 milliliters (P<0.001). This was also associated with a drop in proteinuria by 342 mg/day, even though both plasma renin activity and aldosterone concentration were increased by the low salt intake. The energy intake did not change.
Figure 4. Effect of low salt diet in CKD.
Panels show effects of randomized low salt diet in the setting of chronic kidney disease. In each case, the difference between high salt and low salt intake was statistically significant, with signs of ECF volume reduction being associated with declines in blood pressure. Data from.14
Left ventricular hypertrophy (LVH) is common in CKD and also appears to be associated with ECF volume expansion. In a study of 104 patients with CKD, even stage 2 CKD was associated with excess ECF volume, and ECF volume excess correlated with left ventricular mass index.15 As LVH is associated with poor prognosis in this population,16 it is reasonable to recommend dietary sodium chloride restriction for most patients with CKD. The most recent KDIGO guidelines for individuals with CKD recommend restricting sodium intake to <2 g daily, unless contraindicated.17
Although dietary salt restriction is essential, it is often insufficient to control the blood pressure and ECF volume expansion in CKD. While the use of antihypertensive agents in this situation is beyond the scope of this review, several comments on diuretic usage, as a component of antihypertensive regimens, are warranted. Agarwal tested the effects of the loop diuretics furosemide and torsemide on blood pressure and ECF volume in patients with stage 2 and 3 CKD.18 As in the studies noted above, these investigators found that ECF water was elevated in the individuals with CKD at baseline. Diuretics decreased ECF water, decreases that persisted for up to 3 weeks, leading to reductions in blood pressure. Yet the blood pressure reductions lagged after reduction in the ECF volume. As with salt restriction, discussed above, diuretic-induced decreases in ECF water were associated with the elevations in plasma renin activity and aldosterone, but these were not sufficient to counteract the ECF volume depletion.
Although thiazides and thiazide-like drugs are typically viewed as more effective than loop diuretics to treat hypertension in individuals with normal kidney function, as GFR declines, this relationship may reverse. This belief has both theoretical and observational underpinnings. Thiazides typically increase Na+ excretion to 5–7% of filtered load, while loop diuretics can increase it to 20–25%. As GFR declines, there effectiveness would be expected to decline proportionately (Figure 2). It has been noted19 that, when the GFR is 10 ml/min/M2, for example, an individual must excrete approximately 10% of the filtered Na load (200 mEq) to be in negative salt balance. As the typical limits for thiazide effects are 5–7%, one would not expect these drugs to be very effective. Additionally, some early studies confirmed low effectiveness of thiazides when kidney function was poor.20,21 These considerations have led KDOQI to recommend thiazides for the treatment of hypertension only as long as eGFR is greater than 30 ml/minute/M2.
Yet interest in the continued use of thiazides as CKD progresses has resurfaced recently, acknowledging the superiority of these drugs for hypertension in normal individuals. While large comparative effectiveness trials are lacking, Agarwal reviewed the smaller trials examining this issue.22,23 These generally showed effects of thiazide type diuretics on blood pressure, despite substantial CKD, but several factors should be noted. First, like with loop diuretics, it may be necessary to increase the dose above that recommended for individuals with preserved kidney function. With normal renal function, for example, doses of chlorthalidone are typically recommended to be 12.5–25 mg/day.24 In one small study where the chlorthalidone dose was titrated, individuals with eGFR of 20–45 ml/minute/M2 received an average of 67.5 mg/daily to achieve blood pressure control.25 The second is that several of these studies used thiazides as ‘add on’ treatment for individuals already using loop diuretics. Chronic treatment with loop diuretics activates compensatory processes in the distal nephron that may strikingly increase the percentage of filtered NaCl reabsorbed therein.26 This may be one reason that adding a thiazide or thiazide-like diuretic to a regimen of loop diuretics in the setting of CKD has long been known to be effective.27
Edema in CKD
As noted, many times CKD is associated with mild ECF volume expansion, but not enough to cause substantial edema or other signs of congestion, even when patients approach the need for dialysis. This is because the resulting hypertension drives a pressure natriuresis. In other situations, however, when nephrotic syndrome of heart failure accompanies CKD, then ECF volume expansion is more severe and signs of peripheral and central congestion do occur. These two situations will be considered separately, although the mechanisms involved share many features.
Heart failure is typically categorized as associated with reduced and preserved ejection fraction. While these two syndromes are not entirely distinct, the categorization has proved useful, as the treatment approaches, and treatment successes, are quite different. In the situation of heart failure with reduced ejection fraction, damage to heart muscle reduces cardiac output, leading to hypotension, which is sensed by the kidney and in the vascular system. This leads to activation of the efferent limb of body NaCl homeostasis. Specifically, a decrease in glossopharyngeal and vagal tone from the carotid and aortic receptors to the CNS leads to a rapid increase in sympathetic activity with associated activation of the renin angiotensin aldosterone axis and, when severe, nonosmotic release of vasopressin.28 Additionally, a decrease in pressure at renal baroreceptors, and decreased NaCl delivery to the macula densa, increase renin secretion, and thereby angiotensin II and aldosterone. The resulting increase in systemic vascular resistance and renal sodium and water retention raises venous pressure and restores cardiac output, through the Frank-Starling mechanism. The purpose of these concerted actions, therefore, is to maintain the arterial circulatory integrity and restore the perfusion to the vital organs, but the price paid is expansion of the ECF volume; in this case, edema results because the ECF volume expansion does not raise the arterial pressure about its normal threshold (Figure 1). Therapy is targeted to the neurohormonal factors that contribute to these processes, as well as to the congestion itself, and it has been shown to improve outcomes.
The pathogenesis of heart failure with preserved ejection fraction (HFpEF) is much more poorly understood, and therapy is largely symptomatic. Yet many observers have suggested that, in this situation, CKD may play an important and pathogenic role by causing ECF volume expansion and hypertension, and by damaging the heart and the endothelium29. Yet, despite differences between the two major categories of heart failure regarding pathogenesis, and despite the fact that many treatments have not been found to benefit heart failure with preserved ejection fraction, diuretics play central roles in treatment of both types. Heart failure itself can be a diuretic resistant state, but the combination of CKD and heart failure presents unique challenges for diuretic therapy, as described below.
Nephrotic syndrome, with substantial ECF volume expansion, is another situation in which edema is present in CKD. Two processes that are not mutually exclusive may be involved. The first results directly from the hypoalbuminemia that is a core feature of the syndrome. The fall in plasma oncotic pressure alters the Starling forces, increasing the flux of fluid into the interstitial spaces, leading the circulation to be ‘underfilled’.30,31 In this case, when the blood pressure declines below the renal ‘set point’, NaCl retention is triggered and edema results, much like in heart failure. Patients with minimal-change disease often have a contracted plasma volume and a stimulated renin angiotensin aldosterone system.32 Alternatively, primary renal NaCl retention, resulting from intrinsic renal disease, can contribute to ‘overflow edema’, when renal NaCl retention is driven by intrinsic renal processes. Patients with diabetes and hypertension usually have an expanded plasma volume and a suppressed renin angiotensin aldosterone system33. Yet primary renal NaCl retention alone tends to cause hypertension, with escape from NaCl retention, as discussed above. Thus, even in this situation, a component of abnormal fluid transudation from the plasma into the interstitium is essential for edema to develop. Ebah and colleagues34 detected increased interstitial pressures in CKD patients with edema, especially those in whom the duration was short.
Micropuncture studies of sodium-retaining animal models of the nephrotic syndrome35,36 demonstrate pronounced NaCl reabsorption in the distal nephron and thick ascending limb. The proteinuric kidney of a rat model of unilateral nephrotic syndrome has an enhanced Na+ reabsorption in the collecting duct37 and diminished response to ANP,38 compared with the non proteinuric kidney. Hyperaldosteronism reinforces NaCl reabsorption at these sites. Renin and aldosterone levels are highly variable in patients with the nephrotic syndrome.39 There is also growing evidence that one cause of primary renal NaCl reabsorption in nephrotic syndrome is related to the proteins that are filtered by the abnormal glomerular basement membrane. These may include proteases that activate epithelial sodium channels directly, by cleaving them.40–42 Knowing whether the edema is mostly ‘overflow’ or ‘overfill’ has substantial therapeutic implications.
Kapur and colleagues reported that ECF volume contracted (underfilled) patients had higher BUN, BUN/creatinine ratio, urine osmolality, and lower FeNa (<0.2%) than expanded (overflow) patients; treatment with diuretics alone, without volume expanders, such as albumin, proved effective and safe for the volume expanded group.43 Especially in children with minimal change disease, however, in whom a more ECF volume contracted pattern is common, it is customary to treat resistant edema with albumin combined with loop diuretics, with the goal of achieving transient movement of fluid into the vasculature during the diuresis.32
Nephrotic syndrome itself presents unique challenges to diuretic treatment, regardless of the pattern. Animal studies demonstrate five mechanisms that could impair the responsiveness to loop diuretics in patients with the nephrotic syndrome, including 1) decreased delivery and/or decreased tubular secretion of the diuretic, 2) increased renal diuretic metabolism,44 3) decreased blockade of the Na-K-2Cl cotransporter by the diuretic,45 and 4) increased NaCl reabsorption by other nephron segments. Clinical studies confirm that nephrotic patients have an impaired tubular response to loop diuretics. Hypoalbuminemia decreases the binding of furosemide to plasma proteins and thereby increases its volume of distribution.46 The secretion of loop diuretics by the proximal tubule is reduced by hypoalbuminemia,44 and albumin infusion into nephrotic patients increases renal furosemide excretion in the urine.47 Early work suggested that premixing furosemide with albumin prior to intravenous injection amplified diuresis,48 but this was not confirmed by some others.49–51 A meta-analysis suggested minor and transient benefits of combining albumin with loop diuretics, but noted that the quality of data was poor.52 Indeed, patients with a serum albumin exceeding 2 g/dL can typically deliver normal quantities of furosemide into the urine.53 A more effective approach to diuretic resistance in such patients, especially when signs of volume expansion are present, is to attempt to limit albuminuria with an ACE inhibitor or ARB, while using aggressive loop diuretic regimens. As noted above, any maneuver that reduces ECF volume, including loop diuretics, will provide further reductions in proteinuria.54 Nevertheless, especially for children who appear ECF volume contracted, and with few nephritic signs, combining albumin with loop diuretics remains reasonable.
Another potential cause of diuretic resistance in nephrosis is that albumin filtered through the abnormal glomerulus can restrict the interaction of furosemide with the Na-K-2Cl cotransporter.55 In micropuncture studies of rats, adding albumin to the tubular perfusate of the loop of Henle attenuated the response to furosemide, presumably because of binding to albumin, an effect reversed by co-perfusion with warfarin, which displaces furosemide from its albumin binding site.56 Agarwal and colleagues,53 however, found that displacing furosemide from albumin by co-administration of sulfisoxazole did not affect natriuresis in patients with the nephrotic syndrome, suggesting that this mechanism is not predominant. This study, however, was not definitive, as these patients did not have diuretic resistance.
As noted above, one other cause of primary sodium retention in nephrotic syndrome has received attention recently. Plasma proteins that are filtered abnormally by diseased glomeruli include proteases. It is well established that the epithelial sodium channel, ENaC, is activated when it is cleaved by a diverse group of proteases. This appears to be a physiological pathway, but when abnormal proteins are present in the tubule lumen, it may be activated pathologically.42 This mechanism might help to explain the data presented above, suggesting that activated sodium transport along the collecting duct is important in nephrotic syndrome. It provides a rationale for treating patients with amiloride or triamterene, but adequate clinical studies to support this are lacking.
Salt wasting in CKD
During the last half of the previous century, salt wasting as a complication of CKD was widely discussed, and viewed as a substantial clinical concern.9 Renal salt wasting connotes inappropriate Na and Cl losses in the urine. Because salt (used here to indicate NaCl) excretion is determined largely by the ECF volume and mean arterial pressure, as discussed above, the term renal salt wasting indicates that renal salt excretion continues at an ECF volume at which renal salt excretion normally ceases. Yet renal salt wasting does not necessarily imply unrelenting renal salt losses. For example, the diagnosis of renal salt wasting was often said to require persistent sodium and chloride losses in the face of symptomatic extracellular fluid volume depletion. Yet genetic disruption of several renal ion transport proteins, like the thiazide-sensitive NaCl cotransporter, NCC, in humans with Gitelman syndrome, leads to subtle salt wasting that is not associated with unremitting salt losses or even with easily perceptible extracellular fluid volume depletion, although the blood pressure is slightly low.57
The phenomenon of salt wasting resulting from CKD was first described by Peters.58 Later, the term salt wasting nephritis was proposed to characterize a minority of patients with CKD who lose large amounts of NaCl in their urine.59 The majority of patients with CKD have only a modest tendency to waste salt, as indicated by the fact that they cannot reduce urinary NaCl excretion promptly during dietary salt restriction. Despite this, they often have ECF volume expansion, when consuming a ‘Western’ diet, as discussed above.
Several theories have been advanced to explain this salt wasting tendency. First, natriuresis may result from an increased osmotic load per nephron. The second involves compensatory adaptations from the reduced number of functioning nephrons. When the number of nephrons is reduced, the single nephron glomerular filtration rate of the remaining nephrons increases. An increased single nephron glomerular filtration rate increases sodium delivery to the proximal tubule, which increases sodium reabsorption along the nephron (the phenomenon of glomerulotubular balance). Although glomerulotubular balance tends to maintain urinary salt excretion within the normal range, it cannot compensate fully for the rise in sngfr, so some excess of sodium may escape reabsorption.
A third mechanism involves damage to kidney tubules leading to defective salt transport. This mechanism is probably most prominent when massive salt wasting (see below) results from tubulointerstitial or medullary cystic disease and can resemble Addison’s disease.59 According to Bricker and colleagues,9 many cases of salt wasting associated with CKD are reversible, if the dietary salt deprivation is imposed gradually. This type of salt-wasting tendency likely contributes to the predisposition to AKI in patients with CKD. Whereas mild sudden ECF volume depletion can elicit prompt sodium chloride conservation in normal settings, the patient with CKD will be more susceptible to AKI because NaCl excretion will persist for longer. This phenomenon also has implications for treatment with dietary salt restriction. While a NaCl restricted diet is recommended for CKD, it may be useful to implement this gradually, to avoid worsening of kidney function.
Massive salt wasting is a rare, but devastating complication of CKD that can lead to cardiovascular collapse and death. In contrast to the mild salt wasting described above, patients with this disorder waste salt even when dietary salt is high. Thorn and colleagues60 described two patients who presented with progressive volume depletion, hemoconcentration, lassitude, and eventually shock. The patients were shown to have CKD and massive salt wasting. The adrenal glands were normal, and adrenal hormone replacement was not effective. The authors coined the term ‘salt losing nephritis’ to describe this syndrome. Enticknap found that chronic interstitial nephritis disease, primarily of the renal medulla, was responsible for most cases of massive salt wasting.61 Many patients also demonstrate cystic changes in the renal medulla, although the inherited medullary cystic disease can present in a very similar manner and might be confused with this presentation clinically. Patients with salt-wasting typically present with weakness and tiredness, together with polyuria and nocturia. Hundreds of millimoles of NaCl may be lost in the urine daily.62
Many patients with massive salt wasting and renal failure have tubulointerstitial disease, as noted above, and often medullary calcifications. Among the disorders reported to lead to salt wasting are the milk-alkali syndrome and hyperparathyroidism salt wasting.63 Patients with the milk-alkali syndrome can develop profound extracellular fluid volume depletion owing to salt wasting, but renal salt wasting often improves following correction of the alkalosis and hypercalcemia. Thus, once the calcium and acid/base disorder are corrected, the patients will retain salt normally, falsely suggesting that depletion of the extracellular fluid volume did not result from renal salt wasting. Other interstitial renal diseases also lead to salt wasting on occasion. These include multiple myeloma,64 analgesic nephropathy,65 and amyloidosis.64 Many of these have been associated with nephrocalcinosis.
Practical Approach to Treatment of Salt Retention in CKD
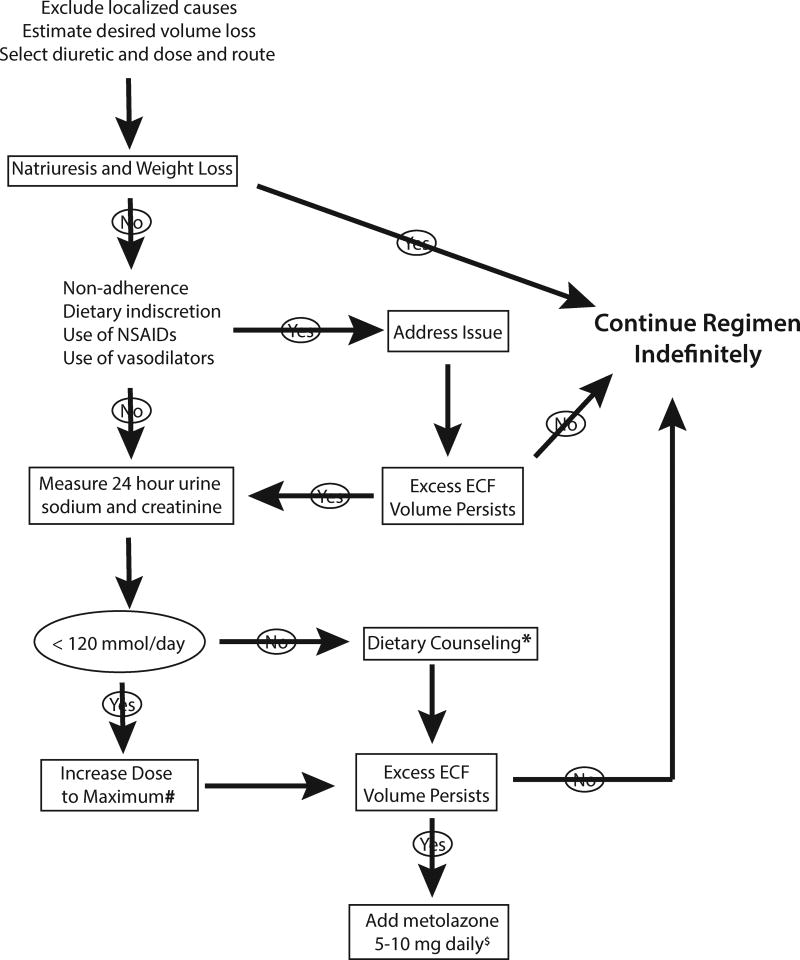
When a patient with CKD presents with signs and symptoms of ECF volume expansion, it is always important to determine first whether nephrotic syndrome is contributing. Nephrotic patients often tolerate aggressive diuresis quite well, especially if they appeared to be ‘overfilled’, as noted above. Most clinicians introduce a loop diuretic, as first line treatment for ECF volume expansion in CKD, although a thiazide may be a reasonable choice in the setting of hypertension. Starting doses of 40–80 mg twice daily are reasonable for patients with stages 3 and 4 CKD, with dose escalation thereafter.66 Because both CKD and nephrotic syndrome are diuretic resistant states, the most frequent error in treating such patients is using a dose that is too low. Many clinicians use furosemide, although pharmacokinetic considerations, including longer half-life and better bioavailability, suggest that torsemide should be preferred. Based on the short half-life, most patients should receive the drug twice daily.
While equipotent doses of bumetanide to furosemide are typically cited as 1:40 in patients with normal kidney function, the ratio declines to 1:20, as a result of non-renal bumetanide clearance, in the presence of stage 4–5 CKD.67 Many patients, especially if previously untreated, will experience a gratifying natriuresis and a return of the ECF volume towards normal, when diuretics are initiated. An approach for those who do not is provided in Figure 5. As noted, this includes dose escalation. A maximal oral dose of furosemide is 160–320 mg, depending on the severity of the CKD, but many clinicians would add second line agents before raising the dose this far.
Figure 5. Algorithm for diuretic treatment in CKD.
* indicates that dietary counseling may not be effective in reducing sodium intake, in which case more aggressive diuretic approaches are warranted. # indicates that dose ranges for loop diuretics are given in in the text. $ indicates that other thiazides are also effective, but metolazone is often selected as first line treatment.
As shown on Figure 5, an important tool can be the measurement of urinary Na+ excretion during 24 hours (with creatinine collected to confirm the collection adequacy). This will indicate both that the patient is responding adequately to the diuretic, and that he or she is ingesting too much salt, when the value exceeds 120 mmol/day (which is equivalent to 2.8 grams of sodium). The treatment in this case is to reduce dietary salt intake. While adhering to strict sodium restriction is not always achievable, this simple ‘biofeedback’ can prove helpful.
When maximal doses of loop diuretics still are not effective, most physicians add a thiazide type drug. Metolazone has been popular in this setting, and data from several trials in heart failure argue for its effectiveness.68 Yet there is also evidence that other thiazides are effective, as well.27 Continued resistance in this setting often suggests further diagnostic evaluation, and may be an indication to initiate dialysis.
Clinical Summary.
Chronic kidney disease is most commonly associated with expansion of the extracellular fluid volume, which typically contributes to hypertension.
Loop diuretics are often required to reduce extracellular fluid volume and correct hypertension, but thiazide and thiazide-like diuretics may be more useful than previously appreciated.
Nephrotic syndrome presents additional challenges to diuretic treatment, owing to the hypoalbuminemia and albuminuria
Acknowledgments
The author has received support from the following sources for some of this work: NIDDK R01 DK051496, R01DK054983, and VA Merit Review I01 BX002228-02.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author declares that there is no conflict of interest.
References
- 1.Lerchl K, Rakova N, Dahlmann A, et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension. 2015;66:850–7. doi: 10.1161/HYPERTENSIONAHA.115.05851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titze J. A different view on sodium balance. Current opinion in nephrology and hypertension. 2015;24:14–20. doi: 10.1097/MNH.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 3.Schneider MP, Raff U, Kopp C, et al. Skin Sodium Concentration Correlates with Left Ventricular Hypertrophy in CKD. Journal of the American Society of Nephrology : JASN. 2017 doi: 10.1681/ASN.2016060662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakova N, Juttner K, Dahlmann A, et al. Long-term space flight simulation reveals infradian rhythmicity in human Na(+) balance. Cell Metab. 2013;17:125–31. doi: 10.1016/j.cmet.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Titze J, Dahlmann A, Lerchl K, et al. Spooky sodium balance. Kidney international. 2014;85:759–67. doi: 10.1038/ki.2013.367. [DOI] [PubMed] [Google Scholar]
- 6.Guyton AC. Blood pressure control-Special role of the kidneys and body fluids. Science. 1991;252:1813–6. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 7.Epstein M, Hollenberg NK. Age as a determinant of renal sodium conservation in normal man. J Lab Clin Med. 1976 Mar;87:411–7. [PubMed] [Google Scholar]
- 8.Ellison DH. Disorders of sodium balance. In: Berl T, editor. Atlas of Diseases of the Kidney. Philadelphia: Blackwell Science; 1998. pp. 2.1–2.22. [Google Scholar]
- 9.Danovitch GM, Bourgoignie J, Bricker NS. Reversibility of the 'salt-losing' tendency of chronic renal failure. NEnglJMed. 1977;296:14–9. doi: 10.1056/NEJM197701062960104. [DOI] [PubMed] [Google Scholar]
- 10.Vallon V, Rieg T, Ahn SY, Wu W, Eraly SA, Nigam SK. Overlapping in vitro and in vivo specificities of the organic anion transporters OAT1 and OAT3 for loop and thiazide diuretics. American journal of physiology Renal physiology. 2008;294:F867–73. doi: 10.1152/ajprenal.00528.2007. [DOI] [PubMed] [Google Scholar]
- 11.Shankar SS, Brater DC. Loop diuretics: from the Na-K-2Cl transporter to clinical use. American journal of physiology Renal physiology. 2003;284:F11–21. doi: 10.1152/ajprenal.00119.2002. [DOI] [PubMed] [Google Scholar]
- 12.Felker GM, Lee KL, Bull DA, et al. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blumberg A, Nelp WB, Hegstrom RM, Scribner BH. Extracellular volume in patients with chronic renal disease treated for hypertension by sodium restriction. Lancet. 1967;2:69–73. doi: 10.1016/s0140-6736(67)92061-2. [DOI] [PubMed] [Google Scholar]
- 14.McMahon EJ, Bauer JD, Hawley CM, et al. A randomized trial of dietary sodium restriction in CKD. Journal of the American Society of Nephrology : JASN. 2013;24:2096–103. doi: 10.1681/ASN.2013030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essig M, Escoubet B, de Zuttere D, et al. Cardiovascular remodelling and extracellular fluid excess in early stages of chronic kidney disease. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2008;23:239–48. doi: 10.1093/ndt/gfm542. [DOI] [PubMed] [Google Scholar]
- 16.Zoccali C, Benedetto FA, Mallamaci F, et al. Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney international. 2004;65:1492–8. doi: 10.1111/j.1523-1755.2004.00530.x. [DOI] [PubMed] [Google Scholar]
- 17.Verbeke F, Lindley E, Van Bortel L, et al. A European Renal Best Practice (ERBP) position statement on the Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for the management of blood pressure in non-dialysis-dependent chronic kidney disease: an endorsement with some caveats for real-life application. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2014;29:490–6. doi: 10.1093/ndt/gft321. [DOI] [PubMed] [Google Scholar]
- 18.Vasavada N, Agarwal R. Role of excess volume in the pathophysiology of hypertension in chronic kidney disease. Kidney international. 2003;64:1772–9. doi: 10.1046/j.1523-1755.2003.00273.x. [DOI] [PubMed] [Google Scholar]
- 19.Sica DA. Diuretic use in renal disease. Nat Rev Nephrol. 2011;8:100–9. doi: 10.1038/nrneph.2011.175. [DOI] [PubMed] [Google Scholar]
- 20.Schreiner GE. Chlorothiazide in renal disease. Ann N Y Acad Sci. 1958;71:420–9. doi: 10.1111/j.1749-6632.1958.tb46769.x. [DOI] [PubMed] [Google Scholar]
- 21.Reubi FC, Cottier PT. Effects of reduced glomerular filtration rate on responsiveness to chlorothiazide and mercurial diuretics. Circulation. 1961;23:200–10. doi: 10.1161/01.cir.23.2.200. [DOI] [PubMed] [Google Scholar]
- 22.Sinha AD, Agarwal R. Thiazides are useful agents in CKD. Journal of the American Society of Hypertension : JASH. 2016;10:288–9. doi: 10.1016/j.jash.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Sinha AD, Agarwal R. Thiazide Diuretics in Chronic Kidney Disease. Current hypertension reports. 2015;17:13. doi: 10.1007/s11906-014-0525-x. [DOI] [PubMed] [Google Scholar]
- 24.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults: Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2013 doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Sinha AD, Pappas MK, Ammous F. Chlorthalidone for poorly controlled hypertension in chronic kidney disease: an interventional pilot study. American journal of nephrology. 2014;39:171–82. doi: 10.1159/000358603. [DOI] [PubMed] [Google Scholar]
- 26.Subramanya AR, Ellison DH. Distal convoluted tubule. Clinical journal of the American Society of Nephrology : CJASN. 2014;9:2147–63. doi: 10.2215/CJN.05920613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fliser D, Schröter M, Neubeck M, Ritz E. Coadministration of thiazides increases the efficacy of loop diuretics even in patients with advanced renal failure. Kidney Int. 1994;46:482–8. doi: 10.1038/ki.1994.298. [DOI] [PubMed] [Google Scholar]
- 28.Schrier RW, Gurevich AK, Cadnapaphornchai MA. Pathogenesis and management of sodium and water retention in cardiac failure and cirrhosis. Seminars in nephrology. 2001;21:157–72. doi: 10.1053/snep.2001.20933. [DOI] [PubMed] [Google Scholar]
- 29.Afsar B, Rossignol P, van Heerebeek L, et al. Heart failure with preserved ejection fraction: a nephrologist-directed primer. Heart Fail Rev. 2017 doi: 10.1007/s10741-017-9619-2. [DOI] [PubMed] [Google Scholar]
- 30.Orth SR, Ritz E. The nephrotic syndrome. The New England journal of medicine. 1998;338:1202–11. doi: 10.1056/NEJM199804233381707. [DOI] [PubMed] [Google Scholar]
- 31.Schrier RW, Fassett RG. A critique of the overfill hypothesis of sodium and water retention in the nephrotic syndrome. Kidney international. 1998 May;53:1111–7. doi: 10.1046/j.1523-1755.1998.00864.x. [DOI] [PubMed] [Google Scholar]
- 32.Bircan Z, Kervancioglu M, Katar S, Vitrinel A. Does albumin and furosemide therapy affect plasma volume in nephrotic children? Pediatr Nephrol. 2001;16:497–9. doi: 10.1007/s004670100576. [DOI] [PubMed] [Google Scholar]
- 33.Meltzer JI, Keim HJ, Laragh JH, Sealey JE, Jan KM, Chien S. Nephrotic syndrome: vasoconstriction and hypervolemic types indicated by renin-sodium profiling. Annals of internal medicine. 1979;91:688–96. doi: 10.7326/0003-4819-91-5-688. [DOI] [PubMed] [Google Scholar]
- 34.Ebah LM, Wiig H, Dawidowska I, et al. Subcutaneous interstitial pressure and volume characteristics in renal impairment associated with edema. Kidney international. 2013;84:980–8. doi: 10.1038/ki.2013.208. [DOI] [PubMed] [Google Scholar]
- 35.Bernard DB, Alexander EA, Couser WG, Levinsky NG. Renal sodium retention during volume expansion in experimental nephrotic syndrome. Kidney international. 1978;14:478–85. doi: 10.1038/ki.1978.152. [DOI] [PubMed] [Google Scholar]
- 36.Kirchner KA, Voelker JR, Brater DC. Intratubular albumin blunts the response to furosemide-A mechanism for diuretic resistance in the nephrotic syndrome. JPharmacolExpTher. 1990;252:1097–101. [PubMed] [Google Scholar]
- 37.Ichikawa I, Rennke HG, Hoyer JR, et al. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephrotic syndrome. J Clin Invest. 1983;71:91–103. doi: 10.1172/JCI110756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Humphreys MH. Mechanisms and management of nephrotic edema. Kidney international. 1994;45:266–81. doi: 10.1038/ki.1994.33. [DOI] [PubMed] [Google Scholar]
- 39.Brown EA, Sagnella G, Jones BE, Markandu ND, MacGregor GA. Evidence that some mechanism other than the renin system causes sodium retention in nephrotic syndrome. Lancet. 1982:1237–40. doi: 10.1016/s0140-6736(82)90102-7. [DOI] [PubMed] [Google Scholar]
- 40.Andersen H, Friis UG, Hansen PB, Svenningsen P, Henriksen JE, Jensen BL. Diabetic nephropathy is associated with increased urine excretion of proteases plasmin, prostasin and urokinase and activation of amiloride-sensitive current in collecting duct cells. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30:781–9. doi: 10.1093/ndt/gfu402. [DOI] [PubMed] [Google Scholar]
- 41.Svenningsen P, Friis UG, Versland JB, et al. Mechanisms of renal NaCl retention in proteinuric disease. Acta Physiol (Oxf) 2013;207:536–45. doi: 10.1111/apha.12047. [DOI] [PubMed] [Google Scholar]
- 42.Svenningsen P, Bistrup C, Friis UG, et al. Plasmin in nephrotic urine activates the epithelial sodium channel. Journal of the American Society of Nephrology : JASN. 2009;20:299–310. doi: 10.1681/ASN.2008040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kapur G, Valentini RP, Imam AA, Mattoo TK. Treatment of severe edema in children with nephrotic syndrome with diuretics alone--a prospective study. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:907–13. doi: 10.2215/CJN.04390808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pichette V, Geadah D, du Souich P. The influence of moderate hypoalbuminaemia on the renal metabolism and dynamics of furosemide in the rabbit. Br J Pharmacol. 1996 Nov;119:885–90. doi: 10.1111/j.1476-5381.1996.tb15755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirchner KA, Voelker JR, Brater DC. Tubular resistance to furosemide contributes to the attenuated diuretic response in nephrotic rats. Journal of the American Society of Nephrology : JASN. 1992;2:1201–7. doi: 10.1681/ASN.V271201. [DOI] [PubMed] [Google Scholar]
- 46.Keller E, Hoppe-Seyler G, Schollmeyer P. Disposition and diuretic effect of furosemide in the nephrotic syndrome. Clin Pharmacol Ther. 1982;32:442–9. doi: 10.1038/clpt.1982.187. [DOI] [PubMed] [Google Scholar]
- 47.Sjöström PA, Odlind BG, Beermann BA, Karlberg BE. Pharmacokinetics and effects of frusemide in patients with the nephrotic syndrome. EurJClinPharmacol. 1989;37:173–80. doi: 10.1007/BF00558227. [DOI] [PubMed] [Google Scholar]
- 48.Inoue M, Okajima K, Itoh K, et al. Mechanism of furosemide resistance in analbuminemic rats and hypoalbuminemic patients. Kidney Int. 1987;32:198–203. doi: 10.1038/ki.1987.192. [DOI] [PubMed] [Google Scholar]
- 49.Akcicek F, Yalniz T, Basci A, Ok E, Dorhut Mees EJ. Diuretic effect of frusemide in patients with nephrotic syndrome: is it potentiated by albumin? BMJ. 1995;310:162–3. doi: 10.1136/bmj.310.6973.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chalasani N, Gorski JC, Horlander JC, Sr, et al. Effects of albumin/furosemide mixtures on responses to furosemide in hypoalbuminemic patients. Journal of the American Society of Nephrology : JASN. 2001;12:1010–6. doi: 10.1681/ASN.V1251010. [DOI] [PubMed] [Google Scholar]
- 51.Fliser D, Zurbruggen I, Mutschler E, et al. Coadministration of albumin and furosemide in patients with the nephrotic syndrome [In Process Citation] Kidney international. 1999;55:629–34. doi: 10.1046/j.1523-1755.1999.00298.x. [DOI] [PubMed] [Google Scholar]
- 52.Kitsios GD, Mascari P, Ettunsi R, Gray AW. Co-administration of furosemide with albumin for overcoming diuretic resistance in patients with hypoalbuminemia: a meta-analysis. J Crit Care. 2014;29:253–9. doi: 10.1016/j.jcrc.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 53.Agarwal R, Gorski JC, Sundblad K, Brater DC. Urinary protein binding does not affect response to furosemide in patients with nephrotic syndrome. Journal of the American Society of Nephrology : JASN. 2000 Jun;11:1100–5. doi: 10.1681/ASN.V1161100. [DOI] [PubMed] [Google Scholar]
- 54.Esnault VL, Ekhlas A, Delcroix C, Moutel MG, Nguyen JM. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. Journal of the American Society of Nephrology : JASN. 2005;16:474–81. doi: 10.1681/ASN.2004060505. [DOI] [PubMed] [Google Scholar]
- 55.Green TP, Mirkin BL. Furosemide disposition in normal and proteinuric rats: urinary drug-protein bindings as a determinant of drug excretion. JPharmacolExpTher. 1981;218:122–7. [PubMed] [Google Scholar]
- 56.Kirchner KA, Voelker JR, Brater DC. Binding inhibitors restore furosemide potency in tubule fluid containing albumin. Kidney international. 1991;40:418–24. doi: 10.1038/ki.1991.228. [DOI] [PubMed] [Google Scholar]
- 57.Cruz DN, Simon DB, Nelson-Williams C, et al. Mutations in the Na-Cl cotransporter reduce blood pressure in humans. Hypertension. 2001;37:1458–64. doi: 10.1161/01.hyp.37.6.1458. [DOI] [PubMed] [Google Scholar]
- 58.Peters JP, Wakeman AM, Lee C. Total acid-base equilibrium of plasma in health and disease. JClinInvest. 1929;6:551–75. doi: 10.1172/JCI100211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sawyer WH, Solez C. Salt losing nephritis simulating adrenocortical insufficiency. New Engl J Med. 1949;240:210–5. doi: 10.1056/NEJM194902102400603. [DOI] [PubMed] [Google Scholar]
- 60.Thorn GW, Koepf GF, Clinton M., Jr Renal failure simulating adrenocortical insufficiency. New Engl J Med. 1944;231:76–85. [Google Scholar]
- 61.Enticknap JB. The condition on the kidneys in salt-losing nephritis. Lancet. 1952;2:458–61. doi: 10.1016/s0140-6736(52)90245-6. [DOI] [PubMed] [Google Scholar]
- 62.Stanbury SW, Mahler RF. Salt-wasting renal disease. Q J Med. 1959;28:425–47. [PubMed] [Google Scholar]
- 63.Bell NH, Del Greco F, Colwell JA. Primary hyperparathyroidism and salt-wasting nephropathy. J Chronic Dis. 1975 Dec;28:601–7. doi: 10.1016/0021-9681(75)90073-9. [DOI] [PubMed] [Google Scholar]
- 64.Kahn T, Levitt MF. Salt wasting in myeloma. Archives of internal medicine. 1970 Oct;126:664–7. [PubMed] [Google Scholar]
- 65.Cove-Smith JR, Knapp MS. Sodium handling in analgesic nephropathy. Lancet. 1973 Jul 14;2:70–2. doi: 10.1016/s0140-6736(73)93263-7. [DOI] [PubMed] [Google Scholar]
- 66.Brater DC. Diuretic therapy. N Engl J Med. 1998;339:387–95. doi: 10.1056/NEJM199808063390607. [DOI] [PubMed] [Google Scholar]
- 67.Voelker JR, Cartwright-Brown D, Anderson S, et al. Comparison of loop diuretics in patients with chronic renal insufficiency. Kidney Int. 1987;32:572–8. doi: 10.1038/ki.1987.246. [DOI] [PubMed] [Google Scholar]
- 68.Grodin JL, Stevens SR, de Las Fuentes L, et al. Intensification of Medication Therapy for Cardiorenal Syndrome in Acute Decompensated Heart Failure. J Card Fail. 2016;22:26–32. doi: 10.1016/j.cardfail.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walser M. Phenomenological analysis of renal regulation of sodium and potassium balance. Kidney Int. 1985;27:837–41. doi: 10.1038/ki.1985.89. [DOI] [PubMed] [Google Scholar]