Abstract
EBV expresses a number of viral noncoding RNAs (ncRNAs) during latent infection, many of which have known regulatory functions and can post-transcriptionally regulate viral and/or cellular gene expression. With recent advances in RNA sequencing technologies, the list of identified EBV ncRNAs continues to grow. EBV-encoded RNAs (EBERs), the BamHI-A rightward transcripts (BARTs), a small nucleolar RNA (snoRNA), and viral microRNAs (miR-NAs) are all expressed during EBV infection in a variety of cell types and tumors. Recently, additional novel EBV ncRNAs have been identified. Viral miRNAs, in particular, have been under extensive investigation since their initial identification over ten years ago. High-throughput studies to capture miRNA targets have revealed a number of miRNA-regulated viral and cellular transcripts that tie into important biological networks. Functions for many EBV ncRNAs are still unknown; however, roles for many EBV miRNAs in latency and in tumorigenesis have begun to emerge. Ongoing mechanistic studies to elucidate the functions of EBV ncRNAs should unravel additional roles for ncRNAs in the viral life cycle. In this chapter, we will discuss our current knowledge of the types of ncRNAs expressed by EBV, their potential roles in viral latency, and their potential involvement in viral pathogenesis.
1 Introduction
Latent EBV infection is causally linked to a variety of lymphoid and epithelial malignancies in vivo including Burkitt’s lymphoma (BL), Hodgkin’s lymphoma(HL) and non-Hodgkin’s lymphoma (NHL), rare NK and T cell lymphomas, nasopharyngeal carcinoma (NPC), and a subset of gastric carcinomas (GC). Over 80 protein-coding open reading frames (ORFs) have been identified within the EBV genome as well as ~30 different ncRNAs (Fig. 1). The EBV transcriptome is complex and consists of many alternatively spliced transcripts which yield various gene products. During latency, only a subset of viral genes is expressed. At least three distinct latency programs have been described for EBV, which are characterized by different patterns of coding and noncoding viral gene expression. During latency III, which occurs in vivo predominantly in the context of immunosuppression, such as in AIDS-associated NHL and in post-transplant lymphoproliferative disease (PTLD), as well as in B cells infected in vitro, nine latent viral genes and all EBV ncRNAs described to date are expressed. Interestingly, the majority of EBV ncRNAs are also expressed in the other latency stages, while EBV protein expression is more limited, suggesting that ongoing EBV ncRNA expression contributes to the maintenance of viral latency. In latency II, commonly observed in EBV+ NPCs, in EBV+ HL, and in EBV+ primary effusion lymphoma (PEL) cells that are co-infected with another herpesvirus, Kaposi’s sarcoma-associated herpesvirus (KSHV), the episomal maintenance protein EBV nuclear antigen 1 (EBNA-1), three latent membrane proteins (LMP1, LMP2A, and LMP2B), and abundant levels of Epstein-Barr virus-encoded RNAs (EBERs), BamHI-A rightward transcripts (BARTs), and BART microRNAs (miR-NAs) are expressed. In the most restricted stage of latency, latency I, which is the characteristic of BL, only the EBNA-1 protein is expressed, while low levels of BART miRNAs are also detectable.
Fig. 1.
Genomic origin of latent EBV transcripts, including EBV noncoding RNAs (shaded). The noncoding RNAs include the EBERs, miRNAs, BART transcripts, and an EBV snoRNA. The 25 EBV precursor miRNAs are clustered in the BHRF1 and BART regions of the genome
Exact functions for many EBV ncRNAs remain unknown; however, many studies suggest that a number of EBV ncRNAs—particularly viral miRNAs—may contribute to the establishment and persistence of viral latency, EBV-driven B cell immortalization in vitro, and potentially, the development of cancer in vivo. Here, we discuss the types of ncRNAs expressed by EBV and their known functions.
2 Epstein-Barr Virus-Encoded RNAs (EBERs)
The EBERs (EBER1 and EBER2), separated by ~160 nt in the EBV genome, are expressed individually as non-polyadenylated RNA polymerase III transcripts that remain stable within the nucleus of EBV-infected cells. Both EBER1 and EBER2 contain intragenic A and B box transcriptional control elements that are characteristic of many RNA pol-III transcripts; upstream Sp1 and ATF binding sites as well as TATA-like sequences are necessary for efficient transcription (Howe and Shu 1989). The EBER transcripts are highly abundant and expressed between one to five million copies per cell (Lerner et al. 1981), making them the most abundant viral RNA species present in presumably all EBV-infected cell types. Due to their high expression levels, the EBERs are often utilized as in situ biomarkers for EBV infection in clinical samples. Their presence in virtually all EBV-associated tumors makes the EBERs potential therapeutic targets for EBV-associated cancers.
Exact function(s) of the EBERs remain both controversial and obscure. Despite their prevalence in all EBV-infected cells, tumors, and other clinical samples, their biological roles, particularly in vivo, are poorly understood. Since the initial identification of EBERs nearly thirty-five years ago, studies from a number of groups have provided conflicting results as to their activities in B cells and epithelial cells during EBV infection. Recombinant Akata-derived viruses in which the EBERs are mutationally inactivated exhibit an ~100-fold decrease in their ability to induce LCL outgrowth compared to wild-type virus, a phenotype which may or may not be specific to EBER2 (Yajima et al. 2005; Wu et al. 2007; Gregorovic et al. 2011). The role of the EBERs in lymphomagenesis has also been tested in vivo. EBER1-expressing transgenic mice develop lymphoid hyperplasia, some of which progress to B cell lymphomas (Repellin et al. 2010). Contradicting these results, deletion of the EBERs in the B95-8 background had no measurable effect on human B cell transformation efficiency or LCL growth rates in vitro (Swaminathan et al. 1991). The inconsistencies in reported EBER-associated phenotypes are possibly due to the differences in EBV strains utilized; EBER-related phenotypic effects have been observed for Akata-derived viruses, which contain additional viral transcripts and miRNAs that are absent from the EBV B95-8 (utilized by Gregorovic et al. 2011) and P3HR1 strains (utilized by Swaminathan et al. 1991). Regardless, all of these results have suggested that EBERs are not essential for, but likely contribute to, B cell transformation and the establishment and maintenance of latency.
The EBERs were also shown to be dispensable for lytic replication (Swaminathan et al. 1991), suggesting that they exert their functions primarily during latency. Furthermore, deletion of EBER1 or EBER2 individually in EBV B95-8 correlates with specific gene expression changes in LCLs. Among EBER dependent, differentially expressed genes were genes with functional roles in membrane signaling, the regulation of apoptosis, and interferon responses (Gregorovic et al. 2011). Consistent with these data, the EBERs can protect EBV-infected BL cells from interferon alpha-induced apoptosis (Ruf et al. 2005; Nanbo et al. 2002).
Further insight into EBER function may come from the unique secondary structures adopted by these two RNAs, which can facilitate interactions with host proteins. EBER1 (167 nt) and EBER2 (172 nt) adopt well-defined, evolutionarily conserved RNA secondary structures consisting of multiple stem-loop domains and have an uncapped 5′ tri-phosphate and a 3′ polyuridylate region that are characteristics of RNA pol-III transcripts (Fig. 2a). The 3′ stretch of U nucleotides is thought to facilitate interactions with cellular factors. A number of cellular proteins are known to interact with the EBERs to form ribonucleoprotein complexes. Both EBERs have been shown to bind the auto-antigen La (Lerner et al. 1981), a nuclear phosphoprotein that is known to bind many RNA pol-III transcripts, and the retinoic acid-inducible gene I (RIG-I) protein (Samanta et al. 2006), a detector of double-stranded RNAs and activator of type I interferon signaling. EBER2 may provide additional structured RNA elements for binding to other as yet undefined host factors. Indeed, new studies demonstrate interactions between EBER2, the EBV terminal repeats, and the B cell transcription factor PAX5 which can mediate LMP expression (Lee et al. 2015).
Fig. 2.
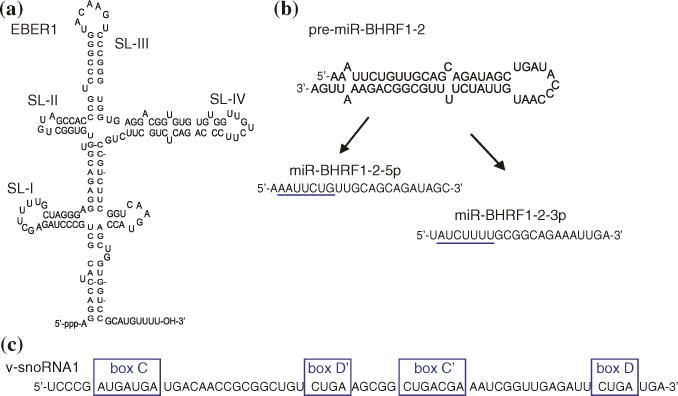
Structural features of select EBV ncRNAs. a EBER1 structure. SL indicates the four major “stem-loops.” b Predicted folding structure of the precursor miRNA for miR-BHRF1-2; highlighted are the mature miRNAs derived from the 5p and 3p arms of the pre-miRNA with their underlined seed sequences that mediate initial interactions between RISC and target mRNAs. C v-snoRNA1 sequence with outlined canonical C/D boxes
EBER1 can bind human ribosomal protein L22, which results in the relocalization of L22 from the cytoplasm to the nucleoplasm (Fok et al. 2006b; Toczyski et al. 1994). EBER1 also binds host hnRNPs (A1, A2/B1, and AUF1/D) (Lee et al. 2012). AUF1 (AU-rich element (ARE) binding factor 1) interactions with ARE-containing mRNAs usually result in enhanced mRNA destabilization and decay (reviewed in White et al. 2013). High levels of EBER1 can interfere with AUF1 binding to ARE-rich mRNAs, and ~15 % of the transcripts that are down-regulated upon deletion of EBER1 contain AREs, suggesting that EBER1 may contribute to the stability of these mRNAs (Lee et al. 2012; Gregorovic et al. 2011).
The EBERs are similar in size and structure to two well-characterized adenovi-ral small ncRNAs, termed VAI and VAII, which are essential for adenovirus replication and have been shown to inhibit PKR-mediated shutdown of translation. Intriguingly, the EBERs can functionally substitute for VAI/II and partly rescue replication of adenoviruses lacking VAI/II (Bhat and Thimmappaya 1983). EBERs have been reported to interact with the normally cytoplasmic PKR protein in vitro (Clarke et al. 1991); however, given their nuclear localization and the lack of an effect on PKR phosphorylation in EBV-infected BL cells (Ruf et al. 2005), such interactions seem unlikely to occur in vivo.
Both VAI and VAII are processed by Dicer into functional miRNAs (Furuse et al. 2013; Andersson et al. 2005; Aparicio et al. 2006; Lu and Cullen 2004), which has raised the question of whether the EBERs might also undergo miRNA processing. Small RNAs mapping to the EBERs have been reported in a number of small RNA sequencing experiments (Lung et al. 2009; Skalsky et al. 2012, 2014; Riley et al. 2012). Some of these EBER-derived RNA fragments are associated with RISC (Riley et al. 2012; Skalsky et al. 2012, 2014); however, current evidence argues against these EBER-derived RNA fragments as bona fide miRNAs. EBER fragments lack the precise 5′ end and specific length that is characteristic of miRNAs (Skalsky et al. 2014). Both EBERs are confined to the nucleus (Fok et al. 2006a) and therefore lack access to miRNA biogenesis machinery, namely Dicer, in the cytoplasm. Lastly, in vitro experiments have revealed that EBER1 is resistant to Dicer cleavage (Sano et al. 2006). A more likely explanation for the observed RISC-associated EBER fragments is that these small RNAs arise from EBER RNA breakdown products.
3 BamHI-A Rightward Transcripts (BARTs)
The BARTs represent another abundant, stable viral RNA species present in all infected cell types. The BARTs were originally identified in the C15 NPC xenograft tumor that is serially propagated in nude mice (Gilligan et al. 1990; Hitt et al. 1989). The transcripts were readily detectable by Northern blot in a number of NPC cell lines and patient biopsies (Gilligan et al. 1991) and found to arise from regions antisense to several lytic genes, including BALF5 (Karran et al. 1992). Additional studies have revealed that the BARTs are detectable during lytic infection and latent infection, in the peripheral blood of EBV-infected individuals, in B and T cell lymphomas, in B cell lines infected in vitro, and in epithelial carcinomas, especially NPCs (Edwards et al. 2008; Al-Mozaini et al. 2009; Chen et al. 2005). Interestingly, the B95-8 laboratory strain of EBV bears a deletion that removes almost the entire BART region in EBV yet B95-8 fully retains the ability to immortalize primary B cells in culture, arguing that the BARTs are dispensable for B cell transformation (Robertson et al. 1994). While the level of BART transcripts can vary dramatically between cell types, BARTs are consistently highly abundant in latency II NPCs (Marquitz and Raab-Traub 2012) and thus are thought to play a contributing role in NPC pathogenesis.
The BARTs are a complex family of alternatively spliced, polyadenylated RNAs that remain stable in the nucleus following processing of longer primary transcript(s). The entire BART locus is ~20 kbp and includes seven exons; all BARTs contain exon VII and therefore share the same 3′ end (reviewed in Marquitz and Raab-Traub 2012). Two TATA-less promoters, P1 and P2 (Fig. 1), located ~400 nt upstream of BART exon 1 are responsible for BART mRNA transcription (Chen et al. 2005). These promoters can be regulated by a number of transcription factors. P1 is upregulated by Jun family members, which can bind to a consensus AP-1 site directly upstream of P1, and is suppressed by IRF-5 and IRF-7, which bind an IRF site following their induction by type I interferon (Chen et al. 2005). P2 contains putative binding sites for c-Myc and C/EBP family members, and it has been suggested that high levels of C/EBP proteins in NPCs may contribute to the high levels of BARTs detectable in these tumors (Chen et al. 2005). Epigenetic mechanisms, including methylation of the P2 region, further regulate BART transcription, and levels of the BART miRNAs that arise from the first four BART introns (see Sect. 6) have been shown to correlate with the level of promoter methylation. BL cell lines express comparatively low levels of BART miRNAs and exhibit high BART promoter methylation, while LCLs express higher levels of BART miRNAs and lower promoter methylation (Kim do et al. 2011). Consistent with these observations, treatment of EBV-infected B cells with a DNA methyltransferase inhibitor leads to the induction of BART miRNA expression (Kim do et al. 2011). The BART promoter region in NPC tumors and cell lines is reportedly hypomethylated (Al-Mozaini et al. 2009; de Jesus et al. 2003), which may further explain the abundance of BARTs in NPCs.
The protein-coding potential of the BARTs remains controversial. Several ORFs have been suggested for the BARTs including BARF0, RK-BARF0, RPMS1, and A73 (Gilligan et al. 1990; Smith et al. 2000). Exon VII contains a small, putative ORF predicted to encode a 174 amino acid protein called BARF0 (BamH1 A rightward frame 0). An alternatively spliced transcript encompassing exon V and exon VII was predicted to encode a larger, 279 aa protein termed RK-BARF0 (Kienzle et al. 1999). BARF0, generated via in vitro translation, could be detected by Western blot using serum from NPC patients, which initially suggested that NPC patients might produce antibodies to BARF0 (Gilligan et al. 1991). Contradicting these results, BL cells expressing a BARF0 recombinant protein failed to elicit a cytotoxic T cell (CTL) response when using CTLs from EBV-seropositive patients (Kienzle et al. 1998). Additional experiments have been inconsistent in providing firm evidence for the existence of BARF0 and RK-BARF0 protein products. Antibodies raised against BARF0 peptides can detect in vitro generated BARF0 and RK-BARF0; however, these antibodies also cross-react with cellular HLA-DR in EBV-negative cells (reviewed in Marquitz and Raab-Traub 2012). Recombinant proteins RPMS1 and A73, and 103 aa and 126 aa, respectively, can be artificially expressed in Escherichia coli from spliced BART exons (Smith et al. 2000). Binding assays and yeast two-hybrid screens with these recombinant proteins indicate interactions with cellular proteins such as RBP-Jk/CBF1 for RPMS1 and the calcium-regulator RACK1 for A73 (Smith et al. 2000; Zhang et al. 2001); such interactions have not been confirmed in the context of infection.
Despite the ability to experimentally generate recombinant BART-origin proteins in vitro as well as observe phenotypes associated with their expression in tissue culture (reviewed in Marquitz and Raab-Traub 2012), no BART protein products have been detected in naturally infected cells in vivo to date (Smith et al. 2000; Al-Mozaini et al. 2009) and it remains unclear whether any of the predicted BART ORFs are indeed translated. Given the predominant nuclear localization of the BARTs and lack of clear evidence for BART protein products, it is possible that the BARTs represent noncoding regulatory RNAs that function similar to cellular long ncRNAs (lncRNAs) to regulate viral and/or cellular gene expression. A major role for the intronic regions of these transcripts may also be to produce the viral BART miRNAs (Sect. 6). While the function of BART transcripts therefore remains undefined, they are indisputably abundant in EBV epithelial tumors and therefore thought to be a contributing factor in NPC pathogenesis.
4 Viral snoRNA1
Small nucleolar RNAs (snoRNAs) are ~60–200-nt stable, noncoding RNAs that localize to the nucleolus, a sub-nuclear compartment, and form snoRNA:protein complexes (snoRNPs) that guide the chemical modifications of other RNAs. EBV encodes a single, ~65-nt canonical box C/D snoRNA (Fig. 2c), termed v-snoRNA1, that is located within the BART region, ~100 bp downstream of miR-BART2 (Fig. 1), and is detectable by Northern blot in latently infected B cell lines (Hutzinger et al. 2009). V-snoRNA1 binds canonical core ribonucleoproteins including fibrillarin, Nop65, and Nop58 and is thus thought to assemble with these proteins into a functional snoRNP to guide RNA modifications (Hutzinger et al. 2009). A 24-nt viral RNA with miRNA-like activity has been proposed to be processed from v-snoRNA (Hutzinger et al. 2009) and is detectable by Northern blot at varying levels in EBV-infected B cells and epithelial cells (Lung et al. 2013). Contrary to these studies, RISC immunoprecipitation/deep sequencing experiments in LCLs and EBV+ BLs and deep sequencing experiments in NPCs have failed to capture a v-snoRNA-derived RNA species with miRNA-like features (Riley et al. 2012; Skalsky et al. 2012, 2014; Chen et al. 2010). Nucleotide differences in the v-snoRNA1 region have been noted in different EBV strains (Lung et al. 2013) which may account for differences in these studies. Additionally, the 24-nt v-snoRNA-derived RNA may be present only in epithelial cells during lytic infection (Lung et al. 2013).
5 EBV-sisRNA-1 and Other Viral ncRNAs with Potential Regulatory Activities
RNA sequencing analysis of nuclear RNAs from latently infected B cells recently uncovered several novel EBV ncRNAs including a stable intronic-sequence RNA (ebv-sisRNA-1) that arises from the W repeat region in the genome and is predicted to form a conserved loop structure with two small hairpins (Moss and Steitz 2013). Spliced introns are normally degraded in the nucleus; however, the unusually stable 81-nt ebv-sisRNA-1 is abundantly detectable in latently infected cells by Northern blot at levels comparable to EBER2. Functions for the newly described ebv-sisRNA-1 have yet to be determined. Studies on cellular sisRNAs in Xenopus oocytes have revealed that many sisRNAs are stable for at least 48 h post-transcription (Gardner et al. 2012; Talhouarne and Gall 2014). Interestingly, injection of Xenopus oocytes with SV40 polyomavirus DNA yields a similar, unusually stable nuclear, non-capped, non-polyadenylated, and intronic viral RNA (Michaeli et al. 1988), raising the possibility that other DNA tumor viruses might encode sisRNAs. In fact, lariat-derived, stable intronic RNAs have been described for other herpesviruses, such as the ~2-kb latency-associated transcript (LAT) expressed by herpes simplex virus (Bloom 2004), a conserved ~5-kb intron expressed by human cytomegalovirus (hCMV) (Kulesza and Shenk 2004), and a 7.2-kb ortholog, RNA7.2, expressed by mouse CMV, which facilitates persistent viral replication in vivo (Kulesza and Shenk 2006).
Recent RNA sequencing analysis of poly(A)-selected or rRNA-depleted RNA from Mutu I BL cells and lytically reactivated Akata BL cells revealed bidirectional transcription in many regions of the EBV genome (Concha et al. 2012; O’Grady et al. 2014). Hundreds of novel viral transcripts and stable introns arising from complex, alternative splicing events were detectable during lytic infection. Most of these RNAs lack predicted protein-coding potential. While many of these RNAs may result from RNA degradation, some of the transcripts may represent authentic viral ncRNAs and play a role in the viral transcriptional program or epigenetic regulation similar to what has been reported for cellular long ncRNAs. Additional studies are needed to characterize and define roles for these RNAs during EBV infection.
6 EBV microRNAs
One of the most recently identified and now widely studied forms of EBV ncRNA are the viral miRNAs. miRNAs are an important class of small, ~22-nt regulatory ncRNAs that post-transcriptionally regulate gene expression by guiding the RNA-induced silencing complex (RISC) to partially complementary sequences on target mRNAs. Depending on the degree of complementarity between the miRNA and the target mRNA sequence, miRNA-loaded RISC binding can induce the immediate degradation of a target mRNA or result in translational inhibition often followed by mRNA destabilization (Ambros 2004). miRNAs are expressed by all metazoans and more recently have been identified in many viruses—in particular, the herpesviruses (reviewed in Skalsky and Cullen 2010). miRNAs require only limited sequence complementarity in order to interact with a target mRNA. Predominantly, complementarity to nt 2–7 or 8 of the mature miRNA, termed the miRNA “seed” sequence, is required for target interactions (Bartel 2009). As such, individual miRNAs are able to regulate upward of 200 different transcripts (Friedman et al. 2009) and collectively regulate >30 % of protein-coding transcripts (Carthew and Sontheimer 2009), and thus have a wide impact on gene expression. Cellular miRNAs are implicated in a number of critical cell signaling networks and biological processes including homeostasis, hematopoiesis, and the development of immunological responses. Furthermore, deregulated miRNA expression has been causatively linked to a number of cancers and disease states (Adams et al. 2014).
EBV was the first virus shown to encode viral miRNAs (Pfeffer et al. 2004). Five viral miRNAs were originally identified during cloning of small RNAs from EBV B95-8-infected Burkitt’s lymphoma (BL) cells (Pfeffer et al. 2004). The EBV B95-8 strain has a 12-kb deletion within the BART region and thus lacks many EBV miRNAs. Additional sequencing efforts to examine miR-NAs in EBV/KSHV-infected BC-1 cells (Cai et al. 2006; Gottwein et al. 2011), EBV B95-8 and wild-type EBV LCLs (Skalsky et al. 2012, 2014), and NPC samples infected with wild-type EBV strains, supplemented with bioinformat-ics analysis (Zhu et al. 2009; Chen et al. 2010; Grundhoff et al. 2006; Edwards et al. 2008), have revealed a total of 25 EBV precursor miRNAs (pre-miRNAs) from which ~40 mature miRNAs are processed. Three BHRF1 pre-miRNAs are encoded adjacent to the BHRF1 ORF, which encodes a viral Bcl2 homolog. The remaining pre-miRNAs are in introns located in the BART region; these consist of two large clusters together encompassing 21 BART miRNAs, as well as the more isolated pre-miRNA for miR-BART2, which lies antisense to the EBV BALF5 gene that encodes the viral DNA polymerase (Fig. 1).
Notably, the EBV miRNAs are highly conserved in other lymphocryptoviruses (LCVs) of the gamma-herpesvirus family (Riley et al. 2010; Walz et al. 2009; Cai et al. 2006; Skalsky et al. 2014). The closely related rhesus LCV, which is separated from EBV by ~13 million years of evolution, encodes 34 pre-miRNAs, twenty one of which share extensive sequence identity with EBV miRNAs and are located in homologous regions of the viral genome (Cai et al. 2006; Walz et al. 2009). Three additional LCVs that infect Old World non-human primates, Herpesvirus pan, H. papio, and Pan paniscus LCV1, encode homologs of the BHRF1 and BART cluster I miRNAs (Skalsky et al. 2014; Aswad and Katzourakis 2014; R.L. Skalsky, unpublished). Additional LCV sequences are currently lacking; however, other LCVs are also predicted to encode BART cluster II miRNA homologs based on the analysis of LCV LMP1 3′UTRs, which contain evolutionarily conserved binding sites for multiple BART miRNA homologs (Skalsky et al. 2012, 2014; Riley et al. 2012; Lo et al. 2007). With the exception of several miRNA seed-sequence mimics (see below and Fig. 4), none of the EBV miRNAs exhibit homology otherwise to known cellular miRNAs.
Fig. 4.
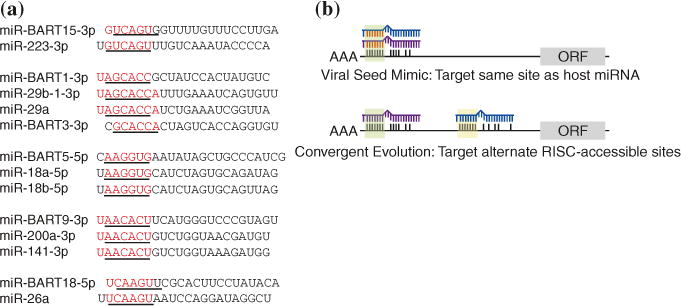
a EBV miRNAs exhibit full as well as offset seed-sequence homology to human miR-NAs. The seed (nt 2–7) of each mature miRNA is underlined. b EBV can usurp existing miRNA regulatory networks by (i) encoding mimics of cellular miRNAs (see a), (ii) perturbing cellular miRNA expression patterns, and (iii) targeting RISC-accessible sites on cellular RNAs involved in conserved biological pathways (convergent evolution)
6.1 miRNA Biogenesis
EBV miRNAs are dependent entirely on the cellular miRNA processing machinery for their biogenesis. To date, no viral factors are known to be involved in EBV miRNA processing. Comparable to their cellular counterparts, EBV miRNAs arise from long, nuclear RNA polymerase II primary miRNA (pri-miRNA) transcripts, which form stem-loop structures that are cleaved by the microprocessor complex, a heterodimer consisting of the RNAse III-like enzyme Drosha and its cofactor DGCR8 (Fig. 3). This cleavage results in a ~60-nt pre-miRNA, which contains an imperfect ~22 bp RNA stem with a 2 nt 3′ overhang and a terminal loop of at least 10 nt (Figs. 2b and 3). Pre-miRNA export from the nucleus into the cytoplasm is mediated by Exportin-5. Subsequent cleavage of the pre-miRNA by cytoplasmic Dicer yields a ~22-bp duplex intermediate, with 2 nt 3′ overhangs, consisting of the mature miRNA and the miRNA passenger strand. One strand of the duplex is incorporated into RISC, which minimally consists of an Argonaute (Ago) family protein, such as Ago2, and a mature miRNA (miRNA processing is reviewed in Ambros 2004; Bartel 2004; Skalsky and Cullen 2010). Ago proteins have two RNA-binding domains: a PIWI domain that binds the miRNA 5′ end and a PAZ domain that binds the 3′ end of the miRNA (Yang and Yuan 2009). Mature, functional miRNAs can be derived from either the 5′ or 3′ arm of a pre-miRNA and are denoted 5p or 3p based on their origin (Ambros et al. 2003) (Fig. 2b). miRNA-loaded RISC subsequently binds to sites on target mRNAs, preferentially in 3′UTRs, and attenuates mRNA translation and/or stability. Ago2 has endonuclease activity and is able to cleave target transcripts directly, depending on the level of miRNA complementarity (Pillai et al. 2007).
Fig. 3.
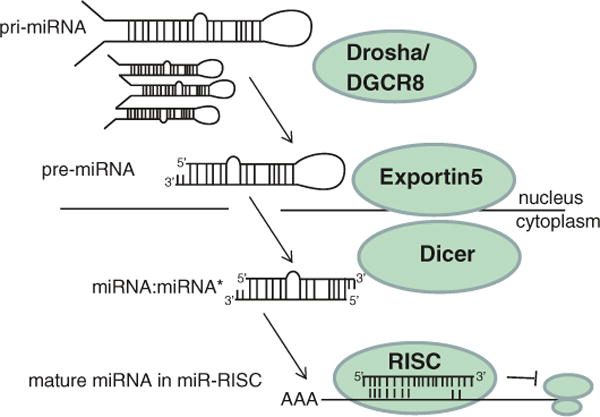
Canonical miRNA biogenesis. EBV miRNAs utilize the cellular miRNA biogenesis machinery and arise from long primary miRNA (pri-miRNA) transcripts in the nucleus that are cleaved into precursor miRNAs (pre-miRNAs) and exported into the cytoplasm. Subsequent cleavage by Dicer yields a miRNA duplex, one strand of which is incorporated into the RNA-induced silencing complex (RISC) to guide RISC to sites in 3′UTRs of target mRNAs. miR-RISC binding results in translational silencing of the target mRNA
6.2 Expression of EBV miRNAs
High-throughput sequencing and PCR-based miRNA arrays have significantly advanced our understanding of the types of EBV miRNAs expressed in virally infected tissues and tumors. The BHRF1 and BART miRNA clusters are transcribed by different promoters: the major latency promoters, Cp and Wp, for the BHRF1 miRNAs and the BART P1 and P2 promoters for the BART miRNAs (Fig. 1), and thus, the miRNAs are differentially expressed depending on the particular viral latency program within the infected cell type.
Latency III occurs in LCLs in vitro and in many EBV+ B cell tumors, including a subset of NHL and EBV+ AIDS diffuse large B cell lymphomas. High levels of BHRF1 miRNAs are detectable by qRT-PCR and by next-generation sequencing in all latency III EBV+ B cells where the Cp and Wp promoters are active (Amoroso et al. 2011; Xia et al. 2008; Skalsky et al. 2012; Pratt et al. 2009). Additionally, BHRF1 miRNAs are detectable in Wp-restricted BL cell lines, which may contribute to the observed resistance of Wp-restricted BL to cell death stimuli compared to latency I BL cells (Amoroso et al. 2011). Unlike in latency II DLBCLs, the BHRF1 miRNAs constitute a substantial portion of the miRNA population in latency III AIDS-DLBCLs, suggesting they may contribute to ongoing pathogenesis in a subset of lymphomas (Imig et al. 2011; Barth et al. 2011; R.L. Skalsky et al., unpublished observations).
Northern blot assays indicate that the BHRF1 miRNAs are most likely derived from introns following splicing of latent Cp and/or Wp transcripts that also generate LTIII BHRF1 mRNAs during latent infection (Xing and Kieff 2007, 2011). During lytic replication, an alternative promoter (BHRF1p) drives BHRF1 mRNA expression. Kinetic studies show that miR-BHRF1-2 and miR-BHRF1-3 levels, in particular, increase during lytic reactivation and correlate with expression of the lytic BHRF1 transcript, suggesting that these two BHRF1 miRNAs can additionally be generated from Drosha cleavage of the lytic BHRF1 transcript (Xing and Kieff 2007, 2011; Amoroso et al. 2011). miR-BHRF1-1, on the other hand, overlaps the BHRF1 promoter region, and thus, expression of miR-BHRF1-1 appears to be entirely dependent on Cp/Wp activity (Amoroso et al. 2011). Coordination between Drosha processing and the splicing machinery that processes LTIII BHRF1 RNAs is further required for miR-BHRF1-1 expression (Xing and Kieff 2011).
BART miRNAs are detectable during all forms of latency; however, their expression levels vary dramatically depending on the infected cell type (Qiu et al. 2011). As the BART miRNAs derive from introns of the longer BART ncRNAs (Edwards et al. 2008), they are thought to share the BART ncRNA expression pattern, being found at especially high levels in latency II that occurs in transformed epithelial cells, including NPCs and GCs, as well as in EBV+/KSHV+ PELs (Cai et al. 2006; Gottwein et al. 2011; Chen et al. 2010; Lung et al. 2009; Zhu et al. 2009; Marquitz et al. 2014). EBV-associated B cell tumors in vivo undergoing latency I and II programs express the BART but not the BHRF1 miR-NAs, and low levels of BART miRNAs are detectable in latency I BL cell lines in vitro (Vereide et al. 2014; Pratt et al. 2009; Amoroso et al. 2011; Qiu et al. 2011). Intriguingly, even though BART miRNAs are expressed as clusters, the relative abundance of individual BART miRNAs within each cluster can vary dramatically (reviewed in Marquitz and Raab-Traub 2012). Some BART miRNAs are reported to be present at thousands of copies per cell, while others are present at less than one hundred copies per cell. There are inconsistencies in the specific abundance of a given BART miRNA in different studies, which is likely due to the different EBV strains and cell types examined as well as methods utilized to detect miRNA expression (Edwards et al. 2008; Marquitz et al. 2014; Skalsky et al. 2012, 2014; Amoroso et al. 2011; Cai et al. 2006; Zhu et al. 2009; Chen et al. 2010; Pratt et al. 2009; Gottwein et al. 2011; Qiu et al. 2011). Nevertheless, BART miRNAs are consistently abundant in EBV-infected epithelial cells, and similar to BHRF1 miR-NAs, the BART miRNAs can be upregulated in response to lytic reactivation (Cai et al. 2006; Amoroso et al. 2011).
6.3 Sequence Polymorphisms in EBV miRNAs
RNA secondary structure plays an important role in miRNA expression. Nucleotide changes that disrupt stem pairing in the pre-miRNA can abrogate Drosha or Dicer cleavage and thereby miRNA expression (Gottwein et al. 2006). Many EBV pre-miRNAs are able to tolerate nucleotide changes within their terminal loops, and cell line-dependent sequence differences for BHRF1 miRNAs, in particular, have been noted (Amoroso et al. 2011); however, these sequence changes do not affect the overall pre-miRNA stem-loop structure that acts as a substrate for Dicer; thus, expression of the mature miRNA is unaffected. miR-BHRF1-3, for example, exhibits sequence variations in the seed sequence (notably a C to U change that affects seed pairing) in several BL cell lines (Ava, Kem, Glor, Sal, and Sav) that are compensated by changes in the opposite passenger strand (Amoroso et al. 2011). Interestingly, miR-BHRF1-3 is not as well conserved at the miRNA sequence level as the other lymphocryptovirus BHRF1 miRNAs (Skalsky et al. 2014), although the flanking regions surrounding all three BHRF1 pre-miRNAs are highly conserved, suggesting that additional sequence requirements may contribute to viral miRNA processing. Nucleotide polymorphisms have also been reported in the regions flanking miR-BART21 and miR-BART22, which may affect the expression levels of these miRNAs in certain cell types (Lung et al. 2009).
Post-transcriptional editing events can also have an effect on miRNA expression. The primary transcripts for four viral miRNAs, miR-BHRF1-1, miR-BART6, miR-BART8, and miR-BART16, were reported to undergo editing at specific nucleotide sites, none of which are located in the miRNA seed regions (Iizasa et al. 2010). Pri-miR-BART6, in particular, undergoes A-to-I editing—most likely by the ADAR1 enzyme—at specific adenosine residues that affect Drosha cleavage and miRNA processing when accompanied by uridine nucleotide deletions in the pre-miRNA terminal loop (Iizasa et al. 2010). Such uridine deletions are observed in the primary BART6 transcripts for Daudi and C666-1 viral strains compared to other wild-type EBV strains and may regulate the ability of miR-BART6 to target host mRNAs encoding Dicer (Iizasa et al. 2010). Notably, the miR-BART6 editing events are also detectable in the Akata and MutuI BL cell lines (Lin et al. 2013b). A second EBV miRNA, miR-BART3-5p, has recently been reported to undergo A-to-I editing in epithelial carcinoma cells, which may alter its ability to target the DICE1 mRNA (Lei et al. 2013); however, deep sequencing experiments have not detected significant levels of edited miR-BART3-5p in EBV-infected AGS epithelial carcinoma cells, NPCs, or LCLs (Skalsky et al. 2012; Chen et al. 2010; Marquitz et al. 2014). Further studies are needed to understand how these editing events, which appear to occur at quite low levels, might contribute to miRNA phenotypes in vivo.
6.4 Viral miRNAs as Biomarkers and in Exosomes
Multiple studies have shown that miRNAs have tremendous potential as biomark-ers for disease states and for monitoring responses to therapies. qRT-PCR analysis of the level of miR-BART2-5p, miR-BART6-5p, and miR-BART17-5p in serum from NPC patients and healthy control individuals demonstrated that EBV miRNA expression correlates with NPC status, strongly indicating that the presence of circulating viral miRNAs could be used as a non-invasive diagnostic or prognostic biomarker for EBV-associated tumors (Wong et al. 2012). miRNAs are highly stable in serum and plasma samples, perhaps due in part to their association with membrane-bound exosomes, which can protect miRNAs from nuclease degradation.
Exosomes are small, extracellular membrane vesicles that contain mRNAs, miRNAs, and proteins, arise through endosome and vesicular trafficking pathways, and are secreted by many different cell types (Valadi et al. 2007). Pegtel et al. first demonstrated that EBV miRNAs were present in CD63+ exosomes purified from the supernatant of EBV-infected cell cultures (Pegtel et al. 2010); the secreted, exosome-associated viral miRNAs were reported to be internalized by recipient cells and present at physiological levels high enough to inhibit a 3′UTR reporter in these cells. Although studies are still in their early stages, exosome-mediated delivery of miRNAs and other virus products, including the EBERs, has been proposed as a method for intercellular communication during infection, and exosomal transfer of viral miRNAs, in particular, could dampen signals in neighboring cells related to immune activation (Pegtel et al. 2010; Gourzones et al. 2010; Meckes et al. 2010; Valadi et al. 2007). However, given that an expression level of at least 0.1 % of the total miRNA pool in a given cell is needed to exert a detectable effect on target mRNA expression (Mullokandov et al. 2012), exosome-delivered viral miRNAs may only be present at limiting levels in recipient cells. Notably, contact-dependent intercellular transfer of EBV miRNAs from latency III Raji BL cells to CD3+ T cells has also been observed, which may introduce a greater amount of viral miRNAs into recipient cells and potentially result in the downregulation of EBV miRNA-targeted transcripts (Rechavi et al. 2009). Thus, it seems that EBV has hijacked or exploited multiple intercellular communication systems in order to alter external signals from neighboring, non-infected cells.
6.5 Functions for EBV miRNAs
Functions for EBV miRNAs have begun to slowly emerge, and it is now apparent that many viral miRNAs may contribute to and/or promote viral latency by targeting viral and cellular factors involved in host cell growth, survival, and signaling pathways as well as cellular factors involved in anti-viral immune responses. Some of the viral miRNA targets involved in these processes are now known (see next sections).
A number of studies point to a role for EBV miRNAs during the initial stages of infection. Unlike KSHV miRNA mutant viruses (Lei et al. 2010), EBV mutants lacking miRNAs do not spontaneously reactivate (Seto et al. 2010; Feederle et al. 2011a, b), arguing that EBV miRNAs, at least in B cells, exert some of their regulation during events leading to latent infection. BHRF1 and BART miRNAs can be detected by two days post-infection of primary B cells in vitro, and EBV miRNA levels continue to increase during the first week of infection as B cells progress to LCLs (Pratt et al. 2009; Amoroso et al. 2011). miR-BHRF1-1 and miR-BHRF1-2, in particular, peak by 3 dpi (Amoroso et al. 2011). Loss-of-function studies show that the EBV BHRF1 miRNAs contribute to LCL outgrowth, B cell immortalization, and cell cycle progression in vitro (Feederle et al. 2011a, b; Seto et al. 2010; Wahl et al. 2013). In line with a role for BHRF1 miRNAs early after infection, a 20-fold to 30-fold reduction in LCL outgrowth was observed for EBV miR-BHRF1 mutants (Feederle et al. 2011a, b; Seto et al. 2010). Established LCLs lacking all three BHRF1 miRNAs continue to exhibit a reduced growth rate when compared to “wild-type” LCLs and exhibit a significant reduction in their ability to enter S-phase (Feederle et al. 2011b). After four weeks in culture, LCLs lacking BHRF1 miRNAs also exhibit an increase in Cp/Wp promoter activity and higher levels of EBNA-LP (Feederle et al. 2011a, b). Since BHRF1 miRNAs do not target EBNA-LP mRNAs, the higher EBNA-LP levels must be an indirect consequence of miR-BHRF1 inactivation. Humanized mouse model studies using CD34+ human fetal liver cell transplants to reconstitute the immune system in NSG mice show that the BHRF1 miRNAs play a role in acute, systemic infection in vivo; mutational inactivation of the BHRF1 miRNAs results in significant delays in viremia (Wahl et al. 2013), further supporting an important role for the BHRF1 miRNAs early after infection.
Recently, phenotypic studies with miRNA mutant recombinant viruses have implicated EBV miRNAs in cell growth and survival, which is relevant for cancer. BART miRNAs downregulate pro-apoptotic and tumor suppressor gene products, enhance the growth transforming properties of EBV-infected epithelial cells, and can enhance metastatic potential in epithelial carcinomas (Marquitz et al. 2011, 2012; Hsu et al. 2014; Choy et al. 2008; Skalsky et al. 2012; Kang et al. 2015; Cai et al. 2015). Expression of BART miRNAs in early-stage LCLs or BL cell lines protects cells from apoptosis (Vereide et al. 2014; Seto et al. 2010). Additionally, individual BART miRNAs can influence NF-kB signaling through the regulation of LMP1 and cellular transcripts that control IkBa stability (Skalsky et al. 2012, 2014; Lo et al. 2007). Thus, EBV-encoded miRNAs can influence multiple signaling pathways in infected cells.
EBV laboratory strains lacking the BART region (i.e., B95-8) and BHRF1 miRNA knockout viruses remain able to effectively immortalize B cell in vitro, demonstrating that EBV miRNAs are not essential for B cell transformation. Furthermore, absence of the BHRF1 miRNAs and the majority of BART miR-NAs had little effect on the oncogenic potential of EBV in humanized immunodeficient mice (Walsh et al. 2010). In immunocompetent human patients in vivo, however, EBV miRNAs may promote viral latency or oncogenesis by modulating cellular mRNAs, especially in cell types and tumors where the viral miRNAs are expressed at high levels, and play a key role in the persistence of latently infected cells by attenuating host immune responses (see Sect. 6.8.1).
Studies on related oncogenic herpesviruses have linked viral miRNAs to cancer formation in vivo. KSHV, a human g-herpesvirus linked to KS and PEL, encodes 12 viral pre-miRNAs, eight of which enhance tumor incidence in nude mouse models (presumably, the viral miRNAs are targeting conserved cellular genes within this context) (Moody et al. 2013). MDV-1, a chicken herpesvirus that causes T cell lymphomas, encodes a viral mimic of miR-155 (Yao et al. 2008). Deletion of the viral miR-155 mimic within the context of the viral genome fully abrogates tumor formation in chickens (Zhao et al. 2011), demonstrating that viral miRNAs can exert robust phenotypes within the context of their natural hosts.
6.6 Identifying Viral miRNA Targets
miRNA target identification continues to be a major hurdle for the field. Methods to first bioinformatically predict miRNA targets based on seed pairing (Lewis et al. 2005), combined with assays to examine transcriptional or translational changes in response to miRNA gain or loss of function, have been successful in determining a handful of viral miRNA targets (Skalsky et al. 2007b; Gottwein et al. 2007; Xia et al. 2008; Lo et al. 2007, 2012; Marquitz et al. 2011; Choy et al. 2008). Transcriptome-wide studies, such as RISC immunoprecipitation followed by microarray-based transcriptome profiling (RIP-Chip), have significantly increased the list of potential viral miRNA targets (Dolken et al. 2010). While techniques such as RIP-Chip have been instrumental in identifying the mRNAs that are RISC-associated during viral infection, these techniques are still unable to distinguish mRNAs targeted by viral miRNAs from those mRNAs that are targeted by cellular miRNAs.
More recently, high-throughput approaches to experimentally isolate and sequence RNAs that are cross-linked to RISC have been highly successful in capturing hundreds of direct viral miRNA targets. Two such methods, PAR-CLIP (photoactivatable ribonucleoside-enhanced cross-linking and immunoprecipitation) and HITS-CLIP (high-throughput sequencing of RNA isolated by cross-linking and immunoprecipitation), have been applied to EBV-infected B cells including LCLs (Skalsky et al. 2012, 2014), latency II BC-1 PEL cells co-infected with EBV and KSHV (Gottwein et al. 2011), and the latency III BL cell line Jijoye (Riley et al. 2012). The HITS-CLIP method utilizes a cross-linking wavelength of UV 245 nm and has an advantage in that it can be applied to both cell lines in vitro as well as tissue samples obtained in vivo (Chi et al. 2009; Haecker et al. 2012; Riley et al. 2012). The PAR-CLIP method relies on growing cells in the presence of a photoactivatable nucleoside analog, such as 4-thiouridine (4SU), that is incorporated into nascent RNAs and allows for efficient cross-linking at a UV wavelength of 365 nm (Hafner et al. 2010; Skalsky et al. 2012; Gottwein et al. 2011). For both methods, following UV cross-linking, RISC-associated RNAs are immu-nopurified using antibodies to an Ago protein and the complexes are digested with an RNase to leave only RISC-protected RNAs, representing miRNA-interaction sites. These RNAs are subsequently ligated to adapters and PCR-amplified prior to high-throughput sequencing. Computational algorithms are then applied to reconstruct miRNA-interaction sites from the CLIP data.
Key to PAR-CLIP is the addition of the 4SU; not only does this enhance cross-linking, but it also marks the cross-linked site, giving the method an advantage in defining a miRNA-interaction site. During PCR amplification of the sequencing library, 4SU pairs with a “G” instead of an “A” which causes a T-to-C conversion in the sequencing read at the cross-linked site (Hafner et al. 2010). Thus, PAR-CLIP, combined with the PARalyzer algorithm designed specifically to extract RNA-binding protein sites from PAR-CLIP sequencing datasets (Corcoran et al. 2011), has a high success rate in defining miRNA binding sites. In fact, over 80 % of miRNA targets captured via PAR-CLIP represent bona fide targets and can be experimentally confirmed (Skalsky et al. 2012; Gottwein et al. 2011).
While both PAR-CLIP and HITS-CLIP techniques are technically and computationally challenging, they have clearly yielded novel insights into the types of miRNA regulation occurring during EBV infection. Consistent with studies examining cellular miRNA function (Lewis et al. 2005; Bartel 2009; Hafner et al. 2010), the majority of EBV miRNA binding sites occur in 3′UTRs, and to a lesser extent, in coding regions, and occur predominantly in cellular mRNAs (Skalsky et al. 2012, 2014; Riley et al. 2012; Gottwein et al. 2011). HITS-CLIP experiments in Jijoye cells indicate that approximately half of the EBV miRNA targets are co-targeted by members of the miR-17/92 cluster (Riley et al. 2012), which is abundantly expressed in BL cells and has a well-established role in BL pathogenesis. Notably, over 75 % of viral targets captured by PAR-CLIP in LCLs are also targeted by B cell miRNAs, and the majority of these co-targeted mRNAs are evolutionarily conserved (Skalsky et al. 2012, 2014), suggesting that viral miRNAs are tying into existing miRNA regulatory networks, such as those involved in B cell activation.
PAR-CLIP experiments with miRNA mutant viruses have further revealed unique features of viral miRNA targeting. For example, analysis of high-confidence cellular target sites for BHRF1 miRNAs revealed that miR-BHRF1-1 utilizes canonical seed-based targeting (pairing with nt 1–8 of the mature miRNA), while other viral miRNAs may tolerate bulge pairing in the seed regions and bind non-canonical sites in addition to seed-match sites (Skalsky et al. 2012; Majoros et al. 2013). While additional experiments are required to confirm these observations, these studies demonstrate that hundreds of both canonical and non-canonical sites on cellular transcripts are bound by EBV miRNAs during infection.
6.7 EBV Transcripts Targeted by miRNAs
EBV miRNAs have described roles during the viral life cycle and can target the 3′UTRs of multiple viral protein-coding mRNAs (Table 1). miR-BART2-5p is perfectly complementary to the BALF5 3′UTR and inhibits expression of the viral DNA polymerase by inducing cleavage of the BALF5 mRNA during lytic reactivation (Pfeffer et al. 2004; Barth et al. 2008). BZLF1 and BRLF1, two lytic gene products that share 3′UTR sequences, were recently reported to be targeted by miR-BART20-5p (Jung et al. 2014). PAR-CLIP experiments captured sites for BART miRNAs in the 3′UTRs of BNRF1 and BALF2 (Skalsky et al. 2014). Analysis of miRNA targets for the closely related rLCV revealed that homologs of BZLF1, BALF2, and other lytic gene products are targeted by multiple LCV miR-NAs (Skalsky et al. 2014), and notably, many of the viral miRNA binding sites are conserved. A number of other herpesvirus miRNAs have been shown to target and inhibit expression of immediate early genes involved in lytic viral replication (reviewed in Skalsky and Cullen 2010). It has been proposed that viral miRNA regulation of lytic mRNAs is a mechanism for stabilizing, maintaining, and/or establishing latency.
Table 1.
EBV transcripts targeted by miRNAs
| EBV target | miRNA(s) | References |
|---|---|---|
| LMP1 | Multiple BART miRNAs, miR-17/106/20/93 family | Lo et al. (2007); Skalsky et al. (2012); Riley et al. (2012); Skalsky et al. (2014) |
| BHRF1 | miR-BART10-3p, miR-17/106/20/93 family | Skalsky et al. (2012); Riley et al. (2012); Skalsky et al. (2014) |
| BALF5 | miR-BART2-5p | Pfeffer et al. (2004); Barth et al. (2008) |
| BZLF1/BRLF1 | miR-BART20-5p | Jung et al. (2014) |
| LMP2A | miR-BART22 | Lung et al. (2009) |
| EBNA2 | ? | Skalsky et al. (2012); Riley et al. (2012) |
| BNRF1 | miR-BART5-5p, miR-BART17-3p | Skalsky et al. (2014) |
| BALF2 | miR-BART1-5p | Skalsky et al. (2014) |
Latency-associated viral gene products are also regulated by viral miRNAs. The BHRF1 3′UTR is targeted by miR-BART10-3p (Skalsky et al. 2014; Riley et al. 2012). Multiple BART miRNAs target the 3′UTRs of LMP1 and LMP2A, which are two highly immunogenic viral proteins that contribute to the proliferation and transformation of EBV-infected cells by activating specific cell signaling pathways (see other Chapters). LMP2A protein levels were reported to be reduced in response to miR-BART22 expression in epithelial cells (Lung et al. 2009). Likewise, BART cluster I miRNAs can downregulate LMP1 protein levels following ectopic expression (Lo et al. 2007; Skalsky et al. 2014). Stringent analysis of the LMP1 3′UTR recently revealed that miR-BART3 and miR-BART5, in particular, directly target the LMP1 3′UTR (Skalsky et al. 2014). Intriguingly, these miR-NAs are conserved in rLCV, and their homologs target the rLCV LMP1 3′UTR in rLCV-infected B cells (Skalsky et al. 2014). PAR-CLIP analysis of rLCV LCLs further revealed that additional viral miRNAs, including a miR-BART20 homolog, interact with the LMP1 3′UTR (Skalsky et al. 2014). The consequences of these interactions have partly been examined; modulation of LMP1 by EBV miRNAs reduces epithelial cell sensitivity to apoptotic stimuli (Lo et al. 2007) and modulates LMP1-mediated NF-kB activation (Skalsky et al. 2014; Lo et al. 2007).
6.7.1 Viral Transcripts Are Targeted by Cellular miRNAs at Conserved Sites
LMP1 has pleiotropic activities to activate multiple cell signaling pathways, such as NF-kB (Young and Rickinson 2004), that promote B cell activation and LMP1 can also induce apoptosis when expressed at high levels (Pratt et al. 2012). In fact, many EBV-induced cellular gene expression changes can be attributed to LMP1 (Cahir-McFarland et al. 2004; Luftig et al. 2003; Soni et al. 2007). Given these activities, is it therefore not surprising that the LMP1 mRNA is subject to intensive regulation by not only viral BART miRNAs, but also conserved cellular miRNAs, namely members of the miR-17 seed family, which includes miR-17, miR-20a/b, miR-106a/b, and miR-93 (Skalsky 2012, 2014; Riley 2012). Inhibition of endogenous miR-17/20/106 activity in LCLs leads to an increase in LMP1 protein levels, demonstrating canonical miRNA-mediated regulation of LMP1 by miR-17 family members (Skalsky et al. 2012). The miR-17/20/106/93 miRNAs arise from three Myc-regulated miRNA clusters (miR-17/92, miR-106b/25, miR-106a/363) and are upregulated in response to transient Myc induction, attributed to EBNA2 expression, shortly following de novo B cell infection (Nikitin et al. 2010; Price et al. 2012). Studies examining LMP1 mRNA and protein levels following de novo infection show a delay in LMP1 protein expression as well as a delay in expression of downstream NF-kB target genes despite the LMP1 transcript being present (Price et al. 2012). This delay may be explained in part due to the activities of miR-17, which may play an important role in transitioning infected B cells between an early Myc-dependent growth program and a later NF-kB-dependent growth program (Faumont et al. 2009; Price et al. 2012).
Regulation of LMP1 by Myc-regulated miRNAs is likely important to lym-phocryptovirus biology since the miR-17/20/106/93 binding site is evolutionarily conserved in the LMP1 3′UTRs of several other LCVs (Skalsky et al. 2014). Intriguingly, another conserved binding site for the miR-17 seed family is present in the 3′UTR of BHRF1, a viral Bcl2 homolog with a role in inhibiting apoptosis (Skalsky et al. 2012, 2014; Riley et al. 2012). BHRF1 is also reported to be regulated by cellular miR-142 (Riley et al. 2012), a miRNA highly expressed in B cells, although this target site is not conserved in other LCVs (Skalsky et al. 2014). The evolutionary conservation of the miR-17 family target sites in LMP1 and BHRF1 indicates that the activities of these two viral gene products are intricately linked to the cellular regulatory pathways controlled by this miRNA family. PAR-CLIP and HITS-CLIP studies of miR-17 family targets as well as studies with transgenic miR-17/92-expressing mice demonstrate that these miRNAs negatively regulate canonical NF-kB activation and inhibit pro-apoptotic genes (Skalsky et al. 2012, 2014; Jin et al. 2013; Riley et al. 2012). Thus, in EBV-infected cells, LMP1, BHRF1, and miR-17 family members have both antagonistic and synergistic roles in relation to one another, which may be resolved through miR-17 control of the viral gene products.
6.8 Cellular Targets of Viral miRNAs
6.8.1 miRNA Targets Involved in Immune Evasion
EBV has evolved multiple strategies to escape recognition by host immune defenses, thereby permitting the virus to latently persist in cells throughout the life of the host. Recently, viral miRNAs have been shown to contribute to EBV-mediated immune evasion strategies by targeting a number of cellular factors involved in immune responses (Table 2). One of the first reported EBV miRNA cellular targets, CXCL11/I-TAC, is an interferon-inducible T cell-attracting chemokine that selectively binds to a T cell chemokine receptor, CXCR3, expressed on NK and Th1 cells. Three non-canonical binding sites in the CXCL11 3′UTR were bioinformatically predicted for miR-BHRF1-3 (Pfeffer et al. 2004), and inhibition of miR-BHRF1-3 in EBV-infected BL cells enhanced CXCL11 mRNA levels (Xia et al. 2008), indicating that miR-BHRF1-3, either directly or perhaps indirectly, regulates CXCL11 expression. TBX21/T-bet, a direct transcriptional activator of IFN-gamma and regulator of IL-2 and Th2 cytokine production, was reported as a target of miR-BART20-5p in invasive EBV+ nasal NK/T cell lymphomas; inhibition of T-bet expression by miR-BART20-5p may contribute to tumor development or allow for EBV replication by inhibiting cytokine production (Lin et al. 2013a). In a similar manner, NLRP3 inflammasome activation and pro-inflammatory cytokine production (IL-1beta) were shown to be inhibited in the presence of miR-BART15-3p (Haneklaus et al. 2012). miR-BART15-3p can target the NLRP3 3′UTR directly, and interestingly, this binding occurs at the miR-223 binding site due to part sequence homology between these two miRNAs (Fig. 4a). MICB, a stress-induced NK cell ligand that is recognized by the NKG2D receptor on NK cells and CD8+ T cells, was reported to be a target of miR-BART2-5p (Nachmani et al. 2009); however, PAR-CLIP and HITS-CLIP studies indicate binding sites for other BART miRNAs in the MICB 3′UTR (Skalsky et al. 2012; Riley et al. 2012). The MICB 3′UTR also contains binding sites for KSHV and HCMV miRNAs; viral miRNA expression leads to a decrease in cell surface expression of MICB and subsequently a reduced cytolytic response following NKG2D activation (Nachmani et al. 2009).
Table 2.
Cellular transcripts targeted by EBV miRNAs
| Cellular target | Function | miRNA(s) | References |
|---|---|---|---|
| BACH1 | Oxidative stress | miR-BHRF1-2 | Skalsky et al. (2012) |
| KDM4B | Histone demethylase | miR-BHRF1-2 | Skalsky et al. (2012) |
| OTUD1 | NF-kB signaling | miR-BART2-5p | Skalsky et al. (2012) |
| PELI1 | NF-kB signaling | miR-BART2-5p | Skalsky et al. (2012); Kang et al. (2015) |
| PDE7A | Cytokine production | Two BART miRNAs | Skalsky et al. (2012) |
| CLEC2D | Immune responses | Two BART miRNAs | Skalsky et al. (2012) |
| LY75 | Immune responses | miR-BART1-5p | Skalsky et al. (2012) |
| SP100 | PML bodies | miR-BART1-5p | Skalsky et al. (2012) |
| ZNF451 | PML bodies | miR-BHRF1-2 | Skalsky et al. (2012) |
| CLIP1 | Immune responses | miR-BART1-5p | Skalsky et al. (2012) |
| GUF1 | GTPase | miR-BHRF1-1 | Skalsky et al. (2012) |
| SCRN1 | Exocytosis | miR-BHRF1-1 | Skalsky et al. (2012) |
| NAT12 | acetyltransferase | miR-BHRF1-1 | Skalsky et al. (2012) |
| DAZAP2 | Wnt signaling | miR-BART3-3p | Skalsky et al. (2012); Kang et al. (2015) |
| CAPRIN2 | Wnt signaling | miR-BART13-3p | Riley et al. (2012) |
| CAND1 | NF-kB signaling | miR-BART3 | Skalsky et al. (2014) |
| FBXW9 | Adaptor protein | miR-BART3 | Skalsky et al. (2014) |
| DICE1 | Tumor suppressor | miR-BART3-5p | Lei et al. (2013); Kang et al. (2015) |
| MAP3K2 | MAPK signaling | miR-BART18-5p | Qiu and Thorley-Lawson (2014) |
| CXCL11 | Immune responses | miR-BHRF1-3 | Xia et al. (2008) |
| DICER | miRNA biogenesis | miR-BART6-5p | Iizasa et al. (2010); Kang et al. (2015) |
| BBC3/PUMA | Apoptosis | miR-BART5? | Choy et al. (2008) |
| BCL2L11/Bim | Apoptosis | Multiple BART miRs | Marquitz et al. (2011) |
| TBX21/T-bet | Immune responses | miR-BART20-5p | Lin et al. (2013a, b) |
| IPO7 | Transport | Two BART miRNAs | Dolken et al. (2010); Skalsky et al. (2012); Riley et al. (2012); Vereide et al. (2014); Kang et al. (2015) |
| TOMM22 | Transport | miR-BART16 | Dolken et al. (2010) |
| MICB | Immune responses | miR-BART2-5p | Nachmani et al. (2009) |
| CASP3 | Apoptosis | Two BART miRNAs | Vereide et al. (2014) |
| WIF1 | Wnt signaling | miR-BART19-3p | Wong et al. (2012) |
| APC | Wnt signaling | Multiple BART miRs | Wong et al. (2012) |
| NLK | Wnt signaling | Multiple BART miRs | Wong et al. (2012) |
| YWHAZ | Protein modifier | miR-BART14 | Grosswendt et al. (2014) |
| NLRP3 | Inflammasome | miR-BART15-3p | Haneklaus et al. (2012) |
| BRUCE | Apoptosis | miR-BART15-3p | Choi et al. (2013) |
| CDH1 | Cell migration | miR-BART9 | Hsu et al. (2014) |
| PTEN | Tumor suppressor | Multiple BART miRs | Cai et al. (2015) |
PAR-CLIP experiments in LCLs have also revealed several EBV miRNA targets related to immune evasion, including SP100, ZNF451, LY75/CD205, PDE7A, and CLEC2D. Both SP100 and ZNF451 are involved in promyelocytic leukemia-nuclear (PML) body formation that occurs in the nucleus during antiviral innate immune responses. A number of herpesviruses use multiple strategies to target PML body formation (Tavalai and Stamminger 2009). LY75 (lymphocyte antigen 75) is a transmembrane receptor that is involved in antigen transport from the cell surface to late endosomes containing MHC class I and II receptors (Gurer et al. 2008). PDE7A is involved in cytokine production and the proliferation of NK cells (Goto et al. 2009). Finally, CLEC2D is another NK ligand expressed on B cell surfaces following B cell receptor signaling or activation of toll-like receptor signaling. CD161, present on NK cells and T cells, recognizes CLEC2D, resulting in the production of IFN-gamma (Germain et al. 2011). EBV miRNA targeting of these transcripts has been confirmed by luciferase reporter assays; however, functional studies are required to understand the biological significance of these interactions—in particular, how inhibition of these transcripts by viral miR-NAs might attenuate host immunological responses to EBV infection.
6.8.2 miRNA Targets Involved in Apoptosis
Many of the identified and confirmed targets for BART miRNAs are pro-apoptotic (Table 2) which supports the observed role of BART miRNAs in protection from apoptotic stimuli (Vereide et al. 2014; Marquitz et al. 2011; Kang et al. 2015). The mRNA of the BH3-only protein, Bim (BCL2L11), was confirmed as a target for multiple BART miRNAs (Marquitz et al. 2011), and subsequent PAR-CLIP experiments in LCLs revealed binding sites for miR-BART-4 and miR-BART15 in the BCL2L11 3′UTR (Skalsky et al. 2012). Bim inhibits the anti-apoptotic function of Bcl-2; consequently, inhibition of Bim expression by BART miRNAs should confer enhanced cell survival (Marquitz et al. 2011). PUMA (BBC3), another BH-3 only protein that facilitates release of cytochrome C from the mitochondria in response to apoptotic stimuli, has been reported as a target of miR-BART5-5p (Choy et al. 2008); however, recent experiments examining miR-BART5 functions in epithelial carcinoma cells have failed to confirm these findings (Kang et al. 2015), and BBC3 transcripts are not enriched in RISC in BART-expressing EBV− negative cells (Vereide et al. 2014).
PAR-CLIP screens identified CASP3 as a potential target of multiple BART miRNAs (Gottwein et al. 2011; Skalsky et al. 2012). Caspase 3, a member of the cysteine-aspartic acid protease family, has an extensively documented role in apoptosis and functions as the executioner caspase. The CASP3 mRNA is decreased in response to BART miRNA expression in epithelial cells (Marquitz et al. 2012). Recent studies using RISC-IP experiments and luciferase reporter assays indicate that CASP3 is a target of miR-BART16 and miR-BART1-3p in BL cells (Vereide et al. 2014) although this could not be confirmed in epithelial cells (Kang et al. 2015). PAR-CLIP and HITS-CLIP studies have identified multiple other cellular factors involved in apoptotic pathways, such as APAF1 (a component of the apoptosome), BCLAF1 (pro-apoptotic), and CAPRIN2 [also related to Wnt signaling (see below)], as well as CASZ1, DICE1, OCT1, CREBBP, SH2B3, PAK2 and TP53INP1 that are targets of EBV miRNAs, further supporting an anti-apoptotic role for EBV miRNAs (Skalsky et al. 2012; Riley et al. 2012; Kang et al. 2015).
6.8.3 miRNA Targets Involved in Multiple Signal Transduction Pathways
As noted above, BART miRNAs can alter NF-kB signaling pathways by directly inhibiting LMP1 expression. Ectopic expression of miR-BART3 or miR-BART1 in the absence of LMP1 disrupts NF-kB activation and stabilizes IkBa (Skalsky et al. 2014), indicating that these miRNAs also target cellular gene products involved in these pathways. Targets potentially involved in IkBa stability include CAND1, an exchange factor for the Skip/Cullin/F-box ubiquitin ligase complex that regulates IkBa, and FBXW9, an evolutionarily conserved F-box protein (Skalsky et al. 2014). Additional targets related to NF-kB signaling include PELI1, a confirmed target of miR-BART2-5p, and E3 ligases cIAP1/XIAP and cIAP2/BIRC3, the deubiquitinase CYLD, A20/TNFAIP3, IKKa/CHUK, and NFKBIZ, all of which are targeted by multiple EBV miRNAs in LCLs (Skalsky et al. 2012, 2014). Notably, these targets are both activators and repressors of NF-kB signaling. An attractive hypothesis is that EBV miRNAs direct and maintain the level of NF-kB activation within a threshold suitable for viral persistence (Skalsky et al. 2014). PELI1 is an E3 ubiquitin ligase that is activated following IL-1beta signaling through IL-1R or through MyD88 (reviewed in Moynagh 2009). PELI1 activity leads to NF-kB activation and the induction of pro-inflammatory cytokines. Thus, knockdown of PELI1 by miR-BART2-5p may also impair innate immune responses (Table 2).
Similar to what is observed for EBV miRNA targets involved in NF-kB signaling, both inhibitors and enhancers of Wnt signaling are regulated by EBV miR-NAs. CAPRIN2, targeted by miR-BART13-3p (Riley et al. 2012), promotes activation of canonical Wnt signaling by stabilizing beta-catenin (Ding et al. 2008). siRNA knockdown of CAPRIN2 decreased Wnt3a-induced LEF1/TCF promoter activity and expression of Wnt target genes (Ding et al. 2008). PAR-CLIP studies in LCLs and microarray experiments in BART-expressing epithelial cell lines identified DAZAP2 as a target of miR-BART3 (Skalsky et al. 2012; Gottwein et al. 2011; Marquitz et al. 2012). DAZAP2 can interact with Tcf/Lef family members, including TCF-4, which transcriptionally activates Wnt-responsive genes. Knockdown of Dazap2 expression reduced Tcf-mediated transcription and responsiveness to Wnt stimulation (Lukas et al. 2009), similar to what has been reported for CAPRIN2 knockdown.
Inhibitors of Wnt signaling are regulated by several BART miRNAs and may play an important role in the proliferation of EBV-infected epithelial cells. Three Wnt antagonists were identified as EBV miRNA targets following profiling of EBV+ NPC tumors (Wong et al. 2012). WIF1 (targeted by miR-BART19-3p), APC (targeted by miR-BART7, miR-BART19-3p, and miR-BART17-5p), and NLK (targeted by miR-BART19-3p, miR-BART14, and miR-BART18-5p) protein and transcript levels were reduced following transient expression of BART miRNA mimics in EBV-negative epithelial cell lines (Wong et al. 2012). Presumably, downregulation of these Wnt antagonists by BART miRNAs would activate Wnt signaling. NLK, activated via MAPK signaling, can block Tcf/Lef transcriptional activation, APC is a direct inhibitor of beta-catenin, and WIF1 can block the induction of the Wnt pathway (Wong et al. 2012). Interestingly, WIF1 is also a positive regulator of miR-200 family members (Ramachandran et al. 2014), which inhibit ZEB1 and ZEB2 expression (Ellis-Connell et al. 2010); both cellular proteins control the switch between latency and lytic reactivation by repressing the BZLF1 promoter. Thus, BART miRNAs may also indirectly regulate lytic viral gene expression through WIF1 targeting.
More recent studies show that targeting of MAP3K2 by miR-BART18-5p plays a role in repressing lytic viral reactivation (Qiu and Thorley-Lawson 2014). MAP3K2 is a central player in multiple signal transduction pathways. Activation of the BZLF1 promoter and initiation of the lytic cascade, at least following B cell receptor cross-linking, occur through several different pathways, including PI3K, Ras, Rac1, and phospholipase C (PLC). Both Rac1 and PLC pathways converge on MAP3K2 to activate p38 and JNK transcriptional regulators. Overexpression of miR-BART18-5p in EBV-infected BL cells inhibited viral reactivation and conversely expression of MAP3K2 in B95-8 LCLs upregulated lytic gene expression (Qiu and Thorley-Lawson 2014), thereby demonstrating a role for miR-BART18-5p in promoting latency by targeting a key signaling molecule.
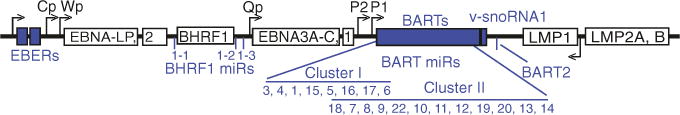
Several mRNAs are now known to be targeted by multiple, different EBV miR-NAs, indicating that EBV miRNAs can synergistically downregulate the expression of specific gene products. In addition to targeting by miR-BART18-5p, the 3′UTR of MAP3K2, for example, is targeted by miR-BART2-5p, BART4, BART5, and BART19-3p (Gottwein et al. 2011; Skalsky et al. 2012). Wnt-inhibitory transcripts as well as pro-apoptotic transcripts, in particular, appear to be co-targeted by multiple EBV miRNAs. Furthermore, multiple components with both activating and repressing potential within a given signal transduction pathway can be collectively targeted by multiple viral miRNAs (Skalsky et al. 2012; Riley et al. 2012; Gottwein et al. 2011). These observations indicate that viral miRNAs cooperatively facilitate a complex, yet finely tuned and directed outcome for signaling events and the transcriptional environment in an infected cell. Future functional studies must therefore consider the combinatorial effects of EBV miRNAs on the full spectrum of targets related to a given pathway.
6.9 EBV Exploits Intrinsic Cellular miRNA Regulatory Networks
Target identification studies have shown that multiple biological processes and cell signaling pathways can be regulated by viral miRNAs, most of which are also intrinsically regulated by cellular miRNAs. EBV is able to tie into these intrinsic miRNA regulatory networks via several means, including (i) by mimicking cellular miRNA sequences (Fig. 4a), (ii) by occupying RISC-accessible sites that are not otherwise occupied by cellular miRNAs (Fig. 4b), and (iii) by altering cellular miRNA expression patterns.
Seed mimicking by a viral miRNA, which presumably enables the viral miRNA to compete for identical seed-match sites on cellular transcripts, was first noted for KSHV miR-K11 and the cellular oncomiR, miR-155 (Skalsky et al. 2007b; Gottwein et al. 2007). miR-155 is upregulated in many B cell lymphomas and critically required for B cell activation and the formation of germinal center reactions (Thai et al. 2007; Rodriguez et al. 2007). The first ten nucleotides of KSHV miR-K11 and miR-155 are identical, and as a result, these miRNAs share a highly overlapping mRNA target repertoire (Skalsky et al. 2007b; Gottwein et al. 2007). To demonstrate that miR-K11 can indeed function as a mimic of miR-155 in vivo, NOD/SCID IL2Rgamma-null mice were injected with CD34+ human cord blood progenitor cells transduced with miR-K11 or miR-155 expression vectors (Boss et al. 2011). Expression of either miR-155 or KSHV miR-K11 led to downregulation of C/EBPbeta, increased production of IL-6, and enhanced B cell proliferation in the spleen, demonstrating that seed mimicking by a viral miRNA can translate into significant consequences in vivo (Boss et al. 2011). Notably, MDV-1, a virus linked to T cell lymphomas in chickens, also encodes a mimic of miR-155, miR-M4, which is involved in tumorigenesis (Zhao et al. 2011) and targets of miR-M4 overlap with targets of miR-155 (Parnas et al. 2014). EBV does not encode a miR-155 seed mimic, but instead strongly induces miR-155 expression directly, which is critically required for the survival and proliferation of EBV-infected cells (Linnstaedt et al. 2010).
Several EBV miRNAs mimic the seed sequences of cellular miRNAs with described tumor suppressor and/or oncomiR activities (Skalsky et al. 2007a; Skalsky and Cullen 2010) (Fig. 4a) and are thus thought to bind cognate sites, although the extent to which these mimics might compete with the cellular miR-NAs for miRNA binding sites is not yet known. The list includes miR-BART9-3p and the miR-200a family, which regulates cell migration and invasion (Bracken et al. 2014; Burk et al. 2008), miR-BART1-3p and the miR-29 family, which is linked to B cell tumors (Pekarsky and Croce 2010), miR-BART5-5p and miR-18-5p, which is a member of the miR-17/92 oncomiR cluster, and lastly, two BART miRNAs which exhibit offset seed homology to cellular miRNAs (miR-BART15-3p and miR-223-3p; miR-BART18-5p and miR-26a-5p) (Haneklaus et al. 2012; Qiu and Thorley-Lawson 2014). MiR-223 is a hematopoietic miRNA with roles in myeloid lineage development and can also inhibit the inflammasome-induced production of IL-1beta. Both miR-223 and miR-BART15-3p reportedly target the NLRP3 3′UTR at the same site, which consequently attenuates inflammasome-mediated activation of IL-1beta production and subsequently reduces inflammation (Haneklaus et al. 2012). miR-BART18-5p reportedly targets a characterized miR-26a binding site in the 3′UTR of MAP3K2 (Qiu and Thorley-Lawson 2014), although this site does not appear to be bound by miR-26a in EBV-infected B cells (Skalsky et al. 2012; Gottwein et al. 2011). miR-BART5 binds a site within the LMP1 3′UTR and can inhibit LMP1 expression (Skalsky et al. 2014; Riley et al. 2012). Experiments with miR-18-5p suggest that only miR-BART5 and not miR-18-5p can bind this site in the LMP1 3′UTR, despite having the same seed sequence (Riley et al. 2012). The lack of LMP1 3′UTR targeting by the miR-18-5p mimic may be attributed to requirements for additional base pairing between the miRNA and the mRNA target outside of the seed region (Riley et al. 2012). Interestingly, a RISC-associated site overlapping this region was identified in LCLs that completely lack miR-BART5, suggesting that miR-18-5p might bind this site in the absence of miR-BART5 (Skalsky et al. 2012).
Common targets for miR-BART9-3p/miR-200a and miR-BART1-3p/miR-29 have not yet been investigated. The miR-200 family is considered to have tumor suppressor activity since miR-200a maintains the epithelial phenotype by suppressing epithelial to mesenchymal transition (EMT) and can regulate a spectrum of targets related to actin cytoskeleton dynamics, focal adhesion, Rho GTPase signaling, and metalloprotease activity (Burk et al. 2008; Bracken et al. 2014). In EBV-infected epithelial cells and carcinomas, miR-200 family members are down-regulated (Lin et al. 2010; Marquitz et al. 2014; Shinozaki et al. 2010) and have been shown to play a role in the EBV latent/lytic switch via the targeting of ZEB1 and ZEB2; induction of miR-200 contributes to lytic replication (Ellis-Connell et al. 2010). Intriguingly, and opposite to what has been documented for miR-200a, miR-BART9 expression can induce a mesenchymal-like phenotype and has been linked to increased metastasis and invasiveness by NPC cells in vitro (Hsu et al. 2014). Thus, while miR-BART9 and miR-200a exhibit homology in the seed regions, miR-BART9 might not be able to bind cognate miR-200a target sites due to sequence differences outside of the miRNA seed region.
miRNA targetome studies indicate that EBV miRNAs predominantly regulate cellular, not viral, gene products (Skalsky et al. 2012, 2014; Gottwein et al. 2011; Riley et al. 2012; Kang et al. 2015). With some exceptions, the sites that are occupied by viral miRNAs on cellular mRNAs are largely not occupied by cellular miRNAs, leading to the hypothesis that viral miRNAs have evolved in part to bind alternate, RISC-accessible sites on cellular gene products that have effector functions pertinent to the viral life cycle. What is further intriguing is that many of these RISC-accessible sites are evolutionarily conserved (Majoros et al. 2013), despite not being occupied by a cellular miRNA—at least, in PAR-CLIP or HITS-CLIP studies. Thus, while a viral miRNA may not bind to the same site(s) as a cellular miRNA, a viral miRNA or combination of viral miRNAs could interact with a set of transcripts specifically regulated by and related to the function of a given cellular miRNA (Fig. 4b). Examples of this include the co-targeting, at distinct sites, of a set of cellular transcripts by EBV miRNAs and members of the miR-17/92 cluster (Riley et al. 2012).
In addition to encoding viral miRNAs, EBV infection dramatically perturbs the cellular miRNA environment and EBV proteins can induce the expression of specific cellular miRNAs that can play pertinent roles in the EBV life cycle (Cameron et al. 2008a, b; Kieff 2007). Most EBV-induced cellular gene expression changes following de novo infection can be attributed to EBNA2 and the CD40 mimic, LMP1. Both EBNA2 and LMP1 initiate dichotomous cell proliferation programs, facilitated through c-Myc and NF-kB activation (Young and Rickinson 2004; Faumont et al. 2009), which also activates expression of known oncomiRs such as the miR-17/92 cluster and miR-155 (Price et al. 2012; Forte and Luftig 2011; Nikitin et al. 2010; Kieff 2007). Recent studies show that transient c-Myc induction is an inherent and critical property of normal B cells to initiate germinal center formation (Dominguez-Sola et al. 2012; Calado et al. 2012), while miR-155 expression is required for completion of GC reactions (Thai et al. 2007; Rodriguez et al. 2007; Xiao and Rajewsky 2009). miR-155, in particular—a miRNA which is linked to hematopoietic malignancies in vivo—is highly induced following EBV infection, in part by LMP1 activation of NF-kB, and is critically required for the growth of LCLs in vitro (Cameron et al. 2008a, b; Linnstaedt et al. 2010). Consequently, EBV-induction of miR-155 can allow EBV to tie into the existing miR-155-regulated pathways. c-Myc can also antagonize NF-kB signaling (Faumont et al. 2009), which may be facilitated in part through Myc-regulated miR-17/92 targeting of NF-kB components (i.e., A20, CYLD, TRAF3, RNF11) (Skalsky et al. 2012; Jin et al. 2013). EBV thus hijacks these miRNA signals to elicit B cell proliferation, which may inadvertently contribute to lymphomagenesis in the absence of appropriate regulatory signals.
7 Summary and Outlook
EBV employs multiple strategies to enable long-term persistence within a host, including expressing viral ncRNAs with described roles in a variety of biological processes. Many EBV ncRNAs are highly evolutionarily conserved among LCVs at both the sequence and structural level, indicating that they have important roles in the virus life cycle and likely favorably shape the host environment to promote viral fitness and facilitate viral persistence.
Since their initial identification over ten years ago, EBV miRNAs and their contributions to viral pathogenesis and oncogenesis have been the subject of intense investigation. Several key signaling pathways, such as the NF-kB and Wnt pathways, and processes, such as apoptosis and immune activation, are now known to be regulated by EBV miRNAs. The next steps are to understand how miRNA-mediated regulation of targets involved in these pathways can contribute to viral infection and to determine the most critical miRNA targets that may be amenable to therapeutic intervention in EBV-associated diseases.
A number of open questions remain regarding the functions for many EBV ncRNAs. For example, do viral ncRNAs act in concert with viral proteins to exert a biological effect? Do EBV miRNAs synergistically target a specific pathway, as has been observed for other herpesvirus miRNAs? What, if any, are the important targets of viral miRNAs during lytic replication? And finally, how do the other viral ncRNAs contribute to the establishment of latent infection?
Future studies should provide mechanistic insight into how viral miRNAs and other ncRNAs might contribute to EBV-mediated oncogenic processes, help elucidate when EBV ncRNAs exert their functions during the natural viral life cycle, and may lead to the rational design of novel, EBV ncRNA-targeted therapies for EBV-associated diseases.
Abbreviations
- EBV
Epstein-Barr virus
- LCV
Lymphocryptovirus
- miRNA
microRNA
- snoRNA
Small nucleolar RNA
- sisRNA
Stable intronic-sequence RNA
- ncRNA
Noncoding RNA
- EBERs
EBV-encoded RNAs
- BARTs
BamHI-A rightward transcripts
- BHRF1
BamHI right forward 1
- UTR
Untranslated region
- HL
Hodgkin’s lymphoma
- BL
Burkitt’s lymphoma
- DLBCL
Diffuse large B cell lymphoma
- PTLD
Post-transplant lymphoproliferative disease
- NPC
Nasopharyngeal carcinoma
- LCL
Lymphoblastoid cell line
- LMP
Latent membrane protein
- EBNA
EBV nuclear antigen
- RNA
Ribonucleic acid
- RISC
RNA-induced silencing complex
- PAR-CLIP
Photoactivatable ribonucleoside-enhanced cross-linking and immunoprecipitation
- HITS-CLIP
High-throughput sequencing of RNA isolated by cross-linking and immunoprecipitation
Contributor Information
Rebecca L. Skalsky, Vaccine and Gene Therapy Institute, Oregon Health and Science University, Beaverton, OR, USA
Bryan R. Cullen, Department of Molecular Genetics and Microbiology, Duke University Medical Center, Durham, NC, USA
References
- Adams BD, Kasinski AL, Slack FJ. Aberrant regulation and function of MicroRNAs in cancer. Curr Biol. 2014;24:R762–R776. doi: 10.1016/j.cub.2014.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mozaini M, Bodelon G, Karstegl CE, Jin B, Al-Ahdal M, Farrell PJ. Epstein-Barr virus BART gene expression. J Gen Virol. 2009;90:307–316. doi: 10.1099/vir.0.006551-0. [DOI] [PubMed] [Google Scholar]
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- Ambros V, Bartel B, Bartel DP, Burge CB, Carrington JC, Chen X, Dreyfuss G, Eddy SR, Griffiths-Jones S, Marshall M, Matzke M, Ruvkun G, Tuschl T. A uniform system for microRNA annotation. RNA. 2003;9:277–279. doi: 10.1261/rna.2183803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso R, Fitzsimmons L, Thomas WA, Kelly GL, Rowe M, Bell AI. Quantitative studies of Epstein-Barr virus-encoded microRNAs provide novel insights into their regulation. J Virol. 2011;85:996–1010. doi: 10.1128/JVI.01528-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson MG, Haasnoot PC, Xu N, Berenjian S, Berkhout B, Akusjarvi G. Suppression of RNA interference by adenovirus virus-associated RNA. J Virol. 2005;79:9556–9565. doi: 10.1128/JVI.79.15.9556-9565.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio O, Razquin N, Zaratiegui M, Narvaiza I, Fortes P. Adenovirus virus-associated RNA is processed to functional interfering RNAs involved in virus production. J Virol. 2006;80:1376–1384. doi: 10.1128/JVI.80.3.1376-1384.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aswad A, Katzourakis A. The first endogenous herpesvirus, identified in the tarsier genome, and novel sequences from primate rhadinoviruses and lymphocryptoviruses. PLoS Genet. 2014;10:e1004332. doi: 10.1371/journal.pgen.1004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth S, Pfuhl T, Mamiani A, Ehses C, Roemer K, Kremmer E, Jaker C, Hock J, Meister G, Grasser FA. Epstein-Barr virus-encoded microRNA miR-BART2 down-regulates the viral DNA polymerase BALF5. Nucleic Acids Res. 2008;36:666–675. doi: 10.1093/nar/gkm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth S, Meister G, Grasser FA. EBV-encoded miRNAs. Biochim Biophys Acta. 2011;1809:631–640. doi: 10.1016/j.bbagrm.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Bhat RA, Thimmappaya B. Two small RNAs encoded by Epstein-Barr virus can functionally substitute for the virus-associated RNAs in the lytic growth of adenovirus 5. Proc Natl Acad Sci USA. 1983;80:4789–4793. doi: 10.1073/pnas.80.15.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom DC. HSV LAT and neuronal survival. Int Rev Immunol. 2004;23:187–198. doi: 10.1080/08830180490265592. [DOI] [PubMed] [Google Scholar]
- Boss IW, Nadeau PE, Abbott JR, Yang Y, Mergia A, Renne R. A Kaposi’s sarcoma-associated herpesvirus-encoded ortholog of microRNA miR-155 induces human splenic B-cell expansion in NOD/LtSz-scid IL2Rgammanull mice. J Virol. 2011;85:9877–9886. doi: 10.1128/JVI.05558-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken CP, Li X, Wright JA, Lawrence D, Pillman KA, Salmanidis M, Anderson MA, Dredge BK, Gregory PA, Tsykin A, Neilsen C, Thomson DW, Bert AG, Leerberg JM, Yap AS, Jensen KB, Khew-Goodall Y, Goodall GJ. Genome-wide identification of miR-200 targets reveals a regulatory network controlling cell invasion. EMBO J. 2014;33:2040–2056. doi: 10.15252/embj.201488641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahir-Mcfarland ED, Carter K, Rosenwald A, Giltnane JM, Henrickson SE, Staudt LM, Kieff E. Role of NF-kappa B in cell survival and transcription of latent membrane protein 1-expressing or Epstein-Barr virus latency III-infected cells. J Virol. 2004;78:4108–4119. doi: 10.1128/JVI.78.8.4108-4119.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Schafer A, Lu S, Bilello JP, Desrosiers RC, Edwards R, Raab-Traub N, Cullen BR. Epstein-Barr virus microRNAs are evolutionarily conserved and differentially expressed. PLoS Pathog. 2006;2:e23. doi: 10.1371/journal.ppat.0020023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Ye Y, Jiang Q, Chen Y, Lyu X, Li J, Wang S, Liu T, Cai H, Yao K, Li JL, Li X. Epstein-Barr virus-encoded microRNA BART1 induces tumour metastasis by regulating PTEN-dependent pathways in nasopharyngeal carcinoma. Nat Commun. 2015;6:7353. doi: 10.1038/ncomms8353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado DP, Sasaki Y, Godinho SA, Pellerin A, Kochert K, Sleckman BP, de Alboran IM, Janz M, Rodig S, Rajewsky K. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol. 2012;13:1092–1100. doi: 10.1038/ni.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JE, Fewell C, Yin Q, McBride J, Wang X, Lin Z, Flemington EK. Epstein-Barr virus growth/latency III program alters cellular microRNA expression. Virology. 2008a;382:257–266. doi: 10.1016/j.virol.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JE, Yin Q, Fewell C, Lacey M, McBride J, Wang X, Lin Z, Schaefer BC, Flemington EK. Epstein-Barr virus latent membrane protein 1 induces cellular MicroRNA miR-146a, a modulator of lymphocyte signaling pathways. J Virol. 2008b;82:1946–1958. doi: 10.1128/JVI.02136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell. 2009;136:642–655. doi: 10.1016/j.cell.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Huang J, Wu FY, Liao G, Hutt-Fletcher L, Hayward SD. Regulation of expression of the Epstein-Barr virus BamHI-A rightward transcripts. J Virol. 2005;79:1724–1733. doi: 10.1128/JVI.79.3.1724-1733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Chen GH, Chen YH, Liu CY, Chang KP, Chang YS, Chen HC. Characterization of Epstein-Barr virus miRNAome in nasopharyngeal carcinoma by deep sequencing. PLoS One. 2010;5:e12745. doi: 10.1371/journal.pone.0012745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, Zang JB, Mele A, Darnell RB. Argonaute HITS-CLIP decodes microRNA-mRNA interaction maps. Nature. 2009;460:479–486. doi: 10.1038/nature08170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy EY, Siu KL, Kok KH, Lung RW, Tsang CM, To KF, Kwong DL, Tsao SW, Jin DY. An Epstein-Barr virus-encoded microRNA targets PUMA to promote host cell survival. J Exp Med. 2008;205:2551–2560. doi: 10.1084/jem.20072581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PA, Schwemmle M, Schickinger J, Hilse K, Clemens MJ. Binding of Epstein-Barr virus small RNA EBER-1 to the double-stranded RNA-activated protein kinase DAI. Nucleic Acids Res. 1991;19:243–248. doi: 10.1093/nar/19.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha M, Wang X, Cao S, Baddoo M, Fewell C, Lin Z, Hulme W, Hedges D, McBride J, Flemington EK. Identification of new viral genes and transcript isoforms during Epstein-Barr virus reactivation using RNA-Seq. J Virol. 2012;86:1458–1467. doi: 10.1128/JVI.06537-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran DL, Georgiev S, Mukherjee N, Gottwein E, Skalsky RL, Keene JD, Ohler U. PARalyzer: definition of RNA binding sites from PAR-CLIP short-read sequence data. Genome Biol. 2011;12:R79. doi: 10.1186/gb-2011-12-8-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jesus O, Smith PR, Spender LC, Elgueta Karstegl C, Niller HH, Huang D, Farrell PJ. Updated Epstein-Barr virus (EBV). DNA sequence and analysis of a promoter for the BART (CST, BARF0) RNAs of EBV. J Gen Virol. 2003;84:1443–1450. doi: 10.1099/vir.0.19054-0. [DOI] [PubMed] [Google Scholar]
- Ding Y, Xi Y, Chen T, Wang JY, Tao DL, Wu ZL, Li YP, Li C, Zeng R, Li L. Caprin-2 enhances canonical Wnt signaling through regulating LRP5/6 phosphorylation. J Cell Biol. 2008;182:865–872. doi: 10.1083/jcb.200803147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolken L, Malterer G, Erhard F, Kothe S, Friedel CC, Suffert G, Marcinowski L, Motsch N, Barth S, Beitzinger M, Lieber D, Bailer SM, Hoffmann R, Ruzsics Z, Kremmer E, Pfeffer S, Zimmer R, Koszinowski UH, Grasser F, Meister G, Haas J. Systematic analysis of viral and cellular microRNA targets in cells latently infected with human gamma-herpesviruses by RISC immunoprecipitation assay. Cell Host Microbe. 2010;7:324–334. doi: 10.1016/j.chom.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Dominguez-Sola D, Victora GD, Ying CY, Phan RT, Saito M, Nussenzweig MC, Dalla-Favera R. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol. 2012;13:1083–1091. doi: 10.1038/ni.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH, Marquitz AR, Raab-Traub N. Epstein-Barr virus BART microRNAs are produced from a large intron prior to splicing. J Virol. 2008;82:9094–9106. doi: 10.1128/JVI.00785-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis-Connell AL, Iempridee T, Xu I, Mertz JE. Cellular microRNAs 200b and 429 regulate the Epstein-Barr virus switch between latency and lytic replication. J Virol. 2010;84:10329–10343. doi: 10.1128/JVI.00923-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faumont N, Durand-Panteix S, Schlee M, Gromminger S, Schuhmacher M, Holzel M, Laux G, Mailhammer R, Rosenwald A, Staudt LM, Bornkamm GW, Feuillard J. c-Myc and Rel/NF-kappaB are the two master transcriptional systems activated in the latency III program of Epstein-Barr virus-immortalized B cells. J Virol. 2009;83:5014–5027. doi: 10.1128/JVI.02264-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feederle R, Haar J, Bernhardt K, Linnstaedt SD, Bannert H, Lips H, Cullen BR, Delecluse HJ. The members of a viral miRNA cluster co-operate to transform B lymphocytes. J Virol. 2011a;85:9801–9810. doi: 10.1128/JVI.05100-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feederle R, Linnstaedt SD, Bannert H, Lips H, Bencun M, Cullen BR, Delecluse HJ. A viral microRNA cluster strongly potentiates the transforming properties of a human herpes-virus. PLoS Pathog. 2011b;7:e1001294. doi: 10.1371/journal.ppat.1001294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok V, Friend K, Steitz JA. Epstein-Barr virus noncoding RNAs are confined to the nucleus, whereas their partner, the human La protein, undergoes nucleocytoplasmic shuttling. J Cell Biol. 2006a;173:319–325. doi: 10.1083/jcb.200601026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fok V, Mitton-Fry RM, Grech A, Steitz JA. Multiple domains of EBER 1, an Epstein-Barr virus noncoding RNA, recruit human ribosomal protein L22. RNA. 2006b;12:872–882. doi: 10.1261/rna.2339606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte E, Luftig MA. The role of microRNAs in Epstein-Barr virus latency and lytic reactivation. Microbes Infect. 2011;13:1156–1167. doi: 10.1016/j.micinf.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse Y, Ornelles DA, Cullen BR. Persistently adenovirus-infected lymphoid cells express microRNAs derived from the viral VAI and especially VAII RNA. Virology. 2013;447:140–145. doi: 10.1016/j.virol.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EJ, Nizami ZF, Talbot CC, Jr, Gall JG. Stable intronic sequence RNA (sisRNA), a new class of noncoding RNA from the oocyte nucleus of Xenopus tropicalis. Genes Dev. 2012;26:2550–2559. doi: 10.1101/gad.202184.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain C, Meier A, Jensen T, Knapnougel P, Poupon G, Lazzari A, Neisig A, Hakansson K, Dong T, Wagtmann N, Galsgaard ED, Spee P, Braud VM. Induction of lectin-like transcript 1 (LLT1) protein cell surface expression by pathogens and interferon-gamma contributes to modulate immune responses. J Biol Chem. 2011;286:37964–37975. doi: 10.1074/jbc.M111.285312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan K, Sato H, Rajadurai P, Busson P, Young L, Rickinson A, Tursz T, Raab-Traub N. Novel transcription from the Epstein-Barr virus terminal EcoRI fragment, DIJhet, in a naso-pharyngeal carcinoma. J Virol. 1990;64:4948–4956. doi: 10.1128/jvi.64.10.4948-4956.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilligan KJ, Rajadurai P, Lin JC, Busson P, Abdel-Hamid M, Prasad U, Tursz T, Raab-Traub N. Expression of the Epstein-Barr virus BamHI A fragment in nasopharyngeal carcinoma: evidence for a viral protein expressed in vivo. J Virol. 1991;65:6252–6259. doi: 10.1128/jvi.65.11.6252-6259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto M, Murakawa M, Kadoshima-Yamaoka K, Tanaka Y, Inoue H, Murafuji H, Hayashi Y, Miura K, Nakatsuka T, Nagahira K, Chamoto K, Fukuda Y, Nishimura T. Phosphodiesterase 7A inhibitor ASB16165 suppresses proliferation and cytokine production of NKT cells. Cell Immunol. 2009;258:147–151. doi: 10.1016/j.cellimm.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Gottwein E, Cai X, Cullen BR. A novel assay for viral microRNA function identifies a single nucleotide polymorphism that affects Drosha processing. J Virol. 2006;80:5321–5326. doi: 10.1128/JVI.02734-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Mukherjee N, Sachse C, Frenzel C, Majoros WH, Chi JT, Braich R, Manoharan M, Soutschek J, Ohler U, Cullen BR. A viral microRNA functions as an orthologue of cellular miR-155. Nature. 2007;450:1096–1099. doi: 10.1038/nature05992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwein E, Corcoran DL, Mukherjee N, Skalsky RL, Hafner M, Nusbaum JD, Shamulailatpam P, Love CL, Dave SS, Tuschl T, Ohler U, Cullen BR. Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe. 2011;10:515–526. doi: 10.1016/j.chom.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourzones C, Gelin A, Bombik I, Klibi J, Verillaud B, Guigay J, Lang P, Temam S, Schneider V, Amiel C, Baconnais S, Jimenez AS, Busson P. Extra-cellular release and blood diffusion of BART viral micro-RNAs produced by EBV-infected nasopharyngeal carcinoma cells. Virol J. 2010;7:271. doi: 10.1186/1743-422X-7-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorovic G, Bosshard R, Karstegl CE, White RE, Pattle S, Chiang AK, Dittrich-Breiholz O, Kracht M, Russ R, Farrell PJ. Cellular gene expression that correlates with EBER expression in Epstein-Barr Virus-infected lymphoblastoid cell lines. J Virol. 2011;85:3535–3545. doi: 10.1128/JVI.02086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosswendt S, Filipchyk A, Manzano M, Klironomos F, Schilling M, Herzog M, Gottwein E, Rajewsky N. Unambiguous identification of miRNA:target site interactions by different types of ligation reactions. Mol Cell. 2014;54:1042–1054. doi: 10.1016/j.molcel.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundhoff A, Sullivan CS, Ganem D. A combined computational and microarray-based approach identifies novel microRNAs encoded by human gamma-herpesviruses. RNA. 2006;12:733–750. doi: 10.1261/rna.2326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurer C, Strowig T, Brilot F, Pack M, Trumpfheller C, Arrey F, Park CG, Steinman RM, Munz C. Targeting the nuclear antigen 1 of Epstein-Barr virus to the human endocytic receptor DEC-205 stimulates protective T-cell responses. Blood. 2008;112:1231–1239. doi: 10.1182/blood-2008-03-148072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haecker I, Gay LA, Yang Y, Hu J, Morse AM, McIntyre LM, Renne R. Ago HITS-CLIP expands understanding of Kaposi’s Sarcoma-associated herpesvirus miRNA function in primary effusion lymphomas. PLoS Pathog. 2012;8:e1002884. doi: 10.1371/journal.ppat.1002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner M, Landthaler M, Burger L, Khorshid M, Hausser J, Berninger P, Rothballer A, Ascano M, Jr, Jungkamp AC, Munschauer M, Ulrich A, Wardle GS, Dewell S, Zavolan M, Tuschl T. Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell. 2010;141:129–141. doi: 10.1016/j.cell.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O’Neill LA, Masters SL. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1beta production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- Hitt MM, Allday MJ, Hara T, Karran L, Jones MD, Busson P, Tursz T, Ernberg I, Griffin BE. EBV gene expression in an NPC-related tumour. EMBO J. 1989;8:2639–2651. doi: 10.1002/j.1460-2075.1989.tb08404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe JG, Shu MD. Epstein-Barr virus small RNA (EBER) genes: unique transcription units that combine RNA polymerase II and III promoter elements. Cell. 1989;57:825–834. doi: 10.1016/0092-8674(89)90797-6. [DOI] [PubMed] [Google Scholar]
- Hsu CY, Yi YH, Chang KP, Chang YS, Chen SJ, Chen HC. The Epstein-Barr virus-encoded microRNA MiR-BART9 promotes tumor metastasis by targeting E-cadherin in nasopharyngeal carcinoma. PLoS Pathog. 2014;10:e1003974. doi: 10.1371/journal.ppat.1003974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzinger R, Feederle R, Mrazek J, Schiefermeier N, Balwierz PJ, Zavolan M, Polacek N, Delecluse HJ, Huttenhofer A. Expression and processing of a small nucleolar RNA from the Epstein-Barr virus genome. PLoS Pathog. 2009;5:e1000547. doi: 10.1371/journal.ppat.1000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iizasa H, Wulff BE, Alla NR, Maragkakis M, Megraw M, Hatzigeorgiou A, Iwakiri D, Takada K, Wiedmer A, Showe L, Lieberman P, Nishikura K. Editing of EBV-encoded BART6 microRNAs controls their dicer targeting and consequently affects viral latency. J Biol Chem. 2010;285:33358–33370. doi: 10.1074/jbc.M110.138362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imig J, Motsch N, Zhu JY, Barth S, Okoniewski M, Reineke T, Tinguely M, Faggioni A, Trivedi P, Meister G, Renner C, Grasser FA. microRNA profiling in Epstein-Barr virus-associated B-cell lymphoma. Nucleic Acids Res. 2011;39:1880–1893. doi: 10.1093/nar/gkq1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HY, Oda H, Lai M, Skalsky RL, Bethel K, Shepherd J, Kang SG, Liu WH, Sabouri-Ghomi M, Cullen BR, Rajewsky K, Xiao C. MicroRNA-17 ~ 92 plays a causative role in lymphomagenesis by coordinating multiple oncogenic pathways. EMBO J. 2013;32:2377–2391. doi: 10.1038/emboj.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung YJ, Choi H, Kim H, Lee SK. MicroRNA miR-BART20-5p stabilizes Epstein-Barr virus latency by directly targeting BZLF1 and BRLF1. J Virol. 2014;88:9027–9037. doi: 10.1128/JVI.00721-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Skalsky RL, Cullen BR. EBV BART MicroRNAs target multiple pro-apoptotic cellular genes to promote epithelial cell survival. PLos Path. 2015;11:e1004979. doi: 10.1371/journal.ppat.1004979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran L, Gao Y, Smith PR, Griffin BE. Expression of a family of complementary-strand transcripts in Epstein-Barr virus-infected cells. Proc Natl Acad Sci USA. 1992;89:8058–8062. doi: 10.1073/pnas.89.17.8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieff E. Epstein-Barr virus and its replication. In: Knipe DMaH PM, editor. Fields virology. 5 2007. [Google Scholar]
- Kienzle N, Sculley TB, Poulsen L, Buck M, Cross S, Raab-Traub N, Khanna R. Identification of a cytotoxic T-lymphocyte response to the novel BARF0 protein of Epstein-Barr virus: a critical role for antigen expression. J Virol. 1998;72:6614–6620. doi: 10.1128/jvi.72.8.6614-6620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienzle N, Buck M, Greco S, Krauer K, Sculley TB. Epstein-Barr virus-encoded RK-BARF0 protein expression. J Virol. 1999;73:8902–8906. doi: 10.1128/jvi.73.10.8902-8906.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Do N, Song YJ, Lee SK. The role of promoter methylation in Epstein-Barr virus (EBV) microRNA expression in EBV-infected B cell lines. Exp Mol Med. 2011;43:401–410. doi: 10.3858/emm.2011.43.7.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza CA, Shenk T. Human cytomegalovirus 5-kilobase immediate-early RNA is a stable intron. J Virol. 2004;78:13182–13189. doi: 10.1128/JVI.78.23.13182-13189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesza CA, Shenk T. Murine cytomegalovirus encodes a stable intron that facilitates persistent replication in the mouse. Proc Natl Acad Sci USA. 2006;103:18302–18307. doi: 10.1073/pnas.0608718103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Pimienta G, Steitz JA. AUF1/hnRNP D is a novel protein partner of the EBER1 noncoding RNA of Epstein-Barr virus. RNA. 2012;18:2073–2082. doi: 10.1261/rna.034900.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, Moss WN, Yario TA, Steitz JA. EBV noncoding RNA binds nascent RNA to drive host PAX5 to viral DNA. Cell. 2015;160:607–618. doi: 10.1016/j.cell.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Bai Z, Ye F, Xie J, Kim CG, Huang Y, Gao SJ. Regulation of NF-kappaB inhibitor IkappaBalpha and viral replication by a KSHV microRNA. Nat Cell Biol. 2010;12:193–199. doi: 10.1038/ncb2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei T, Yuen KS, Xu R, Tsao SW, Chen H, Li M, Kok KH, Jin DY. Targeting of DICE1 tumor suppressor by Epstein-Barr virus-encoded miR-BART3* microRNA in nasopharyngeal carcinoma. Int J Cancer. 2013;133:79–87. doi: 10.1002/ijc.28007. [DOI] [PubMed] [Google Scholar]
- Lerner MR, Andrews NC, Miller G, Steitz JA. Two small RNAs encoded by Epstein-Barr virus and complexed with protein are precipitated by antibodies from patients with systemic lupus erythematosus. Proc Natl Acad Sci USA. 1981;78:805–809. doi: 10.1073/pnas.78.2.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lin Z, Wang X, Fewell C, Cameron J, Yin Q, Flemington EK. Differential expression of the miR-200 family microRNAs in epithelial and B cells and regulation of Epstein-Barr virus reactivation by the miR-200 family member miR-429. J Virol. 2010;84:7892–7897. doi: 10.1128/JVI.00379-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin TC, Liu TY, Hsu SM, Lin CW. Epstein-Barr virus-encoded miR-BART20-5p inhibits T-bet translation with secondary suppression of p53 in invasive nasal NK/T-cell lymphoma. Am J Pathol. 2013a;182:1865–1875. doi: 10.1016/j.ajpath.2013.01.025. [DOI] [PubMed] [Google Scholar]
- Lin Z, Wang X, Strong MJ, Concha M, Baddoo M, Xu G, Baribault C, Fewell C, Hulme W, Hedges D, Taylor CM, Flemington EK. Whole-genome sequencing of the Akata and Mutu Epstein-Barr virus strains. J Virol. 2013b;87:1172–1182. doi: 10.1128/JVI.02517-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnstaedt SD, Gottwein E, Skalsky RL, Luftig MA, Cullen BR. Virally induced cellular miR-155 plays a key role in B-cell immortalization by EBV. J Virol. 2010;84:11670–11678. doi: 10.1128/JVI.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AK, To KF, Lo KW, Lung RW, Hui JW, Liao G, Hayward SD. Modulation of LMP1 protein expression by EBV-encoded microRNAs. Proc Natl Acad Sci USA. 2007;104:16164–16169. doi: 10.1073/pnas.0702896104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo AK, Dawson CW, Jin DY, Lo KW. The pathological roles of BART miRNAs in naso-pharyngeal carcinoma. J Pathol. 2012;227:392–403. doi: 10.1002/path.4025. [DOI] [PubMed] [Google Scholar]
- Lu S, Cullen BR. Adenovirus VA1 noncoding RNA can inhibit small interfering RNA and MicroRNA biogenesis. J Virol. 2004;78:12868–12876. doi: 10.1128/JVI.78.23.12868-12876.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig M, Prinarakis E, Yasui T, Tsichritzis T, Cahir-Mcfarland E, Inoue J, Nakano H, Mak TW, Yeh WC, Li X, Akira S, Suzuki N, Suzuki S, Mosialos G, Kieff E. Epstein-Barr virus latent membrane protein 1 activation of NF-kappaB through IRAK1 and TRAF6. Proc Natl Acad Sci USA. 2003;100:15595–15600. doi: 10.1073/pnas.2136756100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Mazna P, Valenta T, Doubravska L, Pospichalova V, Vojtechova M, Fafilek B, Ivanek R, Plachy J, Novak J, Korinek V. Dazap2 modulates transcription driven by the Wnt effector TCF-4. Nucleic Acids Res. 2009;37:3007–3020. doi: 10.1093/nar/gkp179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung RW, Tong JH, Sung YM, Leung PS, Ng DC, Chau SL, Chan AW, Ng EK, Lo KW, To KF. Modulation of LMP2A expression by a newly identified Epstein-Barr virus-encoded microRNA miR-BART22. Neoplasia. 2009;11:1174–1184. doi: 10.1593/neo.09888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung RW, Tong JH, To KF. Emerging roles of small Epstein-Barr virus derived non-coding RNAs in epithelial malignancy. Int J Mol Sci. 2013;14:17378–17409. doi: 10.3390/ijms140917378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoros WH, Lekprasert P, Mukherjee N, Skalsky RL, Corcoran DL, Cullen BR, Ohler U. MicroRNA target site identification by integrating sequence and binding information. Nat Methods. 2013;10:630–633. doi: 10.1038/nmeth.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz AR, Raab-Traub N. The role of miRNAs and EBV BARTs in NPC. Semin Cancer Biol. 2012;22:166–172. doi: 10.1016/j.semcancer.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz AR, Mathur A, Nam CS, Raab-Traub N. The Epstein-Barr Virus BART microR-NAs target the pro-apoptotic protein Bim. Virology. 2011;412:392–400. doi: 10.1016/j.virol.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz AR, Mathur A, Shair KH, Raab-Traub N. Infection of Epstein-Barr virus in a gastric carcinoma cell line induces anchorage independence and global changes in gene expression. Proc Natl Acad Sci USA. 2012 doi: 10.1073/pnas.1202910109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquitz AR, Mathur A, Chugh PE, Dittmer DP, Raab-Traub N. Expression profile of microRNAs in Epstein-Barr virus-infected AGS gastric carcinoma cells. J Virol. 2014;88:1389–1393. doi: 10.1128/JVI.02662-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meckes DG, Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci USA. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaeli T, Pan ZQ, Prives C. An excised SV40 intron accumulates and is stable in Xenopus laevis oocytes. Genes Dev. 1988;2:1012–1020. doi: 10.1101/gad.2.8.1012. [DOI] [PubMed] [Google Scholar]
- Moody R, Zhu Y, Huang Y, Cui X, Jones T, Bedolla R, Lei X, Bai Z, Gao SJ. KSHV microRNAs mediate cellular transformation and tumorigenesis by redundantly targeting cell growth and survival pathways. PLoS Pathog. 2013;9:e1003857. doi: 10.1371/journal.ppat.1003857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss WN, Steitz JA. Genome-wide analyses of Epstein-Barr virus reveal conserved RNA structures and a novel stable intronic sequence RNA. BMC Genom. 2013;14:543. doi: 10.1186/1471-2164-14-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynagh PN. The Pellino family: IRAK E3 ligases with emerging roles in innate immune signalling. Trends Immunol. 2009;30:33–42. doi: 10.1016/j.it.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Mullokandov G, Baccarini A, Ruzo A, Jayaprakash AD, Tung N, Israelow B, Evans MJ, Sachidanandam R, Brown BD. High-throughput assessment of microRNA activity and function using microRNA sensor and decoy libraries. Nat Methods. 2012;9:840–846. doi: 10.1038/nmeth.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmani D, Stern-Ginossar N, Sarid R, Mandelboim O. Diverse herpesvirus microRNAs target the stress-induced immune ligand MICB to escape recognition by natural killer cells. Cell Host Microbe. 2009;5:376–385. doi: 10.1016/j.chom.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Nanbo A, Inoue K, Adachi-Takasawa K, Takada K. Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt’s lymphoma. EMBO J. 2002;21:954–965. doi: 10.1093/emboj/21.5.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitin PA, Yan CM, Forte E, Bocedi A, Tourigny JP, White RE, Allday MJ, Patel A, Dave SS, Kim W, Hu K, Guo J, Tainter D, Rusyn E, Luftig MA. An ATM/Chk2-mediated DNA damage-responsive signaling pathway suppresses Epstein-Barr virus transformation of primary human B cells. Cell Host Microbe. 2010;8:510–522. doi: 10.1016/j.chom.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Grady T, Cao S, Strong MJ, Concha M, Wang X, Splinter Bondurant S, Adams M, Baddoo M, Srivastav SK, Lin Z, Fewell C, Yin Q, Flemington EK. Global bidirectional transcription of the Epstein-Barr virus genome during reactivation. J Virol. 2014;88:1604–1616. doi: 10.1128/JVI.02989-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas O, Corcoran DL, Cullen BR. Analysis of the mRNA targetome of microRNAs expressed by Marek’s disease virus. MBio. 2014;5:e01060–13. doi: 10.1128/mBio.01060-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekarsky Y, Croce CM. Is miR-29 an oncogene or tumor suppressor in CLL? Oncotarget. 2010;1:224–227. doi: 10.18632/oncotarget.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer S, Zavolan M, Grasser FA, Chien M, Russo JJ, Ju J, John B, Enright AJ, Marks D, Sander C, Tuschl T. Identification of virus-encoded microRNAs. Science. 2004;304:734–736. doi: 10.1126/science.1096781. [DOI] [PubMed] [Google Scholar]
- Pillai RS, Bhattacharyya SN, Filipowicz W. Repression of protein synthesis by miRNAs: how many mechanisms? Trends Cell Biol. 2007;17:118–126. doi: 10.1016/j.tcb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Pratt ZL, Kuzembayeva M, Sengupta S, Sugden B. The microRNAs of Epstein-Barr virus are expressed at dramatically differing levels among cell lines. Virology. 2009;386:387–397. doi: 10.1016/j.virol.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt ZL, Zhang J, Sugden B. The latent membrane protein 1 (LMP1) oncogene of Epstein-Barr virus can simultaneously induce and inhibit apoptosis in B cells. J Virol. 2012;86:4380–4393. doi: 10.1128/JVI.06966-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AM, Tourigny JP, Forte E, Salinas RE, Dave SS, Luftig MA. Analysis of Epstein-Barr virus-regulated host gene expression changes through primary B-cell outgrowth reveals delayed kinetics of latent membrane protein 1-mediated NF-kappaB activation. J Virol. 2012;86:11096–11106. doi: 10.1128/JVI.01069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Thorley-Lawson DA. EBV microRNA BART 18-5p targets MAP3K2 to facilitate persistence in vivo by inhibiting viral replication in B cells. Proc Natl Acad Sci USA. 2014;111:11157–11162. doi: 10.1073/pnas.1406136111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Cosmopoulos K, Pegtel M, Hopmans E, Murray P, Middeldorp J, Shapiro M, Thorley-Lawson DA. A novel persistence associated EBV miRNA expression profile is disrupted in neoplasia. PLoS Pathog. 2011;7:e1002193. doi: 10.1371/journal.ppat.1002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran I, Ganapathy V, Gillies E, Fonseca I, Sureban SM, Houchen CW, Reis A, Queimado L. Wnt inhibitory factor 1 suppresses cancer stemness and induces cellular senescence. Cell Death Dis. 2014;5:e1246. doi: 10.1038/cddis.2014.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi O, Erlich Y, Amram H, Flomenblit L, Karginov FV, Goldstein I, Hannon GJ, Kloog Y. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small RNAs. Genes Dev. 2009;23:1971–1979. doi: 10.1101/gad.1789609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repellin CE, Tsimbouri PM, Philbey AW, Wilson JB. Lymphoid hyperplasia and lymphoma in transgenic mice expressing the small non-coding RNA, EBER1 of Epstein-Barr virus. PLoS ONE. 2010;5:e9092. doi: 10.1371/journal.pone.0009092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley KJ, Rabinowitz GS, Steitz JA. Comprehensive analysis of Rhesus lymphocryptovirus microRNA expression. J Virol. 2010;84:5148–5157. doi: 10.1128/JVI.00110-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley KJ, Rabinowitz GS, Yario TA, Luna JM, Darnell RB, Steitz JA. EBV and human microRNAs co-target oncogenic and apoptotic viral and human genes during latency. EMBO J. 2012;31:2207–2221. doi: 10.1038/emboj.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson ES, Tomkinson B, Kieff E. An Epstein-Barr virus with a 58-kilobase-pair deletion that includes BARF0 transforms B lymphocytes in vitro. J Virol. 1994;68:1449–1458. doi: 10.1128/jvi.68.3.1449-1458.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf IK, Lackey KA, Warudkar S, Sample JT. Protection from interferon-induced apoptosis by Epstein-Barr virus small RNAs is not mediated by inhibition of PKR. J Virol. 2005;79:14562–14569. doi: 10.1128/JVI.79.23.14562-14569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta M, Iwakiri D, Kanda T, Imaizumi T, Takada K. EB virus-encoded RNAs are recognized by RIG-I and activate signaling to induce type I IFN. EMBO J. 2006;25:4207–4214. doi: 10.1038/sj.emboj.7601314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M, Kato Y, Taira K. Sequence-specific interference by small RNAs derived from adenovirus VAI RNA. FEBS Lett. 2006;580:1553–1564. doi: 10.1016/j.febslet.2006.01.085. [DOI] [PubMed] [Google Scholar]
- Seto E, Moosmann A, Gromminger S, Walz N, Grundhoff A, Hammerschmidt W. Micro RNAs of Epstein-Barr virus promote cell cycle progression and prevent apoptosis of primary human B cells. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki A, Sakatani T, Ushiku T, Hino R, Isogai M, Ishikawa S, Uozaki H, Takada K, Fukayama M. Downregulation of microRNA-200 in EBV-associated gastric carcinoma. Cancer Res. 2010;70:4719–4727. doi: 10.1158/0008-5472.CAN-09-4620. [DOI] [PubMed] [Google Scholar]
- Skalsky RL, Cullen BR. Viruses, microRNAs, and host interactions. Annu Rev Microbiol. 2010;64:123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Hu J, Renne R. Analysis of viral cis elements conferring Kaposi’s sarcoma-associated herpesvirus episome partitioning and maintenance. J Virol. 2007a;81:9825–9837. doi: 10.1128/JVI.00842-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Samols MA, Plaisance KB, Boss IW, Riva A, Lopez MC, Baker HV, Renne R. Kaposi’s sarcoma-associated herpesvirus encodes an ortholog of miR-155. J Virol. 2007b;81:12836–12845. doi: 10.1128/JVI.01804-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Corcoran DL, Gottwein E, Frank CL, Kang D, Hafner M, Nusbaum JD, Feederle R, Delecluse HJ, Luftig MA, Tuschl T, Ohler U, Cullen BR. The viral and cellular microRNA targetome in lymphoblastoid cell lines. PLoS Pathog. 2012;8:e1002484. doi: 10.1371/journal.ppat.1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalsky RL, Kang D, Linnstaedt SD, Cullen BR. Evolutionary conservation of primate lymphocryptovirus microRNA targets. J Virol. 2014;88:1617–1635. doi: 10.1128/JVI.02071-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PR, de Jesus O, Turner D, Hollyoake M, Karstegl CE, Griffin BE, Karran L, Wang Y, Hayward SD, Farrell PJ. Structure and coding content of CST (BART) family RNAs of Epstein-Barr virus. J Virol. 2000;74:3082–3092. doi: 10.1128/jvi.74.7.3082-3092.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soni V, Cahir-Mcfarland E, Kieff E. LMP1 TRAFficking activates growth and survival pathways. Adv Exp Med Biol. 2007;597:173–187. doi: 10.1007/978-0-387-70630-6_14. [DOI] [PubMed] [Google Scholar]
- Swaminathan S, Tomkinson B, Kieff E. Recombinant Epstein-Barr virus with small RNA (EBER) genes deleted transforms lymphocytes and replicates in vitro. Proc Natl Acad Sci USA. 1991;88:1546–1550. doi: 10.1073/pnas.88.4.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouarne GJ, Gall JG. Lariat intronic RNAs in the cytoplasm of Xenopus tropicalis oocytes. RNA. 2014;20:1476–1487. doi: 10.1261/rna.045781.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavalai N, Stamminger T. Interplay between herpesvirus infection and host defense by PML nuclear bodies. Viruses. 2009;1:1240–1264. doi: 10.3390/v1031240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A, Rajewsky K. Regulation of the germinal center response by microRNA-155. Science. 2007;316:604–608. doi: 10.1126/science.1141229. [DOI] [PubMed] [Google Scholar]
- Toczyski DP, Matera AG, Ward DC, Steitz JA. The Epstein-Barr virus (EBV) small RNA EBER1 binds and relocalizes ribosomal protein L22 in EBV-infected human B lymphocytes. Proc Natl Acad Sci USA. 1994;91:3463–3467. doi: 10.1073/pnas.91.8.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Vereide DT, Seto E, Chiu YF, Hayes M, Tagawa T, Grundhoff A, Hammerschmidt W, Sugden B. Epstein-Barr virus maintains lymphomas via its miRNAs. Oncogene. 2014;33:1258–1264. doi: 10.1038/onc.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl A, Linnstaedt SD, Esoda C, Krisko JF, Martinez-Torres F, Delecluse HJ, Cullen BR, Garcia JV. A cluster of virus-encoded microRNAs accelerates acute systemic Epstein-Barr virus infection but does not significantly enhance virus-induced oncogenesis in vivo. J Virol. 2013;87:5437–5446. doi: 10.1128/JVI.00281-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KB, Marsolais D, Welch MJ, Rosen H, Oldstone MB. Treatment with a sphingosine analog does not alter the outcome of a persistent virus infection. Virology. 2010;397:260–269. doi: 10.1016/j.virol.2009.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz N, Christalla T, Tessmer U, Grundhoff A. A global analysis of evolutionary conservation among known and predicted gammaherpesvirus microRNAs. J Virol. 2009;84:716–728. doi: 10.1128/JVI.01302-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White EJ, Brewer G, Wilson GM. Post-transcriptional control of gene expression by AUF1: mechanisms, physiological targets, and regulation. Biochim Biophys Acta. 2013;1829:680–688. doi: 10.1016/j.bbagrm.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Kong KL, Tsang JW, Kwong DL, Guan XY. Profiling of Epstein-Barr virus-encoded microRNAs in nasopharyngeal carcinoma reveals potential biomarkers and oncomirs. Cancer. 2012;118:698–710. doi: 10.1002/cncr.26309. [DOI] [PubMed] [Google Scholar]
- Wu Y, Maruo S, Yajima M, Kanda T, Takada K. Epstein-Barr virus (EBV)-encoded RNA 2 (EBER2). but not EBER1 plays a critical role in EBV-induced B-cell growth transformation. J Virol. 2007;81:11236–11245. doi: 10.1128/JVI.00579-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia T, O’Hara A, Araujo I, Barreto J, Carvalho E, Sapucaia JB, Ramos JC, Luz E, Pedroso C, Manrique M, Toomey NL, Brites C, Dittmer DP, Harrington WJ., Jr EBV microR-NAs in primary lymphomas and targeting of CXCL-11 by ebv-mir-BHRF1-3. Cancer Res. 2008;68:1436–1442. doi: 10.1158/0008-5472.CAN-07-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Rajewsky K. MicroRNA control in the immune system: basic principles. Cell. 2009;136:26–36. doi: 10.1016/j.cell.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Xing L, Kieff E. Epstein-Barr virus BHRF1 micro- and stable RNAs during latency III and after induction of replication. J Virol. 2007;81:9967–9975. doi: 10.1128/JVI.02244-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Kieff E. cis-Acting effects on RNA processing and Drosha cleavage prevent Epstein-Barr virus latency III BHRF1 expression. J Virol. 2011;85:8929–8939. doi: 10.1128/JVI.00336-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima M, Kanda T, Takada K. Critical role of Epstein-Barr Virus (EBV)-encoded RNA in efficient EBV-induced B-lymphocyte growth transformation. J Virol. 2005;79:4298–4307. doi: 10.1128/JVI.79.7.4298-4307.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yuan YA. A structural perspective of the protein-RNA interactions involved in virus-induced RNA silencing and its suppression. Biochim Biophys Acta. 2009;1789:642–652. doi: 10.1016/j.bbagrm.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhao Y, Xu H, Smith LP, Lawrie CH, Watson M, Nair V. MicroRNA profile of Marek’s disease virus-transformed T-cell line MSB-1: predominance of virus-encoded microRNAs. J Virol. 2008;82:4007–4015. doi: 10.1128/JVI.02659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- Zhang J, Chen H, Weinmaster G, Hayward SD. Epstein-Barr virus BamHi-a rightward transcript-encoded RPMS protein interacts with the CBF1-associated corepressor CIR to negatively regulate the activity of EBNA2 and NotchIC. J Virol. 2001;75:2946–2956. doi: 10.1128/JVI.75.6.2946-2956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Xu H, Yao Y, Smith LP, Kgosana L, Green J, Petherbridge L, Baigent SJ, Nair V. Critical role of the virus-encoded microRNA-155 ortholog in the induction of Marek’s disease lymphomas. PLoS Pathog. 2011;7:e1001305. doi: 10.1371/journal.ppat.1001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JY, Pfuhl T, Motsch N, Barth S, Nicholls J, Grasser F, Meister G. Identification of novel Epstein-Barr virus microRNA genes from nasopharyngeal carcinomas. J Virol. 2009;83:3333–3341. doi: 10.1128/JVI.01689-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


