Abstract
BACKGROUND
Each year in the U.S., nearly 50,000 prostate cancer patients exhibit a rise in PSA levels, which can indicate disease recurrence. For patients (pts) with biochemically recurrent prostate cancer (PC), we evaluated the effects of white button mushroom (WBM) powder on serum PSA levels; also, the tolerability and biological activity of WBM was determined.
METHODS
Pts with continuously rising PSA levels were enrolled. Dose escalation was conducted in cohorts of 6; this continued provided that no more than one patient per cohort experienced dose limiting toxicity (DLT). The primary objective was to evaluate treatment feasibility and associated toxicity. The secondary objectives were to determine the effect of WBM on both serum PSA and androgen levels; and evaluate WBM’s impact on myeloid-derived suppressor cells (MDSCs) and cytokine levels.
RESULTS
Thirty-six pts were treated; no DLT’s were encountered. Overall PSA response rate was 11%. Two pts receiving 8 and 14 gm/day demonstrated a PSA complete response (CR): their PSA declined to undetectable levels that continue for 49 and 30 months. Two pts, receiving 8 and 12 gm/day, experienced a PSA partial response (PR). After 3 months of therapy, 36% of pts (13/36) experienced some PSA decrease below baseline. Pts with PSA CR and PR demonstrated higher levels of baseline interleukin-15 (IL-15) than nonresponders; for this group, we observed therapy-associated declines in myeloid-derived suppressor cells (MDSCs).
CONCLUSIONS
Therapy with WBM appears to both impact PSA levels and modulate the biology of biochemically recurrent PC by decreasing immunosuppressive factors.
Keywords: cytokines, myeloid-derived suppressor cells (MDSCs), prostate cancer, PSA recurrence, Agaricus bisporus, mushroom
INTRODUCTION
Adenocarcinoma of the prostate causes a significant number of cancer-related deaths in men. This year, an estimated 238,590 men will be diagnosed with PC, and 29,720 men will die from the disease.1 In the past two decades, testing of serum prostate specific antigen (PSA) has markedly increased diagnoses of organ confined PC.2 As a consequence, many men with newly diagnosed disease are treated (with a curative intent) using either radical prostatectomy or radiation therapy. The initial disease stage, PSA level, and Gleason score all influence the rates of recurrence. Within 10–15 years of radical prostatectomy or radiation therapy, approximately 25–30% of men will demonstrate disease recurrence.3 Clinical disease recurrence is usually presaged by elevated PSA levels; the vast majority of patients who relapse initially have no radiographic evidence of metastatic disease. The definition of PSA relapse varies depending on specific local therapy. Following prostatectomy, PSA is expected to be undetectable within 30 days of surgery. Consequently, even a small elevation in PSA (≥ 0.2 ng/mL) is considered a sign of persistent disease, and small levels are associated with subsequent disease progression.3 Conversely, after primary radiation therapy, PSA frequently remains detectable reflecting the presence of residual prostatic tissue. Thus, recurrence after radiation is frequently defined as an increase of PSA by > 2.0 ng/mL above nadir.3 While the natural history of this cohort of patients is variable, it is generally characterized by a protracted clinical course.2, 4, 5 In these patients, the risk of developing metastatic disease depends on several factors: the time to initial biochemical recurrence (2 vs. ≥ 2 yrs); PSA doubling time (< or ≥10 months); and pathologic Gleason's score.2 Patients who have received only one type of local therapy can be occasionally cured with another local modality (radiation or surgery).6, 7 However, patients who received both types of local therapies - yet continue to experience a rise in PSA levels - are considered incurable.
In this setting, the primary treatment option is androgen deprivation therapy. This approach is highly effective in suppressing PC; however, the side effects are potentially significant: fatigue, weight gain, muscle weakness, hot flashes, erectile dysfunction, loss of libido, increased risk of diabetes and cardiovascular problems8. In the context of an otherwise asymptomatic individual, such effects are especially concerning because treatment is applied frequently for many years. Furthermore, androgen deprivation therapy is not curative.9 In PSA-recurrent disease, the ability of androgen deprivation to enhance patient outcomes (especially overall survival) is unclear. Thus, in biochemically recurrent PC, the optimal treatment approach remains undefined; and new therapies with minimal toxicities need to be evaluated for this population. An ideal therapy would be outpatient, orally administered, and have minimal systemic toxicity; these properties facilitate long-term, repeated administration.
According to epidemiologic and basic science evidence, plant-derived phytochemicals may play a significant role in PC prevention, risk of recurrence, and therapy.10 For cancer patients with limited therapeutic options, such phytochemical may represent sources of alternative treatments. In reports on pomegranate juice11, modified citrus pectin12, lycopene, and isoflavone13, 14, these materials demonstrated a modest, favorable effect on PSA kinetics. However, caution is needed when interpreting these studies: a control arm is absent; the clinical significance of the endpoints is uncertain. Nevertheless, for centuries in Asia, a wide variety of plants have been used for medicinal purposes. In particular, several mushroom species have been shown to exhibit anti-cancer effects; these include inhibiting cell proliferation in prostate15–17, colon18 and breast cancer cell lines.15, 19 As suggested by these and other studies, further investigation is warranted into both specific mushroom species and pharmacologically active components of mushroom species.
White button mushroom (WBM, Agaricus bisporus) is the most common edible mushroom in the U.S.A. Accumulated evidence demonstrates that WBM has beneficial effects on various kinds of cancers. Lectins isolated from the WBM increase the sensitivity of lung, colon, and glioblastoma cancer cells to chemotherapeutic drugs.20 In addition, the WBM lectins inhibit colon cancer cell proliferation,20 and they enhance cellular antioxidant defense mechanisms.21 As we previously demonstrated, by inhibiting aromatase activity, both total WBM extract and certain isolated fractions effectively decrease breast cancer cell proliferation.22 In these active WBM fractions, conjugated linoleic acid was an important component; it was an inhibitor of both breast cell proliferation and aromatase activity.22, 23 In addition to breast cancer cells, we evaluated the effect of WBM on PC cell lines in vitro and vivo. According to our results, in all prostate cancer cell lines, WBM extract significantly inhibits cell proliferation; this occurs through induction of apoptosis of cancer cells.24 In mice gavaged with mushroom extract, tumor size and cell proliferation decreased while apoptosis increased. Similarly, for mushroom-fed mice, microarray analysis of tumors identified significant changes in gene expression. Particularly altered were the gene networks involved in cell death; growth and proliferation; lipid metabolism; the TCA cycle; and immune response.22, 23
Based on these preclinical data and a clinical unmet need, we have conducted a Phase I trial of WBM powder in patients with biochemically recurrent PC. All patients enrolled for this trial had (before enrollment) continuously rising PSA levels after previously undergoing definitive local therapy with prostatectomy, radiation therapy or both. Following the intake of WBM by biochemically recurrent prostate cancer patients, correlative serum samples were collected to attempt to answer the question, “What are biological differences between PSA responders and non-responders?”
MATHERIALS AND METHODS
Patient eligibility
A histologically or cytologically confirmed history of adenocarcinoma of the prostate was required for all patients. Any number of local therapies was allowed. Patients had to experience the following PSA failure: a PSA level of ≥ 0.2 ng/mL that increased above nadir after prostatectomy. If no prostatectomy was performed, and radiation or other local therapies were used as a primary therapy, patients had to experience a PSA increase of 2.0 ng/mL above post-therapy nadir. The increase in PSA values had to be established by at least two consecutive measurements (each separated by at least two weeks). No clinical or radiographic evidence of metastatic disease was allowed. In conjunction with their prior primary definitive therapy, patients were permitted to receive up to nine months of neoadjuvant or adjuvant hormone ablation. Androgen deprivation had to be completed at least six months prior to registration, and testosterone levels had to be in the non-castrated level defined as > 50 ng/dL. No complementary or alternative therapy (e.g. St. John’s Wort, PC-SPES, or other herbal remedies taken for the purpose of treating PC) could be given during protocol treatment. For patients who received neoadjuvant and/or adjuvant chemotherapy, such treatments must have been completed at least 6 months prior to protocol registration.
Patients were enrolled between January 2009 and September, 2011. The Phase I clinical trial described in this paper was registered with clinicaltrials.gov (NCT00779168). It was conducted according to the Declaration of Helsinki and its amendments. The study protocol was approved by the City of Hope Institutional Review Board and the Institutional Scientific Peer Review Committee of the City of Hope Comprehensive Cancer Center. All patients gave written informed consent before participation.
Preparation of mushroom tablets
Please see supplementary materials for procedure.
Mushroom tablet treatment
The mushroom tablets were taken twice daily until PSA progression, clinical progression or toxicity. Twenty-eight days constituted one treatment cycle. Dose escalation was conducted in cohorts of six at 6 dose levels: 4 gm, 6 gm, 8 gm, 10 gm, 12 gm and 14 gm daily. If no Dose Limited Toxicities (DLT) were encountered for a cohort of patients during the first 28 days of treatment, the next highest dose level was tested (up to 14 gm daily). Approximately 90% of fresh WBM weight consists of water. Therefore, 4 gm – 14 gm mushroom tablets are equivalent to 40 gm – 140 gm of fresh WBM.
Rationale for dose selection
Our trial examined six dose levels beginning with the dose of 4 gm daily. The maximum dose level was capped at 14 gm daily (28 tablets daily). For chronic ingestion, this was thought to be the highest practical dose. We did not attempt to reach maximum tolerated dose (MTD) since our preclinical data did not indicate a dose-dependent effect.
Efficacy and safety evaluation
Patients were evaluated clinically and with laboratory tests every 28 days while on treatment. At every visit, medication lists including vitamins and supplements were updated. Mushroom tablet pill count was performed at every visit to verify compliance. Toxicity assessment was performed on all patients who began therapy using the NCI Common Terminology Criteria for Adverse Events (CTCAE v 3.0). DLT applied only to cycle one and had to be drug related (possible, probable or definite). Any grade 2 or greater toxicity (excluding allergic rhinitis, fatigue, sweating, weight gain or loss, alopecia, dry skin, nail or pigmentation changes, pruritus, hot flashes, flatulence, mouth dryness, sense of smell or taste disturbance, erectile impotence, decreased libido, oligospermia, and/or insomnia) was considered a DLT. Unless a patient stopped treatment because of toxicity, patients were evaluated for response if they were on therapy for at least 28 days. Otherwise they were replaced. PSA progression was defined as a 100% increase over either the baseline (pretreatment, within 4 weeks of start of treatment) or nadir. To further define PSA progression, the minimum change in PSA needed was an absolute increase of 1 ng/mL, which was confirmed by second value 3–4 weeks later.
Hormone measurement
The ADVIA Centaur Testosterone assay kit (Siemens) was used to measure testosterone in patients’ serum. DHT, DHEA and estrogen were measured by ARUP laboratories.
Cytokine multiplex analysis
Per the manufacturer’s protocol, plasma samples were analyzed for 30 cytokines using Human Cytokine Thirty-Plex Antibody Bead Kit (Invitrogen). Please see supplementary materials for procedure.
T-cell subset analysis
Please see supplementary materials for procedure.
Statistical analysis and data management
This was a single institution Phase I study. The primary objective of this trial was to evaluate the feasibility, toxicity and biological activity of prolonged therapy with WBM powder. Six different dose levels were tested. No MTD was reached. No signal was detected related to dose, resulting in pooled analysis across dose levels for response rate and serum biomarkers. For binomial rates, confidence intervals presented were calculated by the Clopper-Pearson (exact) method. Due to the small number of samples available for serum biomarkers, nonparametric test (Wilcoxon rank sum) was used to compare responders with non-responders. As these biomarker evaluations were exploratory, there was no attempt to adjust for multiple comparisons; thus significant results require a confirmatory study.
RESULTS
Patient characteristics
Thirty-six patients were treated. Demographic characteristics are summarized in Table 1. The median patient age was 68 (53–80). The median PSA level at the onset of therapy was 1.9 ng/mL (0.2–22.2). For all patients, the baseline testosterone level’s median (range) was 295.3 (86.0 – 833.7) ng/dL. All patients had prior radiation therapy, the majority of which took place in the post-prostatectomy salvage setting. Thirty three patients (92%) underwent prior prostatectomy. Eleven patients (30%) underwent earlier hormonal therapy in the neoadjuvant or adjuvant setting.
TABLE 1.
Patient demographic and clinical characteristics (n=36)
| Patient characteristic | Number of patients (%) |
|---|---|
| Age (year) | |
| Median (range) | 68 (53–80) |
| Race | |
| White | 27 (75) |
| African American | 7 (20) |
| Asian | 2 (5) |
| Gleason score | |
| ≤6 | 6 (18) |
| 7 | 14 (44) |
| ≥8 | 12 (38) |
| Baseline PSA (ng/mL) | |
| Median (range) | 1.9 (0.2 – 22.2) |
| Baseline testosterone (ng/dL) | |
| Median (range) | 295.3 (86.0 – 833.7) |
| Prior radiation | 36 (100) |
| Prior prostatectomy | 33 (92) |
| Duration on treatment (months) | |
| Median (range) | 10.2 (0.9 – 57.9+) |
Abbreviations: PSA, prostate-specific antigen
Clinical efficacy
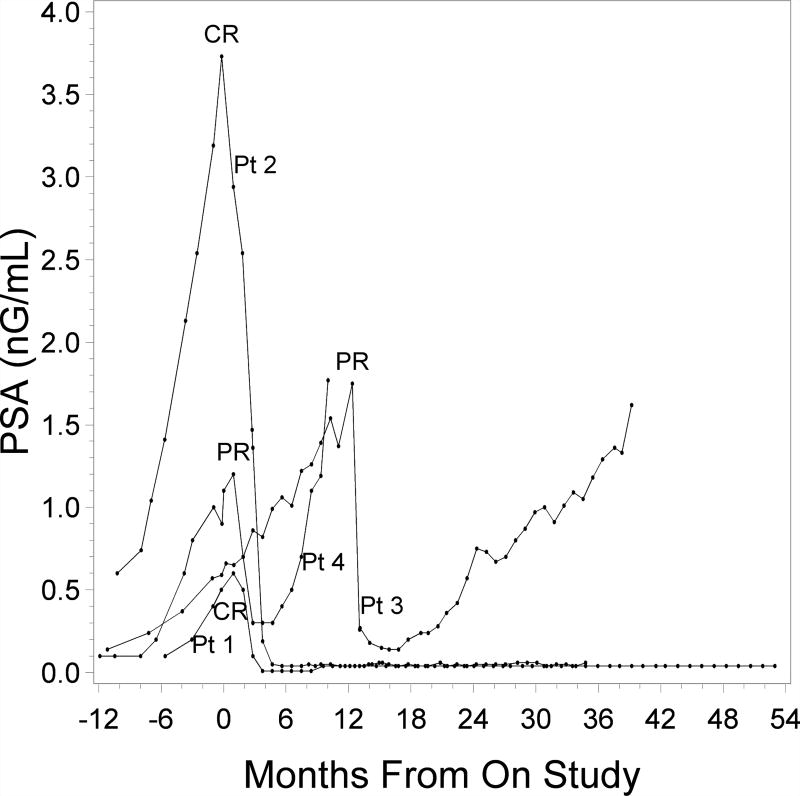
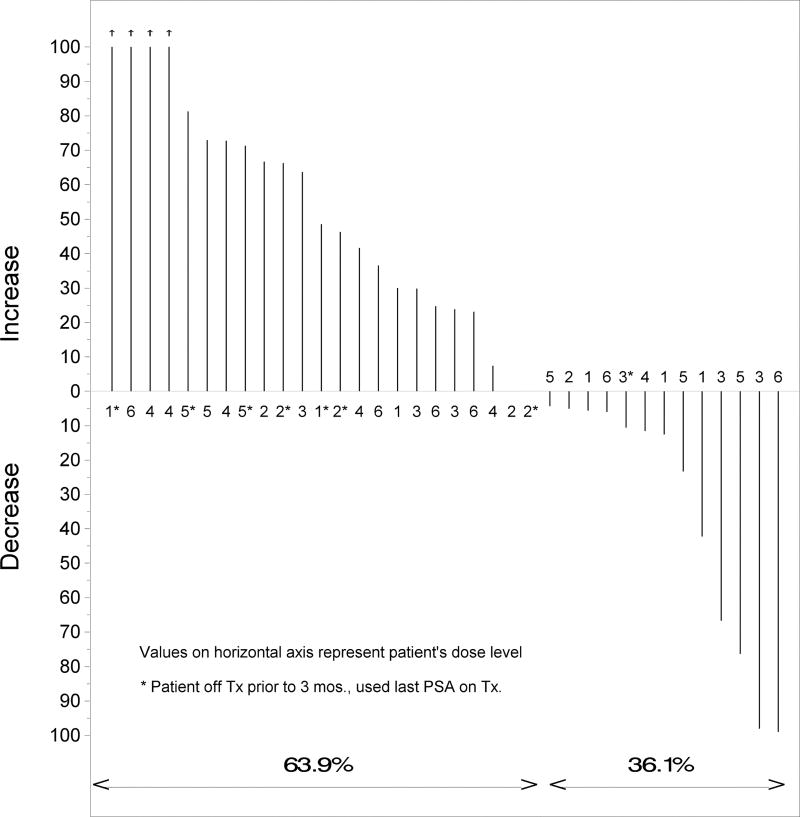
A PSA complete response (CR), defined as a PSA decline to ≤ 0.04 ng/mL (the threshold limit of detection at the City of Hope hospital analytical laboratory: Clinical pathology), was confirmed at least four weeks later. The overall PSA response rate (the sum of partial and complete responders versus nonresponders) was 4/36, or 11% (95% confidence interval (CI): 4%–26%). Two patients (at doses of 8 and 14 gm/day) experienced a prolonged PSA CR that continues to date. At the time of this report, the lengths of these CRs have been 49 and 30 months (Fig. 1). In another two patients, we observed a PSA partial response (PR), which was defined as a 50 % decline from baseline PSA. One patient with a PSA PR demonstrated durable response and remains on the study at 39 months since beginning of therapy. Another partial responder eventually had disease progression and was taken off treatment after 7 months on therapy. At the time when this paper was prepared, an additional 5 patients remained on protocol treatment because of their stable PSA values. Altogether, 8 patients remain on the study. Three out of four responders had a prior prostatectomy and salvage RT that was completed 4, 13 or 16 months prior to starting WBM powder treatment. One patient with a PR had prior brachytherapy followed by salvage prostatectomy. Characteristics of responders are summarized in Table 2. In addition to the partial and complete responses, 36 % of patients demonstrated some decline in PSA within three months after beginning of therapy (Fig. 2). Median duration on therapy for all patients is 10.2 months (0.9 – 57.9+). The longest patient on treatment exhibits a stable PSA level.
Figure 1.
PSA of 4 patients with significant PSA responses beginning 12 months prior to treatment initiation and followed until end of treatment (Pt. 4) or last PSA assessment
TABLE 2.
Characteristics of the 4 patients with PSA PR and CR
| Best PSA response |
Age at diagnosis |
Gleason score |
Prior therapy | PSA before mushroom therapy |
Daily dose | Months of treatment |
|---|---|---|---|---|---|---|
| CR | 61 | 9 | RP, SRT, ADT | 0.5 | 8 gm | 54+ |
| CR | 64 | 7 | RP, SRT | 3.73 | 14 gm | 35+ |
| PR | 65 | 7 | RP, SRT | 0.59 | 12 gm | 39+ |
| PR | 60 | 7 | BRT, RP, ADT | 0.9 | 8 gm | 11 |
Abbreviations: CR, complete response; PR, partial response, RP, radical prostatectomy; SRT, salvage radiation therapy; ADT, neoadjuvant/adjuvant androgen deprivation therapy; BRT, brachytherapy
Figure 2.
Waterfall plot of best PSA variation (%) at 3 months post treatment initiation from baseline (n=36)
Correlation of PSA and testosterone in patients with PSA CR
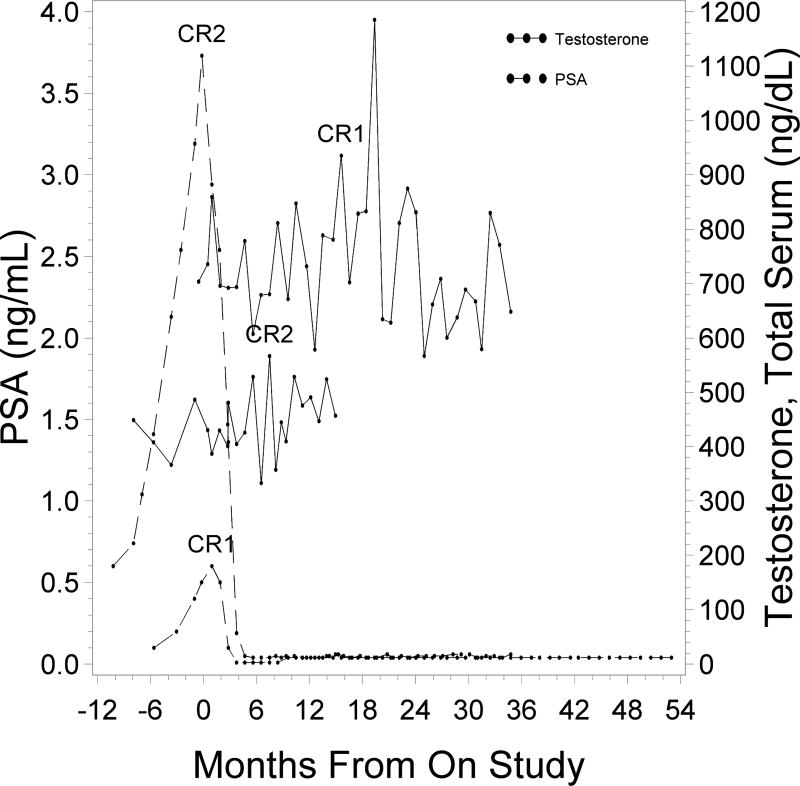
During mushroom treatment for two patients, PSA declined to undetectable levels (≤ 0.04 ng/mL); this was maintained for 49+ and 30+ months. In both patients, total serum testosterone levels fluctuated over the study period; however, there was no noticeable trend. Testosterone levels remained in the non-castrated range (Fig. 3). PSA responses did not correlate with serum testosterone, DHT or DHEA levels (data not shown).
Figure 3.
PSA and testosterone levels of 2 patients with PSA complete responses (CR). Testosterone measurements were limited due to termination of regular test prior to all patients going off study.
Toxicity
Minimal side effects were noted and mostly limited to Grade 1 abdominal bloating (Table 3). Mean compliance with protocol-defined mushroom powder treatment was 98.6 %. One patient at dose level 3 (8 gm daily) experienced grade 3 hyponatremia, possibly related to therapy, and was taken off the protocol for toxicity. This occurred during the second month (cycle 2) of therapy and therefore was not classified as DLT.
TABLE 3.
Adverse events seen in >3% of patients and any grade 3 toxicity
| Toxicity | Grade 1–2; n (%) | Grade ≥3; n (%) |
|---|---|---|
| Abdominal bloating | 13 (36) | 0 |
| SGPT/SGOT elevation | 10 (28) | 0 |
| Dyspepsia | 2 (6) | 0 |
| Leukocytosis | 3 (8) | 0 |
| Lymphopenia | 4 (12) | 0 |
| Fatigue | 3 (8) | 0 |
| Hyponatremia | 8 (22) | 1 (3) |
| Hypocalcemia | 2 (6) | 0 |
| Bilirubin elevation | 3 (8) | 0 |
| Hyperkalemia | 2 (6) | 0 |
| Muscle pain | 2 (6) | 0 |
| Hyperlipidemia | 2 (6) | 0 |
Abbreviations: SGPT, serum glutamic oxaloacetic transaminase; SGOT, serum glutamic pyruvic transaminase
Cytokine multiplex analysis
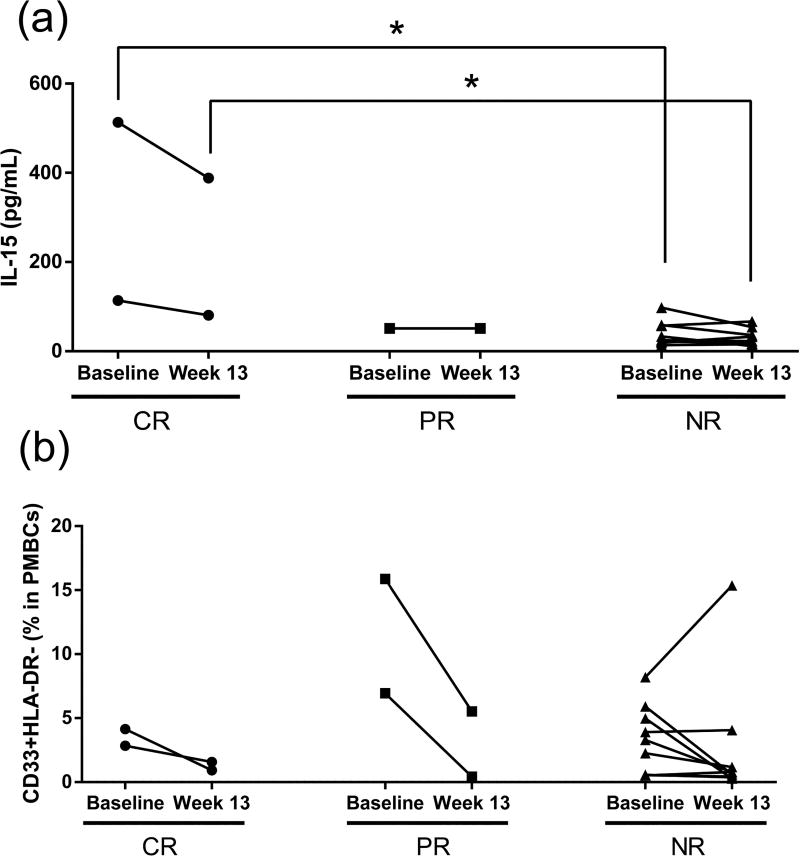
There were no definitive patterns for cytokine levels except for IL-15. In both baseline and post treatment, IL-15 levels of complete responders were significantly higher than levels in non-responders. Compared to baseline, IL-15 levels were not significantly changed after 13 weeks of mushroom intake in both complete-responders and non-responders (Fig. 4A).
Figure 4.
IL15 levels (A) and CD33+DLA-DR- (MDSCs) cell number (B) in patients’ plasma samples for baseline (7 days before enrollment) and during mushroom treatment (week 13). CR: Complete response (n=2), PR: Partial response (n=1 for (A), n=2 for (B)), NR: Non response (n=9). PBMCs: Peripheral blood mononuclear cells. *P < 0.05, Wilcoxon rank sum test.
T cell subset analysis
Compared to the percentage of MDSCs in peripheral blood mononuclear cells (PBMCs) sampled at enrollment, at week 13, the percentage of MDSCs (CD33+HLA-DR) decreased in the four CR and PR patients. Specifically, the levels dropped by 78%, 45%, 94% and 65%. In contrast, for the 9 non-responders at week 13, the percentage did not significantly decrease from enrollment (mean decrease of 25%). In all groups post-mushroom intake, no changes were apparent for CTL Ag4 T (CD4+CD152+); T-reg FoxP3 (CD4+CD25+FoxP3+); and PD-1+ T (CD4+CD279+) cells (Fig. 4B).
DISCUSSION
In this study of 36 men with biochemical recurrence of prostate cancer, WBM powder therapy was associated with declining PSA levels in 36% of patients. Two patients completely responded; and two other patients partially responded. An additional 5 patients remain on protocol treatment to date because of their stable PSA values. These results indicate that mushroom intake can modulate PSA levels in biochemically recurrent prostate cancer. When comparing responders to non-responders, the following factors did not correlate to the mushroom treatment’s response: the daily mushroom dose, Gleason score, baseline PSA level, weight, age, baseline testosterone levels and type of prior therapy. We did not detect any effect of mushroom powder therapy on the levels of circulating androgens: testosterone, DHT and DHEA.
Importantly, in two patients, PSA decreased to undetectable levels after WBM treatment. To the best of our knowledge, this has not been observed in other studies examining phytochemical compounds for biochemically recurrent prostate cancer. Almost all of the patients in our study (92%), including 2 patients with PSA CR, had received prostatectomy and radiation therapy. As such, patients had exhausted a chance for a cure via traditional localized treatment. Compliance with the daily ingestion of up to 28 large mushroom powder tablets was excellent; reflecting lack of significant, chronic toxicity. The most common adverse events were grade 1 abdominal bloating and grade 1 SGPT/SGOT elevation. One patient sustained asymptomatic grade 3 hyponatremia.
We were aware of potential confounding effects of other medications (statins, thiazide diuretics and non - steroidal anti-inflammatory drugs - NSAIDs)25 on PSA levels. As such, on a monthly basis, we verified concurrent medications and supplements. For one patient with prolonged stable PSA, changes in PSA reflected both his cholesterol level and compliance (or lack thereof) with simvastatin. For the rest of the group, PSA responders did not change their baseline medications or supplements. Importantly, they did not ingest any compounds known to affect PSA levels. In addition, we have not discovered any correlation between weight changes and PSA; this was true for patients demonstrating significant PSA responses. According to the literature, WMB not only lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats,26 but it also prevents hepatic steatosis in mice.27 However we have not observed significant changes in blood glucose and lipid levels in patients treated with WBM.
We observed PSA CR and PSA PR from 8 gm daily dose group (Table 2). Our trial examined six dose levels beginning with the dose of 4 gm daily. Maximum dose level was capped at 14 gm daily which was the highest dose that was thought to be practical to ingest over long period of time based on the number of tablets required. As there were no DLTs, dose determination is challenging. In future studies, and especially single product studies on the mushroom powder/tablets in asymptomatic patients, we will start patients on 8 gm a day, the minimum dose where we observed responses, and permit the patient to escalate up to 14 gm a day if well-tolerated.
For decades, the capacity of various mushrooms to both inhibit tumor growth and modulate immune functions has been studied. According to one report, tumor growth was primarily inhibited by stimulating the immune system. In particular, macrophages and natural killer cells were stimulated; so were T cells and their cytokine production.28 Using the Luminex platform, we assayed a variety of serum cytokines involved in immune function, angiogenesis, and lipid and bone metabolism; however, we did not observe a correlation between serum cytokine levels and PSA responses. Despite this, the analysis produced an interesting outcome: for three patients, with either PSA CR or PR, particularly high levels of plasma IL-15 were detected (compared to nonresponsive patients). As it has been suggested, IL-15 may both promote NK cell differentiation and optimize NK cell function.29 Congruent with this, in prostate tumor models, IL-15 has been evaluated as an anticancer immunotherapeutic because it can effectively stimulate lymphocyte subsets: CD-8 T cells, natural killer cells, and natural killer T cells. Consequently, IL-15 can promote tumor suppression. As it is indeed evidenced in the literature, WBM extract enhances natural killer cell activity in mice.30, 31
Several clinical trials of IL-15 in cancer immunotherapy have started recently (ClinicalTrials.gov); it may not be optimal when it is used a single treatment. According to Yu et al., combination therapies--involving both suppression of the immune negative feedback systems and IL-15 therapy--abrogated prostate tumor growth in mice models.32 For the responding patients described herein with high IL-15, we hypothesize that WBM influences immune inhibition. In tumor-bearing mice, β-glucans, which are extracted from mushrooms, can decrease the levels of tumor-associated immunosuppressive cells, such as T-regulatory T-cells.33 Thus, patients with high IL-15 levels may benefit from WBM treatment via suppression of immune negative feedback effects. To both protect the host from pernicious immune stimulation (excessive stimulation during infections) and to control autoimmune responses, there are multiple mechanisms for immune response attenuation. Through these inhibitory mechanisms, cancer cells can escape the immune surveillance and expand their niche. To mediate cancer-related immune suppression, ligands which are expressed by antigen-presenting cells can bind to (a) T cells’ inhibitory receptors (cytotoxic T lymphocyte antigen (CTLA-4) and programmed death 1 (PD1)); and (b) inhibitory cells (T-regs and myeloid-derived suppressor cells).34, 35 Therefore, we performed T cell subset analysis using patient’s PBMCs. Interestingly, the percentage of MDSCs (CD33+HLA-DR-) significantly decreased during mushroom intake in two PSA CR and two PR patients. A direct link between the number of MDSCs and the presence of primary prostate tumor has been reported.36 MDSCs not only possess strong immunosuppressive activities, but they also play an important role in angiogenesis. These cells secrete angiogenic growth factors, and they support tumor metastasis along multiple steps of the process (invasion, embolism and circulation).35 Therefore, patients with high IL-15 levels may benefit from WBM treatment via a decrease in cancer-related immune suppression involving MDSCs.
Dihydrotestosterone (DHT), which is an active androgen, upregulates PSA levels.37 WBM was also found to inhibit 5-alpha reductase, which is the enzyme that produces DHT from testosterone. The WBM extract inhibits both 5α-reductase 1 and 2 (Supplemental Fig. 1). Although we did not detect changes in testosterone and DHT that were freely circulating, in tissues, we cannot rule out inhibitory effects of WBM on 5α reductase (and thus tissue production of DHT).
The trial was carried out with two lots of lyophilized WBM powder. Both lots were obtained from Monterey Mushrooms (Watsonville, California). The standard assay in our laboratory to define the activity of mushrooms or their extract is to determine their anti-aromatase activity. The assay procedure was reported by Kanaya et al.27. The water extract generated from these lots inhibited aromatase in a dose-dependent manner. Our laboratory first reported the ability of WBMs to suppress the activity of aromatase, an enzyme that catalyzes the biosynthesis of estrogen23. The assay is robust and has been the method for us to compare the activity of different mushroom preparations. It is not clear if aromatase activity is a good surrogate for activity related to the effect observed in biochemically recurrent prostate cancer, and efforts will be made to develop assays more relevant to prostate cancer.
A limitation of our analyses, a lack of placebo control, raises the following question: In this population of patients, do the PSA responses simply represent natural variability of relatively low PSA levels? Based on both the literature and experience, we think this is unlikely. Specifically, consecutive rises in PSA to ≥ 0.2 (as required per eligibility criteria) in post prostatectomy patients almost always leads to eventual progression.2–4
In summary, therapy with WBM appears to impact PSA levels and potentially modulates the biology of prostate cancer in some patients, especially in the setting of low tumor burden. Our future efforts will focus on both the isolation and characterization of compound(s) from the WBM with anti- prostate cancer activity; and the development of a placebo controlled clinical trial in patients with early biochemical recurrence of prostate cancer.
Supplementary Material
Acknowledgments
The authors would like to thank Sharon Dension for help with coordinating supply of mushroom tablets. We would also like to thank Ian Talisman, PhD and Gene Hur, PhD for assistance with writing and 5α-reductase assay. Finally, we would like to thank Shu Mi, MD and Vivi Tran in the Clinical Immunobiology Correlative Studies Laboratory for their performance of the cytokine and T-cell analyses.
Support: Mushroom Growers of Australia and North America, the National Institute of Health (ES08258) and National Cancer Institute of the National Institutes of Health (P30CA33572)
Footnotes
Financial disclosures: There are no financial disclosures from any authors.
Disclaimer: We the authors verify that the submitted manuscript has not been published and is not currently submitted for publication elsewhere and confirm that we have no conflict of interest in relation to this work.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 3.Freedland SJ, Moul JW. Prostate specific antigen recurrence after definitive therapy. J Urol. 2007;177(6):1985–91. doi: 10.1016/j.juro.2007.01.137. [DOI] [PubMed] [Google Scholar]
- 4.Rosenbaum E, Partin A, Eisenberger MA. Biochemical relapse after primary treatment for prostate cancer: studies on natural history and therapeutic considerations. J Natl Compr Canc Netw. 2004;2(3):249–56. doi: 10.6004/jnccn.2004.0022. [DOI] [PubMed] [Google Scholar]
- 5.Freedland SJ, Humphreys EB, Mangold LA, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 6.Hayes SB, Pollack A. Parameters for treatment decisions for salvage radiation therapy. J Clin Oncol. 2005;23(32):8204–11. doi: 10.1200/JCO.2005.03.1575. [DOI] [PubMed] [Google Scholar]
- 7.Sanderson KM, Penson DF, Cai J, et al. Salvage radical prostatectomy: quality of life outcomes and long-term oncological control of radiorecurrent prostate cancer. J Urol. 2006;176(5):2025–31. doi: 10.1016/j.juro.2006.07.075. discussion 31–2. [DOI] [PubMed] [Google Scholar]
- 8.Keating NL, O'Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24(27):4448–56. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 9.Crawford ED, Eisenberger MA, McLeod DG, et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med. 1989;321(7):419–24. doi: 10.1056/NEJM198908173210702. [DOI] [PubMed] [Google Scholar]
- 10.Chan JM, Gann PH, Giovannucci EL. Role of diet in prostate cancer development and progression. J Clin Oncol. 2005;23(32):8152–60. doi: 10.1200/JCO.2005.03.1492. [DOI] [PubMed] [Google Scholar]
- 11.Pantuck AJ, Leppert JT, Zomorodian N, et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin Cancer Res. 2006;12(13):4018–26. doi: 10.1158/1078-0432.CCR-05-2290. [DOI] [PubMed] [Google Scholar]
- 12.Guess BW, Scholz MC, Strum SB, Lam RY, Johnson HJ, Jennrich RI. Modified citrus pectin (MCP) increases the prostate-specific antigen doubling time in men with prostate cancer: a phase II pilot study. Prostate Cancer Prostatic Dis. 2003;6(4):301–4. doi: 10.1038/sj.pcan.4500679. [DOI] [PubMed] [Google Scholar]
- 13.Vaishampayan U, Hussain M, Banerjee M, et al. Lycopene and soy isoflavones in the treatment of prostate cancer. Nutr Cancer. 2007;59(1):1–7. doi: 10.1080/01635580701413934. [DOI] [PubMed] [Google Scholar]
- 14.Pendleton JM, Tan WW, Anai S, et al. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer. 2008;8:132. doi: 10.1186/1471-2407-8-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sliva D, Labarrere C, Slivova V, Sedlak M, Lloyd FP, Jr, Ho NW. Ganoderma lucidum suppresses motility of highly invasive breast and prostate cancer cells. Biochem Biophys Res Commun. 2002;298(4):603–12. doi: 10.1016/s0006-291x(02)02496-8. [DOI] [PubMed] [Google Scholar]
- 16.Hsieh TC, Wu JM. Cell growth and gene modulatory activities of Yunzhi (Windsor Wunxi) from mushroom Trametes versicolor in androgen-dependent and androgen-insensitive human prostate cancer cells. Int J Oncol. 2001;18(1):81–8. doi: 10.3892/ijo.18.1.81. [DOI] [PubMed] [Google Scholar]
- 17.deVere White RW, Hackman RM, Soares SE, Beckett LA, Sun B. Effects of a mushroom mycelium extract on the treatment of prostate cancer. Urology. 2002;60(4):640–4. doi: 10.1016/s0090-4295(02)01856-3. [DOI] [PubMed] [Google Scholar]
- 18.Xie JT, Wang CZ, Wicks S, et al. Ganoderma lucidum extract inhibits proliferation of SW 480 human colorectal cancer cells. Exp Oncol. 2006;28(1):25–9. [PubMed] [Google Scholar]
- 19.Zhang M, Chiu LC, Cheung PC, Ooi VE. Growth-inhibitory effects of a beta-glucan from the mycelium of Poria cocos on human breast carcinoma MCF-7 cells: cell-cycle arrest and apoptosis induction. Oncol Rep. 2006;15(3):637–43. [PubMed] [Google Scholar]
- 20.Yu L, Fernig DG, Smith JA, Milton JD, Rhodes JM. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993;53(19):4627–32. [PubMed] [Google Scholar]
- 21.Shi YL, Benzie IF, Buswell JA. Role of tyrosinase in the genoprotective effect of the edible mushroom, Agaricus bisporus. Life Sci. 2002;70(14):1595–608. doi: 10.1016/s0024-3205(01)01546-6. [DOI] [PubMed] [Google Scholar]
- 22.Grube BJ, Eng ET, Kao YC, Kwon A, Chen S. White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J Nutr. 2001;131(12):3288–93. doi: 10.1093/jn/131.12.3288. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Oh SR, Phung S, et al. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus) Cancer Res. 2006;66(24):12026–34. doi: 10.1158/0008-5472.CAN-06-2206. [DOI] [PubMed] [Google Scholar]
- 24.Adams LS, Phung S, Wu X, Ki L, Chen S. White button mushroom (Agaricus bisporus) exhibits antiproliferative and proapoptotic properties and inhibits prostate tumor growth in athymic mice. Nutr Cancer. 2008;60(6):744–56. doi: 10.1080/01635580802192866. [DOI] [PubMed] [Google Scholar]
- 25.Chang SL, Harshman LC, Presti JC., Jr Impact of common medications on serum total prostate-specific antigen levels: analysis of the National Health and Nutrition Examination Survey. J Clin Oncol. 2010;28(25):3951–7. doi: 10.1200/JCO.2009.27.9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong SC, Jeong YT, Yang BK, et al. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr Res. 2010;30(1):49–56. doi: 10.1016/j.nutres.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Kanaya N, Kubo M, Liu Z, et al. Protective effects of white button mushroom (Agaricus bisporus) against hepatic steatosis in ovariectomized mice as a model of postmenopausal women. PLoS One. 2011;6(10):e26654. doi: 10.1371/journal.pone.0026654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borchers AT, Krishnamurthy A, Keen CL, Meyers FJ, Gershwin ME. The immunobiology of mushrooms. Exp Biol Med (Maywood) 2008;233(3):259–76. doi: 10.3181/0708-MR-227. [DOI] [PubMed] [Google Scholar]
- 29.Huntington ND. The unconventional expression of IL-15 and its role in NK cell homeostasis. Immunol Cell Biol. 2014;92(3):210–3. doi: 10.1038/icb.2014.1. [DOI] [PubMed] [Google Scholar]
- 30.Wu D, Pae M, Ren Z, Guo Z, Smith D, Meydani SN. Dietary supplementation with white button mushroom enhances natural killer cell activity in C57BL/6 mice. J Nutr. 2007;137(6):1472–7. doi: 10.1093/jn/137.6.1472. [DOI] [PubMed] [Google Scholar]
- 31.Vannucci L, Krizan J, Sima P, et al. Immunostimulatory properties and antitumor activities of glucans (Review) Int J Oncol. 2013;43(2):357–64. doi: 10.3892/ijo.2013.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu P, Steel JC, Zhang M, et al. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A. 2012;109(16):6187–92. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masuda Y, Inoue H, Ohta H, Miyake A, Konishi M, Nanba H. Oral administration of soluble beta-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int J Cancer. 2013;133(1):108–19. doi: 10.1002/ijc.27999. [DOI] [PubMed] [Google Scholar]
- 34.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–52. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brusa D, Simone M, Gontero P, et al. Circulating immunosuppressive cells of prostate cancer patients before and after radical prostatectomy: profile comparison. Int J Urol. 2013;20(10):971–8. doi: 10.1111/iju.12086. [DOI] [PubMed] [Google Scholar]
- 37.Marks LS, Andriole GL, Fitzpatrick JM, Schulman CC, Roehrborn CG. The interpretation of serum prostate specific antigen in men receiving 5alpha-reductase inhibitors: a review and clinical recommendations. J Urol. 2006;176(3):868–74. doi: 10.1016/j.juro.2006.04.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.