Abstract
Purpose
Clinical studies of breast radiation therapy typically assess breast cosmetic outcome using subjective scales which may lack reproducibility or ability to discriminate subtle changes. To overcome these limitations, quantitative measures of breast cosmesis have been proposed, but their clinical relevance remains unproven. We applied quantitative analysis of digital photographs to measure breast cosmetic outcome within the setting of a randomized trial of conventionally fractionated (CF) and hypofractionated (HF) whole breast irradiation (WBI). Our goals were to identify how quantitative cosmesis metrics were associated with patient- and physician-reported cosmesis and whether they differed by treatment arm.
Materials/Methods
From 2011–2014, 287 women age≥40 with DCIS or early invasive breast cancer were randomized to HF-WBI (42.56Gy/16fx+10-12.5Gy/4-5fx boost) or CF-WBI (50Gy/25fx+10-14Gy/5-7fx). At one year post-treatment, we collected digital photographs, patient-reported cosmesis using the Breast Cancer Treatment and Outcomes Scale (BCTOS), and physician-reported cosmesis using the Radiation Therapy Oncology Group (RTOG) scale. Six quantitative measures of breast symmetry, labeled M1-M6, were calculated from anteroposterior digital photographs. For each measure, values closer to 1 imply greater symmetry, and values closer to 0 imply greater asymmetry. Associations between M1-M6 and patient- and physician-reported cosmesis and treatment arm were evaluated using the Kruskal-Wallis test.
Results
Among 245 evaluable patients, patient-reported cosmesis was strongly associated with M1 (vertical symmetry measure) (P<0.01). Physician-reported cosmesis was similarly correlated with M1 (P<0.01) and also with M2 (vertical symmetry, P=0.01) and M4 (horizontal symmetry, P=0.03). At one-year post-treatment, HF-WBI resulted in better values of M2 (P=0.02) and M3 (P<0.01) than CF-WBI; treatment arm was not significantly associated with M1, M4, M5, or M6 (P≥0.12).
Conclusions
Quantitative assessment of breast photographs reveals similar to improved cosmetic outcome with HF-WBI compared to CF-WBI one year after treatment. Assessing cosmetic outcome using these measures could be useful for future comparative effectiveness studies and outcome reporting.
Keywords: Radiation, breast, cosmesis, hypofractionation
Introduction
Whole breast irradiation (WBI) is a standard of care following breast conserving surgery (BCS) for both ductal carcinoma in situ (DCIS) and early invasive breast cancer. While WBI achieves the dual goal of optimizing oncologic outcomes and facilitating breast preservation [1], it may negatively impact the cosmetic outcome of the treated breast, thereby degrading quality of life [2]. A rigorous, quantitative method for assessing the impact of WBI on cosmetic outcome could facilitate comparison of different approaches to WBI and thus promote optimization of WBI dosing and techniques. Yet to date, cosmetic assessment of the radiated breast has relied primarily on subjective physician assessment, which is prone to meaningful inter- and intra-observer variability [3–6].
To advance understanding of post-radiation breast cosmetic outcome, many quantitative measures of breast symmetry have been proposed [7–11]. These include measures of the symmetry of the breast mound, nipple-areolar complex, and inframammary sulcus. Many of these measures are correlated with subjective assessment of cosmetic outcome by an independent observer [12–14]. An additional quantitative measure has been developed that provides a composite score of breast cosmesis, termed the BCCT.core, and is based on objective measures of color and scar appearance in addition to symmetry [15]. The BCCT.core was shown to have fair correlation with both independent observer assessment [16] and patient assessment [17]. Yet despite the number of studies and their proposed objective parameters, the clinical relevance of these parameters relative to patient satisfaction and quality of life remains unclear. Furthermore, the specific measures of symmetry that have the strongest association with, and therefore the highest relevance to, patient-reported cosmetic assessment have yet to be identified.
We sought to address this knowledge gap within the context of a prospective clinical trial that randomized patients with DCIS or early invasive breast cancer to conventionally fractionated whole breast irradiation (CF-WBI) (50 Gy in 25 fractions + 10–14 Gy boost in 5–7 fractions) or hypofractionated whole breast irradiation (HF-WBI) (42.56 Gy in 16 fractions + 10–12.5 Gy in 4–5 fractions). One year after completing radiation, we obtained 2D photographs and collected patient- and physician-reported cosmetic outcome. The goal of this study was twofold. First, we sought to determine which quantitative measures of cosmetic outcome derived from 2D photographs were the most clinically relevant due to associations with patient- or physician-reported outcomes. Second, we assessed whether quantitative measures of cosmetic outcome varied by treatment arm.
Materials and Methods
Patient cohort
Between March 2011 and February 2014, 287 women age ≥ 40 years with pathologically-confirmed DCIS or early invasive breast cancer (pTis, pT1, pT2, pN0, or pN1) were enrolled after BCS. Patients were randomized to treatment with either HF-WBI or CF-WBI. Full details of patient characteristics, acute toxicity, and 6 month toxicity were recently reported [18]. A total of 258 patients (89.9%) remain in active follow up 18 months after study closure to new patient accrual.
Patient- and physician-reported cosmetic outcome
The Breast Cancer Treatment Outcomes Scale (BCTOS) [19] was administered using either paper or an electronic tablet during the follow up visit one year after completing radiation. The BCTOS cosmetic scale describes patient-reported changes in the treated breast and surrounding area relative to the contralateral breast which serves as an internal control. Items were scored on a 1 to 4 scale, with 1 indicating no difference between the treated breast and untreated contralateral breast, 2 indicating a slight difference, 3 indicating a modest difference, and 4 indicating a large difference. The BCTOS patient-reported cosmetic outcome score is the arithmetic mean of the nine items that assess cosmetic outcome. At the same follow up visit, cosmetic outcome was assessed by the treating physician using the RTOG scale [3]. Numerical outcome scoring for physician-reported cosmetic outcome was as follows: 1 – excellent, 2 – good, 3 – fair, and 4 – poor.
Quantitative measures of cosmesis derived from 2D photographs
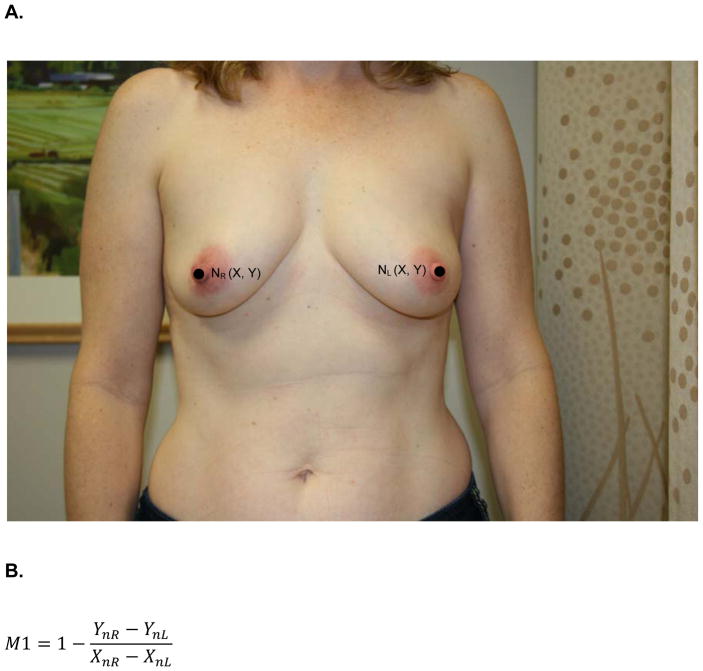
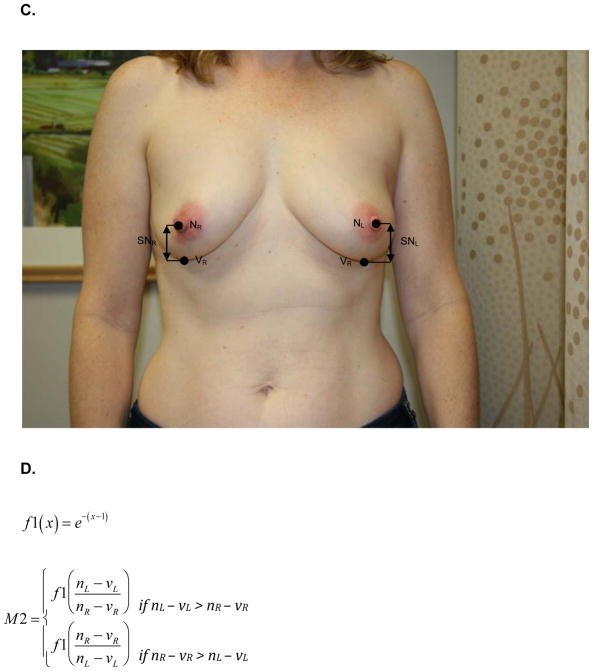
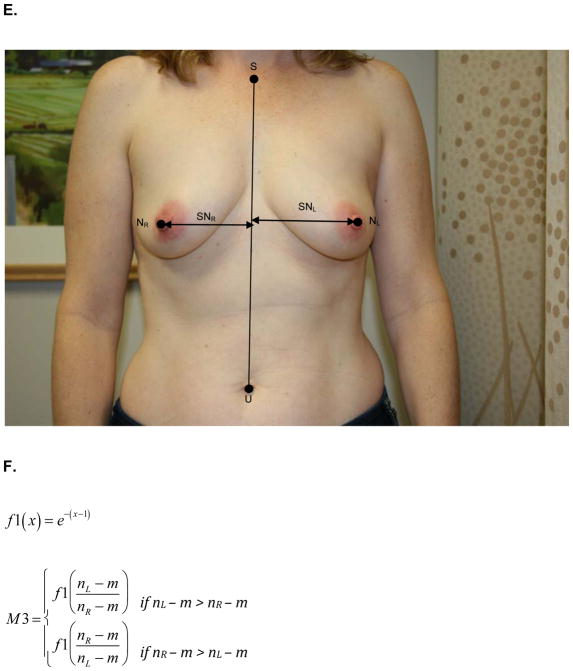
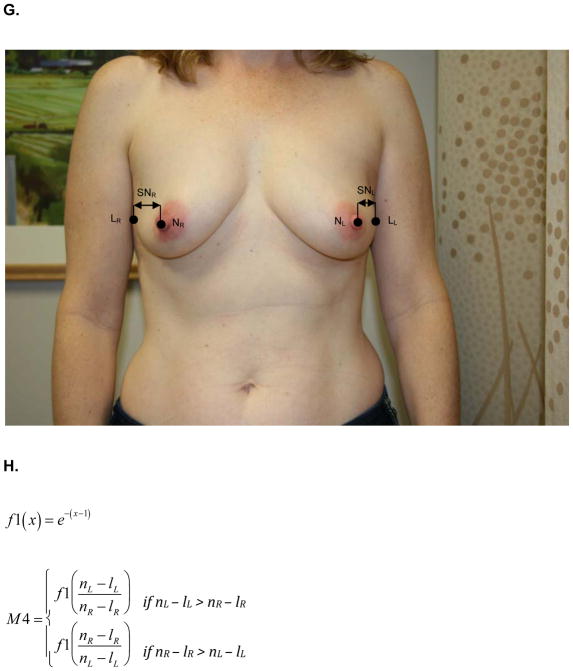
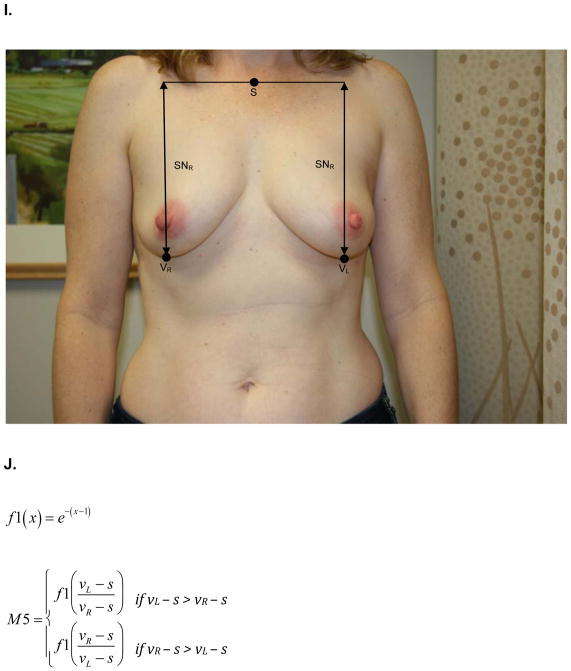
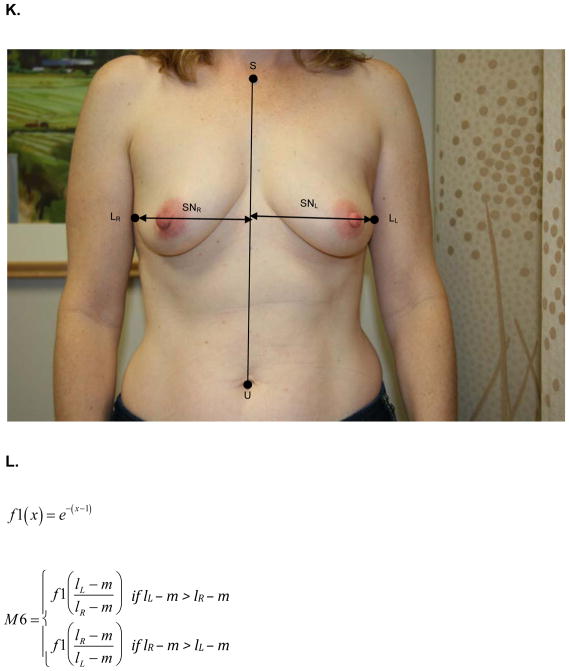
At the same one-year follow up visit, digital photographs were acquired according to criteria specified in the study protocol and supervised by the study PI. To minimize variability between patients, photographs were standardly framed from the low neck to the upper abdomen with arms at their sides. All jewelry was removed prior to photo acquisition. Two investigators (KMN/SCH), blinded to treatment arm, calculated six quantitative metrics from these photographs using MATLAB (MathWorks, Inc, Natick, MA). These measures are: M1, the vertical distance between the nipples relative to the horizontal distance between the nipples; M2, ratio of the vertical distances of the nipples from the most inferior point of the breast; M3, ratio of the horizontal distances of the nipples from the midline of the torso; M4, ratio of the horizontal distances of the nipples from the lateral aspects of the breasts; M5, ratio of the vertical distances of the most inferior points of the breasts from the sternal notch; and M6, ratio of the horizontal distances of the lateral aspects of the breasts from midline of the torso.. Values of M1-M6 closer to 1 indicate more symmetry while values closer to 0 indicate asymmetry. Figure 1 depicts the fiducial points and illustrate how each metric was calculated.
Figure 1.
A) M1- is the ratio of the difference between the vertical positions (Y) of each nipple (N) to the difference between the horizontal positions (X) of each nipple. Larger values near 1 indicate symmetry while smaller values near 0 indicate asymmetry. B) Formula for M1 calculation. C) M2 is a function of the ratio of the vertical displacements of the nipple centroids (N) from the level of the lowest visible point of the breast (V). M2 is bound between 0 and 1. Larger values near 1 indicate symmetry while smaller values near 0 indicate asymmetry. D) Formula for M2 calculation. E) M3 is a function of the ratio of the horizontal displacements of the nipple centroids (N) from midline. M3 is bound between 0 and 1. Larger values near 1 indicate symmetry while smaller values near 0 indicate asymmetry. F) Formula for M3 calculation. G) M4 is a function of the ratio of the horizontal displacements of the nipple centroids (N) from the lateral extent of the breast (L). M4 is bound between 0 and 1. Larger values near 1 indicate symmetry while smaller values near 0 indicate asymmetry. H) Formula for M4 calculation. I) M5 is a function of the ratio of the vertical displacements of the lowest visible points of the breast (V) from the level of sternal notch (S). M5 is bound between 0 and 1. Larger values near 1 indicate symmetry while smaller values near 0 indicate asymmetry. J) Formula for M5 calculation. K) M6 is a function of the ratio of the horizontal displacements of the lateral extents of the breast (L) from the midline. M6 is bound between 0 and 1. Larger values near 1 indicate symmetry while smaller values near 0 indicate asymmetry. L) Formula for M6 calculation.
Statistical methodology
For all analyses, patient-reported cosmetic outcome was grouped into tertiles based on the distribution of responses. For physician-reported cosmetic outcome, the categories of poor and fair were combined into a single category based on the distribution of responses. The relationship between patient- and physician-reported cosmetic outcome was evaluated using Spearman’s correlation coefficient.
The non-parametric Kruskal-Wallis tested the associations of M1-M6 with patient- and physician-reported cosmetic outcome. Recursive partitioning analysis was performed using the RPART package of R to identify clinically relevant cutpoints for quantitative metrics that were significantly associated with cosmetic outcomes in bivariable analyses. Associations of quantitative metrics with treatment arm were tested using the Kruskal-Wallis test for continuous metrics and the chi-square test for categorical metrics. Logistic regression models were used to assess the association of 2D photo measures with cutoff points on the patient- and physician-reported outcomes. The final logistical models included only the 2D photo measures with cutoffs that were of statistical significance. All statistical analyses were conducted using SAS Version 9.3 (Cary, NC) and R version 3.1.0 (the R Foundation for Statistical Computing).
Results
Patient- and Physician-reported cosmesis at one year
A CONSORT diagram describing the number of patients initially assessed for protocol eligibility, those patients registered for the protocol, the number of randomized patients, and the number of patients per randomization arm is shown in Supplemental Figure 1. As previously reported, baseline clinical-pathologic characteristics and patient- and physician-reported cosmetic outcome were well-balanced by treatment arm [18,20]. Of 287 patients randomized and eligible for evaluation, as previously described [18], patient- and physician-reported cosmetic outcome at 1 year post-treatment were available for 240 and 245 patients, respectively.
Regarding patient-reported cosmetic outcome, a BCTOS cosmesis score ranging from 1.0 to 1.5 defined the best tertile (n=84, 35%), a score of 1.5 to 1.9 defined the intermediate tertile (n=80, 33%), and a score of 1.9 to 3.4 defined the lowest tertile (n=76, 32%). The distribution of physician-reported cosmetic outcome was as follows: 88 patients (36%) excellent, 113 patients (46%) good, 41 patients (17%) fair, and 3 patients (1%) poor (Table 1). These two outcome measures were significantly correlated (Spearman coefficient=0.33; P<0.001).
Table 1.
Summary of patient- and physician-reported cosmetic outcomes and 2D photo measures
| N | % | Median | Min | Max | |
|---|---|---|---|---|---|
| Reported Cosmetic Outcome | |||||
| Patient-reported (BCTOS) | 240 | 1.71 | 1 | 3.43 | |
| 1–1.5 (good) | 84 | 35 | |||
| 1.5–1.9 | 80 | 33 | |||
| 1.9–3.4 (poor) | 76 | 32 | |||
| Physician-reported | 245 | ||||
| 1 (excellent) | 88 | 36 | |||
| 2 (good) | 113 | 46 | |||
| 3 (fair) | 41 | 17 | |||
| 4 (poor) | 3 | 1 | |||
| 2D Photo Measures | |||||
| M1 | 246 | 100.0 | 0.95 | 0.71 | 1.00 |
| M2 | 245 | 99.6 | 0.79 | 0.00 | 1.00 |
| M3 | 47 | 19.1 | 0.93 | 0.72 | 0.99 |
| M4 | 246 | 100.0 | 0.75 | 0.01 | 1.00 |
| M5 | 129 | 52.4 | 0.95 | 0.78 | 1.00 |
| M6 | 47 | 19.1 | 0.95 | 0.83 | 1.00 |
2D photo measures of symmetry and cosmetic outcome
Median values and ranges for M1-M6 are presented in Table 1. As illustrated in this table, M3 and M6 could only be calculated for 47 patients, because the umbilicus is used as a reference point in defining the midline of the torso for these measures and it was not readily visualized for 195 photos. Table 2 shows the distribution of 2D photo measures when stratified by patient- and physician-reported cosmetic outcome. One measure of the symmetry of the locations of the nipples (M1) was significantly correlated with both physician-reported cosmesis and patient-reported cosmesis (P<0.001 and P<0.001). One measure of the symmetry of the breast mounds (M5) was significantly correlated with physician-reported cosmesis (P<0.001) and trended toward correlation with patient-reported cosmetic outcome (P=0.14). Two additional measures of the symmetry of the locations of nipples (M2, M4) were significantly associated with physician-reported cosmesis (P = 0.01 and P=0.03, respectively), but not with patient-reported cosmesis. Neither M3 nor M6 were significantly associated with patient- or physician-reported cosmetic outcome (P>0.05, all comparisons). Figure 2 presents photographs of patients across a spectrum of values for M1.
Table 2.
2D Photo measures stratified by patient- and physician-reported cosmetic outcomes
| N | Median (IQR) | N | Median (IQR) | N | Median (IQR) | P value | |
|---|---|---|---|---|---|---|---|
| Patient-reported cosmetic outcome (BCTOS) | 1–1.5 (good) | 1.5–1.9 | 1.9–3.5 (poor) | ||||
| M1 | 84 | 0.96(0.93,0.98) | 80 | 0.95(0.91,0.97) | 76 | 0.93(0.89,0.96) | <0.001 |
| M2 | 83 | 0.83(0.59,0.91) | 80 | 0.82(0.59,0.92) | 76 | 0.76(0.54,0.89) | 0.29 |
| M3 | 15 | 0.93(0.89,0.97) | 14 | 0.92(0.83,0.97) | 17 | 0.94(0.87,0.98) | 0.73 |
| M4 | 84 | 0.77(0.59,0.86) | 80 | 0.69(0.49,0.87) | 76 | 0.77(0.59,0.91) | 0.26 |
| M5 | 39 | 0.97(0.93,0.98) | 45 | 0.95(0.92,0.97) | 40 | 0.95(0.91,0.97) | 0.14 |
| M6 | 15 | 0.94(0.9,0.97) | 14 | 0.95(0.94,0.98) | 17 | 0.97(0.93,0.98) | 0.34 |
| Physician-reported cosmetic outcome | 1 (excellent) | 2 (good) | 3–4 (poor) | ||||
| M1 | 88 | 0.96(0.94,0.98) | 113 | 0.95(0.91,0.98) | 44 | 0.91(0.86,0.94) | <0.001 |
| M2 | 87 | 0.82(0.62,0.91) | 113 | 0.8(0.57,0.93) | 44 | 0.7(0.21,0.85) | 0.01 |
| M3 | 23 | 0.95(0.89,0.98) | 16 | 0.89(0.83,0.95) | 8 | 0.94(0.91,0.97) | 0.11 |
| M4 | 88 | 0.8(0.65,0.92) | 113 | 0.73(0.53,0.86) | 44 | 0.69(0.47,0.86) | 0.03 |
| M5 | 47 | 0.97(0.94,0.98) | 60 | 0.95(0.92,0.98) | 22 | 0.92(0.89,0.95) | <0.001 |
| M6 | 23 | 0.96(0.93,0.98) | 16 | 0.94(0.91,0.97) | 8 | 0.95(0.93,0.97) | 0.48 |
Figure 2.
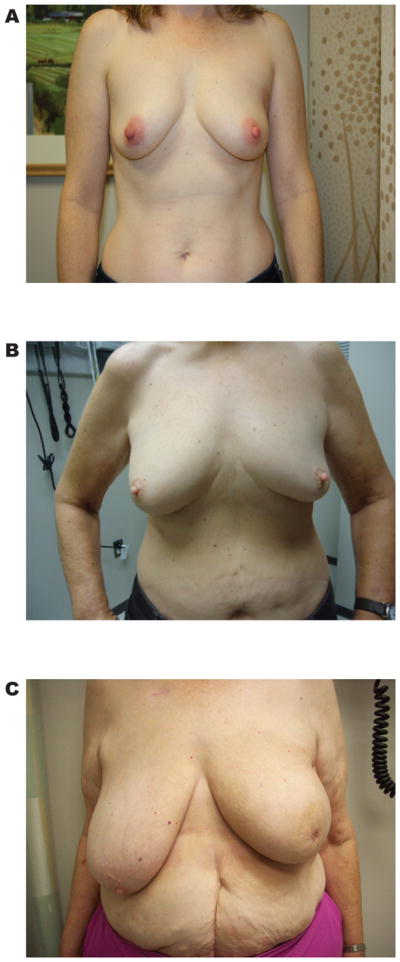
Photographs of patients at the one-year follow up visit across a spectrum of values for M1. Panel A, a patient with a high value of M1 (M1=0.99). Panel B, a patient with an intermediate value of M1 (M1=0.93). Panel C, a patient with the lowest value of M1 in the cohort (M1=0.71).
Defining clinically relevant cutpoints for 2D photo measures
Defining clinically relevant cutpoints for continuous outcomes can facilitate outcome measurement and reporting. Accordingly, we employed a recursive partitioning approach to define such cutpoints for the quantitative metrics most strongly correlated with patient- and physician-reported cosmetic outcome, M1, M2, and M4. M5 could not be obtained for the majority of patients due to the absence of some necessary fiducial points and it bears strong correlation with M1 and M2. As a result, M5 was not included in the subsequent analyses. For M1, this approach yielded a cutpoint of 0.94 for discriminating patient-reported cosmetic outcome and 0.87 for physician-reported outcome. For M2 and M4, this approach yielded cutpoints of 0.83 and 0.90, respectively, for discriminating physician-reported cosmetic outcome.
We then used logistic regression modeling to assess the significance of these cutpoints on both patient- and physician-reported outcomes. M1 ≥ 0.94 continued to be significantly associated with better patient-reported cosmetic outcome scores on multivariable analysis (Table 3). With respect to physician-reported cosmesis, both M1 ≥ 0.87 and M4 ≥ 0.90 were significantly associated better outcomes on multivariable analysis. However, M2 was no longer significantly associated with physician-reported cosmesis and M2 cutpoints were not used for comparisons between randomization arms. Mean patient- and physician-reported outcome values for patients with M1 and M4 values above and below these cutpoints are presented in Supplemental Table 1.
Table 3.
Multivariable ordinal logistic model for cumulative probability for better patient- and physician-reported cosmetic outcomes
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Reported Cosmetic Outcome | |||
| Patient-reported (BCTOS) | |||
| M1 < 0.94 | 1 | ||
| M1 ≥ 0.94 | 3.45 | 2.09–5.71 | <0.001 |
| Physician-reported | |||
| M1 < 0.87 | 1 | ||
| M1 ≥ 0.87 | 8.57 | 3.57–20.5 | <0.001 |
| M2 < 0.83 | 1 | 17 | |
| M2 ≥ 0.83 | 1.17 | 0.71–1.93 | 0.53 |
| M4 < 0.90 | |||
| M4 ≥ 0.90 | 2.02 | 1.11–3.68 | 0.02 |
Comparison of 2D photo measures between randomization arms
Following the identification of relevant 2D photo measures and their optimal cutpoints, we next examined distribution of these metrics between randomization arms in the clinical trial. Median values of M1-6 stratified by treatment arm are listed in Table 4. At one year after radiation, there was no statistically significant difference by treatment arm in the values of M1 and M4-M6 when treated as continuous variables or in the values of M1 or M4 when dichotomized at previously defined cutpoints (P>0.05 for all). However, median values of both M2 and M3 were significantly higher in the HF-WBI arm as compared to the CF-WBI arm (M2: 0.84 vs 0.75, P=0.02; M3: 0.97 vs 0.89, P<0.01).
Table 4.
2D Photo measures stratified by treatment arm
| CF-WBI | HF-WBI | ||||
|---|---|---|---|---|---|
| 2D Photo Measure - continuous | N | Median (IQR) | N | Median (IQR) | P value |
| M1 | 130 | 0.95 (0.91,0.98) | 110 | 0.95 (0.9,0.97) | 0.53 |
| M2 | 129 | 0.75 (0.53,0.89) | 110 | 0.84 (0.63,0.94) | 0.02 |
| M3 | 23 | 0.89 (0.83,0.94) | 23 | 0.97 (0.93,0.98) | <0.01 |
| M4 | 130 | 0.73 (0.5,0.87) | 110 | 0.77 (0.6,0.9) | 0.12 |
| M5 | 67 | 0.95 (0.93,0.98) | 57 | 0.95 (0.91,0.98) | 0.37 |
| M6 | 23 | 0.95 (0.93,0.98) | 23 | 0.95 (0.9,0.99) | 0.82 |
| 2D Photo Measure – dichotomized | N | % | N | % | P value |
| M1 < 0.87 | 13 | 50 | 13 | 50 | |
| M1 ≥ 0.87 | 122 | 55.5 | 98 | 44.5 | 0.60 |
| M1 < 0.94 | 59 | 54.6 | 49 | 45.4 | |
| M1 ≥ 0.94 | 76 | 55.1 | 62 | 44.9 | 0.94 |
| M4 < 0.90 | 110 | 57 | 83 | 43 | |
| M4 ≥ 0.890 | 25 | 47.2 | 28 | 52.8 | 0.20 |
Discussion
Using 2D digital photographs acquired as part of a prospective clinical trial comparing CF-WBI to HF-WBI, we found that several quantitative measures of symmetry were strongly correlated with cosmetic outcome one year after completing radiation therapy. For example, increasing values of M1 (difference in vertical positions of the nipples relative to difference in horizontal positions of the nipples) predicted improved cosmesis as assessed by both the patient and her radiation oncologist. M4 (ratio of the horizontal distances of the nipples from the lateral aspects of the breasts) was significantly correlated with physician-reported cosmesis. Interestingly, neither M1 nor M4 differed by treatment arm at the one-year follow up visit. In contrast, median values of M2 (symmetry of the locations of the nipples in reference to the lowest visible points of the breasts) and M3 (symmetry of the locations of the nipples in reference to the midline of the torso) were significantly improved in the HF-WBI arm compared to the CF-WBI arm. To the best of our knowledge, this is the first study to compare, in a quantitative manner, breast cosmetic outcome in a randomized trial of two different radiation therapy treatment schedules for early breast cancer. Our novel results provide important confirmation that cosmetic outcome at 1 year following WBI is similar to improved with HF-WBI in comparison to CF-WBI.
The recent publication of large randomized trials from Canada and the United Kingdom has established the safety and efficacy of hypofractionated whole breast irradiation with follow up extending past 10 years [4,5]. Nevertheless, clinical practice in the United States has been relatively slow to embrace hypofractionated schedules in the delivery of WBI [21]. One potential concern raised regarding trials of HF-WBI has been the absence of a gold standard to measure cosmetic outcome of the treated breast [22]. Results from the current study help to fill that gap, demonstrating equivalence in quantitative breast cosmesis. The importance of this finding is magnified by the unique features of this study, including uniform use of a tumor bed boost and a high prevalence of obesity (approximately 50%) [18], which mirror current practice patterns and patient populations in the United States.
From a clinical perspective, the finding that M1 is highly correlated with both patient- and physician-reported outcome demonstrates the importance of attaining symmetry of the locations of the nipples to optimize cosmetic outcome from both a patient and physician perspective. Several clinical factors are likely to contribute to symmetry of the locations of the nipples, including baseline breast size, tumor size and location, volume of resection, use of concomitant reduction mammoplasty or mastopexy, radiation dose homogeneity, and potentially other treatment and host factors. The importance of symmetry of the locations of the nipples as illustrated in this study underscores the importance of developing a surgical and radiation treatment plan with the express purpose of optimizing long-term symmetry in order to optimize cosmetic outcome as experienced by the patient and physician.
From a research perspective, to our knowledge this is the first study to establish the direct clinical relevance of quantitative measurements of breast cosmetic outcome and/or symmetry. A number of quantitative metrics of symmetry have been reported in the literature [7–11], and many have correlated these measures to independent observer subjective assessment [12–14,16]. However, none have identified symmetry of the locations of the nipples as the critical factor in overall patient cosmetic assessment. In addition, only the BCCT.core metric, which is a composite of multiple symmetry measures as well as breast and scar appearance, has been demonstrated to have correlation to patient-reported cosmetic outcome [17]. Though useful, the BCCT.core does not provide insight into which aspects of cosmetic outcome are most relevant to patients and/or physicians. This limitation is addressed by our finding of the importance of symmetry of the locations of the nipples as assessed by M1 to patients and their physicians. Also of research interest is the finding that M4, a measure of the symmetry of the locations of the nipples in reference to the lateral extents of the breasts, is highly correlated to physician-reported outcome but not patient-reported outcome. This difference hints at possible reasons for the occasional lack of correlation observed between patient and physician cosmetic assessments. It is conceivable that patient perception is shaped by discrepancies in the amount of up-down vs. right-left displacement of the nipple of the affected breast relative to the unaffected breast, while physician assessments also consider the locations of the nipples in reference to other landmarks of the breast. If true, this would inform interpretation of future clinical trials that employ subjective assessments of breast cosmesis.
Identifying these metrics, particularly M1 and M4, as clinically relevant outcomes also addresses a substantial gap in both the randomized and non-randomized literature on radiation treatment for early breast cancer, which to date has relied solely on subjective measures of cosmesis [23–26]. While subjective measures are important, they are likely prone to significant intra- and inter-observer variability and, for patient-reported cosmetic outcome, to vary according to a patient’s underlying body image investment [27]. These limitations of subjective cosmetic outcome assessment are likely to blunt measured effects, thereby biasing comparative effectiveness studies toward the null hypothesis. We propose that future comparative effectiveness research and clinical trials investigating cosmetic outcomes in breast cancer should consider collecting data on quantitative cosmetic outcome, particularly M1 and M4, which given their purely quantitative nature may be more sensitive to subtle differences in treatment effects than subjective measures of cosmetic outcome.
From a policy perspective, the clinically relevant cutpoints for M1 and M4 could facilitate, for the first time, transparent and rigorous reporting of cosmetic outcome in patients with early breast cancer. Physician-reported cosmetic outcome is clearly insufficient for outcome reporting, as treating physicians are likely to demonstrate bias in favor of their own outcomes. Patient-reported cosmetic outcome is also suboptimal, however, as it is likely prone to variability based on patient socioeconomic status, demographics, age, and image investment. In contrast, the cutpoints for M1 and M4 offer clear metrics to define good cosmesis. For example, facilities could simply report the proportion of their patients with M1 ≥ 0.87 at certain defined timepoints. It is likely that M1 could be easily adjusted for baseline variables also associated with cosmetic outcome, for example BMI or tumor size. Such case-mix adjustment could thus facilitate comparison of M1 outcomes across doctors or treatment facilities.
This study has certain important limitations. First, quantitative measures were not taken after surgery and prior to WBI. This may not fully capture the influence of breast-conserving surgery, as well as associated procedures including oncoplastic rearrangement and reexcision, on symmetry and cosmetic outcome at 1 year. Second, all outcomes were collected at 1 year following the completion of WBI and are thus insufficient to fully capture the weight of late radiation toxicity. We plan to validate these initial findings as patients progress through follow up on this trial. Third, M3 and M6 could only be calculated in 47 patients due to limitations in the acquired 2D photographs. It would be inappropriate at this juncture to conclude that these particular measures of symmetry are not clinically relevant, given limited power to measure associations of symmetry with cosmetic outcomes. Future studies should endeavor to ensure that the requisite anatomic landmarks are encompassed in each photograph.
In summary, we identified several quantitative measures of symmetry that were strongly associated with patient- and/or physician-reported breast cosmesis at 1 year following WBI. These measures were similar to improved in patients treated with HF-WBI compared to CF-WBI one year after treatment, providing additional evidence supporting the safety of HF-WBI. Quantitative measures of breast symmetry could be readily incorporated into future comparative effectiveness research studies and used for outcome measurement and reporting.
Supplementary Material
Summary.
We used photographs to calculate six quantitative measures of breast cosmetic outcome in 245 patients participating in a randomized clinical trial comparing hypofractionated versus conventionally fractionated whole breast irradiation. One year after treatment, quantitative cosmetic measures were similar to improved with hypofractionated whole breast irradiation. Several quantitative cosmetic measures were associated with patient- and physician-reported cosmetic outcome and thus could be useful for future comparative effectiveness research and outcomes reporting.
Acknowledgments
This study was supported by a grant from the Conquer Cancer Foundation (ASCO) funded by the Breast Cancer Research Foundation. It was also funded by the National Cancer Institute, grants R01CA203984, R01CA143190, R01 R01CA207216, and P30CA016672; and by the MD Anderson Center for Radiation Oncology Research. Dr. Smith receives research funding from Varian Medical Systems, but this did not support the current project. Dr. Shaitelman receives research funding from Elekta, but this did not support the current project.
References
- 1.Early Breast Cancer Trialists’ Collaborative G. Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn EA, Segawa E, Kaiser K, et al. Health-related quality of life among women with ductal carcinoma in situ or early invasive breast cancer: Validation of the fact-b (version 4) Expert Review of Quality of Life in Cancer Care. 2016;1:99–109. [Google Scholar]
- 3.Harris JR, Levene MB, Svensson G, et al. Analysis of cosmetic results following primary radiation therapy for stages i and ii carcinoma of the breast. Int J Radiat Oncol Biol Phys. 1979;5:257–261. doi: 10.1016/0360-3016(79)90729-6. [DOI] [PubMed] [Google Scholar]
- 4.Group ST, Bentzen SM, Agrawal RK, et al. The uk standardisation of breast radiotherapy (start) trial b of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group ST, Bentzen SM, Agrawal RK, et al. The uk standardisation of breast radiotherapy (start) trial a of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. The lancet oncology. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 7.Kim MS, Reece GP, Beahm EK, et al. Objective assessment of aesthetic outcomes of breast cancer treatment: Measuring ptosis from clinical photographs. Comput Biol Med. 2007;37:49–59. doi: 10.1016/j.compbiomed.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Kim MS, Sbalchiero JC, Reece GP, et al. Assessment of breast aesthetics. Plast Reconstr Surg. 2008;121:186e–194e. doi: 10.1097/01.prs.0000304593.74672.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pezner RD, Patterson MP, Hill LR, et al. Breast retraction assessment: An objective evaluation of cosmetic results of patients treated conservatively for breast cancer. International Journal of Radiation Oncology*Biology*Physics. 1985;11:575–578. doi: 10.1016/0360-3016(85)90190-7. [DOI] [PubMed] [Google Scholar]
- 10.Tepper OM, Unger JG, Small KH, et al. Mammometrics: The standardization of aesthetic and reconstructive breast surgery. Plast Reconstr Surg. 2010;125:393–400. doi: 10.1097/PRS.0b013e3181c4966e. [DOI] [PubMed] [Google Scholar]
- 11.Fitzal F, Krois W, Trischler H, et al. The use of a breast symmetry index for objective evaluation of breast cosmesis. Breast. 2007;16:429–435. doi: 10.1016/j.breast.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Van Limbergen E, van der Schueren E, Van Tongelen K. Cosmetic evaluation of breast conserving treatment for mammary cancer. 1. Proposal of a quantitative scoring system. Radiother Oncol. 1989;16:159–167. doi: 10.1016/0167-8140(89)90016-9. [DOI] [PubMed] [Google Scholar]
- 13.Vrieling C, Collette L, Bartelink E, et al. Validation of the methods of cosmetic assessment after breast-conserving therapy in the eortc “boost versus no boost” trial. Eortc radiotherapy and breast cancer cooperative groups. European organization for research and treatment of cancer. Int J Radiat Oncol Biol Phys. 1999;45:667–676. doi: 10.1016/s0360-3016(99)00215-1. [DOI] [PubMed] [Google Scholar]
- 14.Sacchini V, Luini A, Tana S, et al. Quantitative and qualitative cosmetic evaluation after conservative treatment for breast cancer. Eur J Cancer. 1991;27:1395–1400. doi: 10.1016/0277-5379(91)90019-a. [DOI] [PubMed] [Google Scholar]
- 15.Cardoso MJ, Cardoso J, Amaral N, et al. Turning subjective into objective: The bcct. Core software for evaluation of cosmetic results in breast cancer conservative treatment. Breast. 2007;16:456–461. doi: 10.1016/j.breast.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Cardoso MJ, Cardoso JS, Wild T, et al. Comparing two objective methods for the aesthetic evaluation of breast cancer conservative treatment. Breast cancer research and treatment. 2009;116:149–152. doi: 10.1007/s10549-008-0173-4. [DOI] [PubMed] [Google Scholar]
- 17.Heil J, Dahlkamp J, Golatta M, et al. Aesthetics in breast conserving therapy: Do objectively measured results match patients’ evaluations? Ann Surg Oncol. 2011;18:134–138. doi: 10.1245/s10434-010-1252-4. [DOI] [PubMed] [Google Scholar]
- 18.Shaitelman SF, Schlembach PJ, Arzu I, et al. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol. 2015;1:931–941. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanton AL, Krishnan L, Collins CA. Form or function? Part 1. Subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91:2273–2281. [PubMed] [Google Scholar]
- 20.Swanick CW, Lei X, Shaitelman SF, et al. Longitudinal analysis of patient-reported outcomes and cosmesis in a randomized trial of conventionally fractionated versus hypofractionated whole-breast irradiation. Cancer. 2016 doi: 10.1002/cncr.30121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekelman JE, Sylwestrzak G, Barron J, et al. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the united states, 2008–2013. JAMA. 2014;312:2542–2550. doi: 10.1001/jama.2014.16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goffman TE, Glatstein E. Hypofractionation redux? J Clin Oncol. 2004;22:589–591. doi: 10.1200/JCO.2004.07.174. [DOI] [PubMed] [Google Scholar]
- 23.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 24.Haviland JS, Owen JR, Dewar JA, et al. The uk standardisation of breast radiotherapy (start) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. The lancet oncology. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 25.Bartelink H, Maingon P, Poortmans P, et al. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. The lancet oncology. 2015;16:47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 26.Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from rapid: A randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol. 2013;31:4038–4045. doi: 10.1200/JCO.2013.50.5511. [DOI] [PubMed] [Google Scholar]
- 27.Cash TF, Melnyk SE, Hrabosky JI. The assessment of body image investment: An extensive revision of the appearance schemas inventory. Int J Eat Disord. 2004;35:305–316. doi: 10.1002/eat.10264. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.