Abstract
Hypersensitivity reactions are the most frequent dose-limiting adverse reactions to Escherichia coli-derived asparaginase in pediatric acute lymphoblastic leukemia (ALL) patients. The aim of the present study was to identify associations between sequence-based Human Leukocyte Antigen Class II region alleles and asparaginase hypersensitivity in a Hungarian ALL population. Four-digit typing of HLA-DRB1 and HLA-DQB1 loci was performed in 359 pediatric ALL patients by using next-generation sequencing method. Based on genotypic data of the two loci, haplotype reconstruction was carried out. In order to investigate the possible role of the HLA-DQ complex, the HLA-DQA1 alleles were also inferred. Multivariate logistic regression analysis and a Bayesian network-based approach were applied to identify relevant genetic risk factors of asparaginase hypersensitivity. Patients with HLA-DRB1*07:01 and HLA-DQB1*02:02 alleles had significantly higher risk of developing asparaginase hypersensitivity compared to non-carriers [P=4.56×10−5; OR=2.86 (1.73–4.75) and P=1.85×10−4; OR=2.99 (1.68–5.31); n=359, respectively]. After haplotype reconstruction, the HLA-DRB1*07:01-HLA-DQB1*02:02 haplotype was associated with an increased risk. After inferring the HLA-DQA1 alleles the HLA-DRB1*07:01–HLA-DQA1*02:01–HLA-DQB1*02:02 haplotype was associated with the highest risk of asparaginase hypersensitivity [P=1.22×10−5; OR=5.00 (2.43–10.29); n=257]. Significantly fewer T-cell ALL patients carried the HLA-DQB1*02:02 allele and the associated haplotype than did pre-B-cell ALL patients (6.5%; vs. 19.2%, respectively; P=0.047). In conclusion, we identified a haplotype in the Human Leukocyte Antigen Class II region associated with a higher risk of asparaginase hypersensitivity. Our results confirm that variations in HLA-D region might influence the development of asparaginase hypersensitivity.
Introduction
Asparaginase is a pivotal component of pediatric acute lymphoblastic leukemia (ALL) treatment. It converts asparagine to aspartic acid and ammonia. Lymphoblast cells lose the ability to produce asparagines; hence the loss of exogenous asparagine leads to cell death. However, the use of asparaginase can be challenging as either hypersensitivity reactions or neutralizing antibody formation can occur against this heterologous enzyme. Both can consequently lead to lower exposure to asparaginase which may result in suboptimal treatment response.1–3 In addition, hypersensitivity reactions to asparaginase range in severity from mild, transient (flushing or rash) to generalized anaphylaxis, which can be potentially life-threatening.4
Few studies have investigated the genetic basis of asparaginase hypersensitivity (AH). In a genome-wide association study (GWAS) of 485 pediatric ALL patients of European ancestry, Chen et al. found that rs4958351 of the glutamate receptor gene GRIA1 located at 5q33 associated with AH.5 Recently, in a GWAS of a multiethnic cohort of 3308 patients, Fernandez et al. replicated the finding between the rs4958351 variant and AH and found that the association was stronger in patients treated with native Escherichia coli-derived asparaginase (E. coli asparaginase) than in those receiving pegylated asparaginase.6 However, the significance of the association (P=0.03) did not reach the genome-wide threshold (P=5 × 10−8). This association has been replicated in a relatively small Slovenian population of 146 patients.7 In our previous candidate-gene study, we found that although genetic variants of GRIA1 influenced the risk to AH, significant differences were observed according to sex and patient subgroup (T-cell or pre-B-cell ALL).8
The association of Human Leukocyte Antigen (HLA)-DRB1*07:01 allele with an increased risk of AH was revealed in a candidate-gene study of Fernandez et al. of 1870 pediatric ALL patients of European ancestry.9 Later, in the subsequent GWAS of the same group on AH, a SNP linked to HLA-DRB1*07:01 also acted as a risk allele in patients of diverse ancestry. In this study, the rs6021191 variant in NFATC2 was also associated with a higher risk of AH at the genome-wide significance threshold (P=4.1 × 10−8, OR=3.11).6 The minor allele frequency (MAF) of rs6021191 was only 0.001 among patients of European descent; therefore, this result has more relevance in patients of non-European ancestry.
HLA class II alleles are involved in presentation of peptides derived from extracellular proteins to T cells and subsequent activation of the immune response. They are located in the Major Histocompatibility Complex region on chromosome 6 and are expressed in antigen presenting cells. This genetic region is highly polymorphic with considerable linkage disequilibrium.
The primary goal of our study was to test the associations of HLA class II alleles with E. coli AH in a Hungarian population of 359 pediatric ALL patients using next-generation sequencing (NGS)-based HLA typing of HLA-DRB1 and HLA-DQB1 alleles. In addition, we aimed to evaluate the possible role of the HLA-DRB1–HLA-DQA1–HLA-DQB1 haplotypes and the polymorphic amino acid positions located in the peptide-binding groove of the HLA-DQ complex in the mechanism of AH.
Methods
Patients
DNA samples from 359 pediatric ALL patients were available to investigate the association of HLA class II alleles with AH. The patients were treated with protocols from the Berlin-Frankfurt-Münster Study Group (accrued patients from 1990 to 2011): ALL-BFM 90 (n=72), ALL-BFM 95 (n=165), ALL IC-BFM 2002 (n=117), and ALL IC-BFM 2009 (n=5). The combined chemotherapy regimens contained E. coli asparaginase (Kidrolase or Asparaginase medac) as first-line treatment. The dosing schedules of E. coli asparaginase have been described in detail previously.8
Written informed consent was obtained from the study participants or from the next of kin, carers, or guardians on behalf of the minors/children who took part in the study. The study was conducted according to the Declaration of Helsinki and approved by the Hungarian Scientific and Research Ethics Committee of the Medical Research Council (ETT TUKEB; case n.: 8-374/2009-1018EKU 914/PI/08).
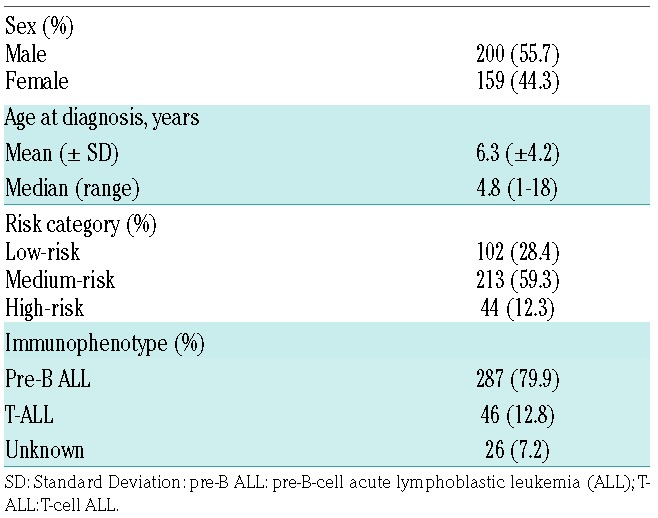
The patients had a median age of 4.8 years at diagnosis (range 1–18 years) (Table 1). The overall incidence of E. coli AH was 39.0%. Data collection was carried out retrospectively from medical records. The National Cancer Institute Common Toxicity Criteria (CTC) system v.3.0 was used to assess the grade of hypersensitivity. We regarded a case as AH when signs of allergic reactions or anaphylactic reactions CTC grade 1 and above were noted, as described earlier.8
Table 1.
Patients’ characteristics.
Sequence-based typing of HLA-DRB1 and DQB1
For sequencing, DNA samples from pediatric ALL patients with peripheral blood or bone marrow origin were available.8 Only those patients whose DNA sample met the quality control criteria of NGS-based typing method were enrolled in this study. All of the 359 patients were typed at high-resolution level for HLA-DRB1 and HLA-DQB1, in the Beijing Genomics Institute (BGI) using an NGS-based genotyping method that applies a massively parallel paired-end sequencing on Illumina MiSeq, effective variant phasing and haploid sequence assembling pipeline, as previously described.10 Based on this, exon 2 was sequenced in HLA-DRB1, and exons 2 and 3 were sequenced in HLA-DQB1 genes.
Estimation of HLA-DRB1–HLA-DQA1–HLA-DQB1 haplotypes
HLA-DRB1–HLA-DQB1 haplotypes were estimated based on HLA-DRB1 and HLA-DQB1 4-digit typing data of ALL patients by using the PHASE software (v.2.1.1). In addition, the HLA-DRB1 and HLA-DQB1 genotypic data was used to search for three-gene HLA-DRB1–HLA-DQA1–HLA-DQB1 haplotypes in the Allele Frequency Net Database-HLA Haplotype Frequency Search (http://www.allelefrequencies.net/hla6003a.asp).13 Using this approach, it was possible to infer independently the most likely haplotypes (including information about the HLA-DQA1 locus) using haplotype data pertaining to Caucasoid ethnic origin as reference panel (Online Supplementary Text S1).
Inference of polymorphic amino acid positions in HLA class II alleles
Amino acid sequences encoded by the HLA-DRB1, HLA-DQA1 and HLA-DQB1 exon 2 were inferred using the four-digit sequencing results of HLA-DRB1 and HLA-DQB1 as well as the inference results for HLA-DQA1. The Alignment Viewer of Database of Major Histocompatibility Complex (dbMHC; https://www.ncbi.nlm.nih.gov/gv/mhc/align.fcgi?cmd=aligndisplay&user_id=0&probe_id=0&source_id=0&locus_id=0&locus_group=1&proto_id=0&kit_id=0&banner=1) was used to assess the polymorphic amino acid positions in HLA-DRB1, HLA-DQB1 and HLA-DQA1 chains.
Statistical analysis
Frequentist methods
Multivariate logistic regression was used to test the associations of HLA class II alleles, HLA-DRB1–HLA-DQA1–HLA-DQB1 haplotypes and polymorphic amino acid positions with E. coli AH. Sex, ALL immunophenotype (pre-B or TALL), age at diagnosis (≤10 or >10 years), risk group (standard- or medium- or high-risk), and treatment protocol were included in the model as categorical covariates. Assuming an additive genetic model, odds ratios (ORs) and 95% confidence intervals (CIs) were obtained to estimate risks for each variable to AH. To account for multiple testing Bonferroni correction was used (P≤2.66 × 10−4, based on a total of 66 HLA class II alleles, 38 haplotypes and 84 polymorphic amino acid positions tested). R statistical software (3.1.2) and IBM SPSS Statistic (v.20) software were used for analyses.
BN-BMLA method
We also applied Bayesian network based Bayesian multilevel analysis of relevance (BN-BMLA) to extend our genetic association study by estimating a posteriori probabilities of strong relevance (posteriors).14–17 The BN-BMLA method was explained in detail in our previous studies.18–20 Briefly, it computes the a posteriori probability of the strongly relevant variable sets with respect to a target variable (e.g. a dichotomous variable (yes/no) describing the AH status of the patients). The strongly relevant variables have a direct influence on the target. Values for posterior (P) range from 0 to 1, where P=1 and P=0 mean that the probability of the target being dependent on a predictor (e.g. HLA allele) is 100% and 0%, respectively. There were two types of strong relevance: direct relevance (e.g. casual genetic variant) and pure interaction (e.g. epistasis). For analyses, HLA-DRB1, HLA-DQB1 and HLA-DQA1 alleles, HLA-DRB1–HLA-DQA1–HLA-DQB1 haplotypes, sex, ALL immunophenotype, age at diagnosis, risk group, and treatment protocol were included in the model as predictors.
Results
Association of HLA-DRB1 and HLA-DQB1 alleles with asparaginase hypersensitivity
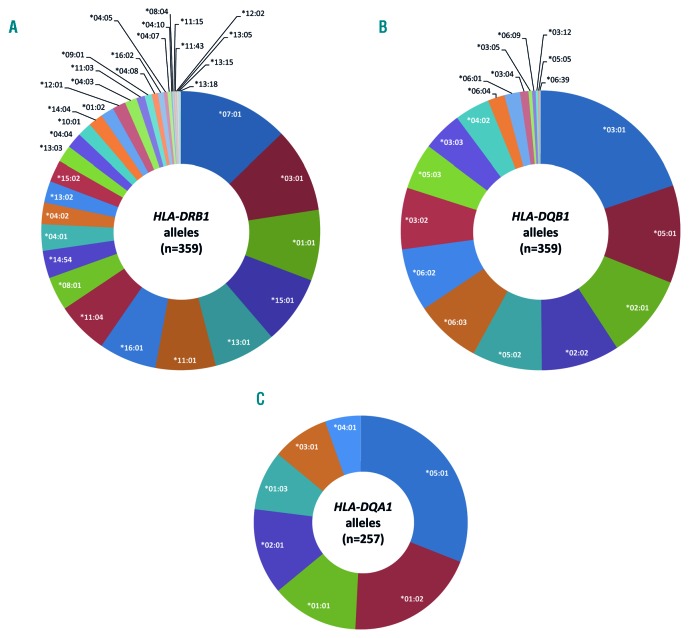
We investigated the associations of HLA class II alleles (HLA-DRB1, HLA-DQB1 and HLA-DQA1) and haplotypes with hypersensitivity reactions to E. coli asparaginase in pediatric ALL patients. Among 359 patients, the high-resolution sequence-based typing resulted in 35 unique HLA-DRB1 and 19 unique HLA-DQB1 alleles (Figure 1A and B).
Figure 1.
Frequencies of HLA-DRB1, HLA-DQB1 and HLA-DQA1 alleles in pediatric patients with acute lymphoblastic leukemia. High-resolution sequence-based typing of HLA class II alleles resulted in 35 unique HLA-DRB1 (A) and 19 unique HLA-DRB1 (B) alleles in 359 patients. HLA-DQA1 alleles were inferred in 257 patients and resulted in 7 unique alleles (C).
Univariate and multivariate logistic regression was performed to test the potential risk factors for AH (Online Supplementary Table S1). The incidence of AH varied by risk group (P=5.7 × 10−5), ranging from 31% for medium-risk group to 77% for the high-risk group and treatment protocol (P=3.36 × 10−3), ranging from 26% for ALL-BFM 90 to 60% for ALL IC-BFM 2002 experimental protocol (Online Supplementary Table S1). Using multivariate analysis risk group and treatment protocol also showed significant association with AH (Online Supplementary Table S1). For analyses with genetic data, we included all risk factors in the model as categorical covariates.
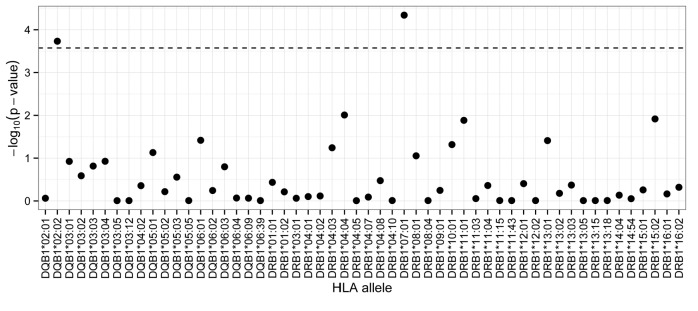
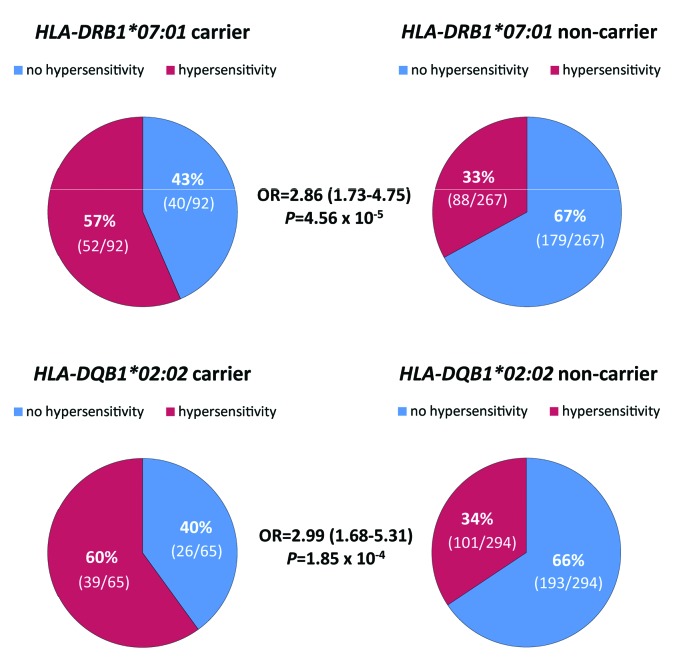
Applying Bonferroni correction (P≤2.66×10−4) in multivariate logistic regression analyses HLA-DRB1*07:01 and HLA-DQB1*02:02 alleles showed significant associations with E. coli AH in 359 pediatric ALL patients (Figure 2). None of the patients was homozygous for either allele. Patients with HLA-DRB1*07:01 allele had significantly higher risk of developing AH compared to non-carriers [P=4.56×10−5; OR=2.86 (1.73–4.75)] (Figure 3). Similarly, patients harboring HLA-DQB1*02:02 were at greater risk of having AH [P=1.85×10−4; OR=2.99 (1.68–5.31)] (Figure 3). Allelic odds ratios and the AH rate of patients depending on the carrier state are shown in Online Supplementary Table S2.
Figure 2.
Association of HLA-DQB1 and HLA-DQB1 alleles with E. coli asparaginase hypersensitivity. Multivariate logistic regression was applied to assess the association of HLA class II alleles with asparaginase hypersensitivity. Sex, age, treatment protocol, risk group, acute lymphoblastic leukemia (ALL) immunophenotype were included in the analysis as categorical covariates. The dashed horizontal line indicates the Bonferroni-corrected statistical significance threshold. HLA-DRB1*07:01 and HLA-DQB1*02:02 alleles were significantly associated with asparaginase hypersensitivity (n=359; P=4.56×10−5 and 1.85×10−4, respectively).
Figure 3.
Patients carrying the HLA-DRB1*07:01 or HLA-DQB1*02:02 alleles had strong association with asparaginase hypersensitivity. The incidence of hypersensitivity reactions was 57% (52 of 92) and 33% (88 of 267) for patients carrying HLA-DRB1*07:01 allele and for non-carriers, respectively. In the case of patients with HLA-DQB1*02:02 allele, the incidence of asparaginase hypersensitivity was 60% (39 of 65), while it was 34% (101 of 294) for patients who did not have the allele. Odds ratios (OR) and 95% confidence intervals were estimated by using multivariate logistic regression analysis.
There was a significant difference in the proportion of HLA-DQB1*02:02 carriers between patients with pre-B-cell and T-cell ALL (19.2% vs. 6.5%; in patients with pre-B-cell and T-cell ALL, respectively; P=0.047). No such difference could be detected in the case of HLA-DRB1*07:01 carriers.
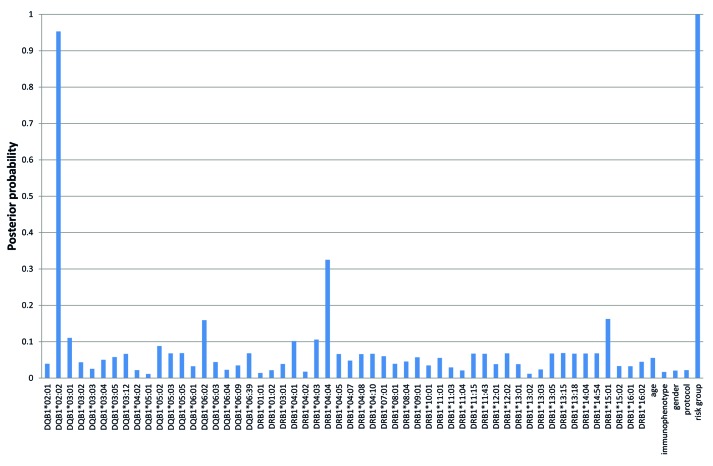
The BN-BMLA approach was used to investigate the different dependency relations between HLA alleles, age, ALL immunophenotype, sex, treatment protocol, risk group, and AH. The analysis revealed that if both HLA-DRB1 and HLA-DQB1 alleles were included in the Bayesian Network, among genetic factors only HLA-DQB1*02:02 allele had direct strong relevance to AH (P=0.95) beside the influence of the risk group status (P=1.00) (Figure 4). There was no interaction or redundancy between predictors.
Figure 4.
A posteriori strong relevance of predictors to asparaginase hypersensitivity. The BN-BMLA approach was used to investigate the different dependency relations between HLA alleles, age, acute lymphoblastic leukemia (ALL) immunophenotype, sex, treatment protocol, risk group and asparaginase hypersensitivity. HLA-DQB1*02:02 allele and risk group had direct strong relevance to asparaginase hypersensitivity (P=0.95 and 1.00, respectively).
Haplotype estimation and the inference of HLA-DQA1 alleles
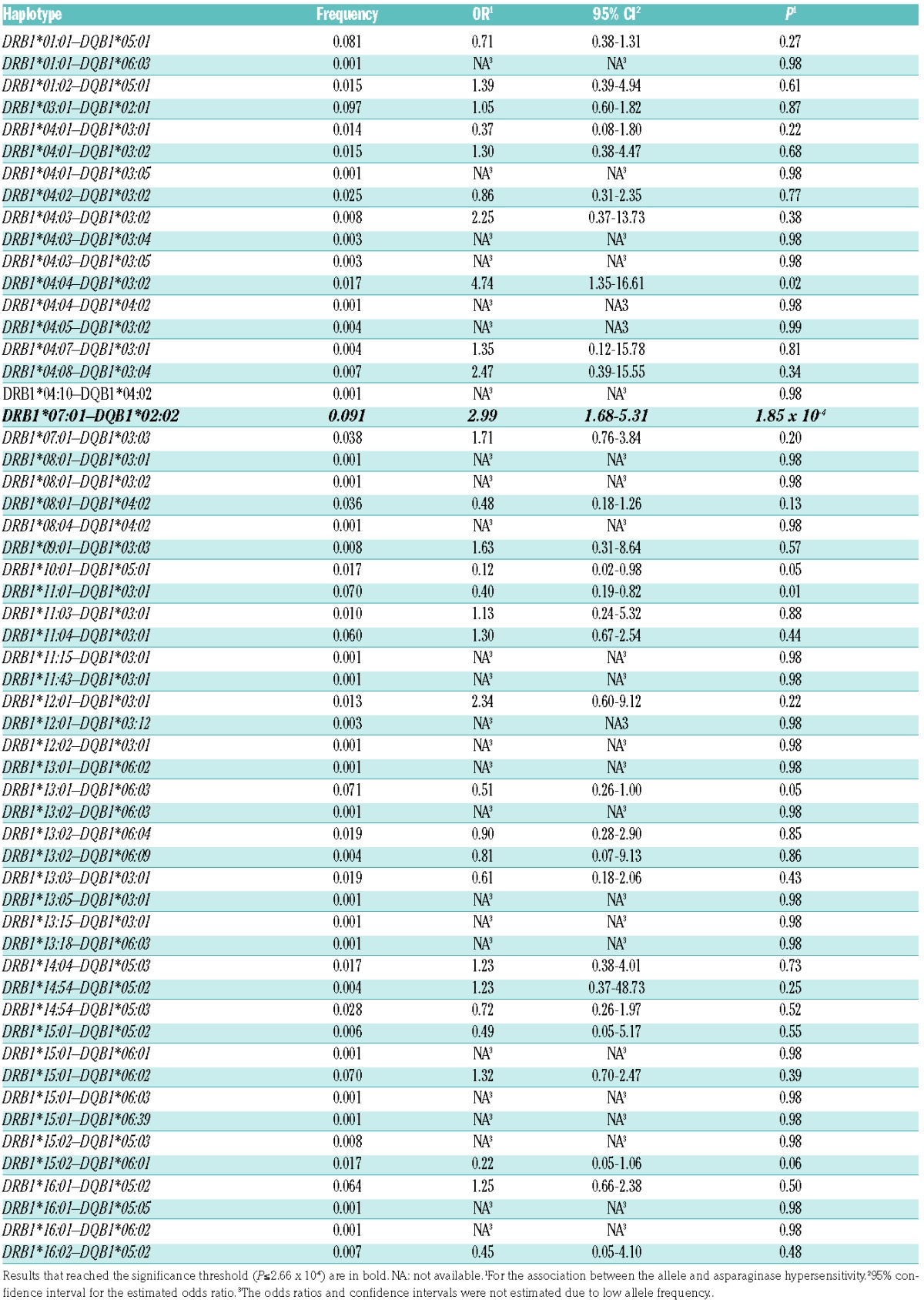
In order to further investigate the relationship between HLA-DRB1*07:01 and HLA-DQB1*02:02 alleles associated with AH, we estimated haplotypes using the PHASE software (v.2.1.1). Using this method, the haplotype reconstruction resulted in 56 different haplotypes (Table 2). Two haplotypes containing HLA-DRB1*07:01 allele were estimated among patients: HLA-DRB1*07:01–HLA-DQB1*02:02 and HLA-DRB1*07:01–HLA-DQB1*03:03. Out of these, only HLA-DRB1*07:01–HLA-DQB1*02:02 showed a positive association with AH [P=1.85 × 10−4; OR=2.99 (1.68–5.31)] (Table 2). According to this, the HLA-DRB1*07:01 and HLA-DQB1*02:02 are risk alleles and in contrast to the HLA-DRB1*07:01–HLA-DQB1*03:03 haplotype, HLA-DRB1*07:01–HLA-DQB1*02:02 reached statistical significance. This is in agreement with the result of the BN-BMLA, i.e. in respect of AH, the presence of HLA-DQB1*02:02 seems to be important.
Table 2.
Association of HLA-DRB1–HLA-DQB1 haplotypes with E. coli asparaginase hypersensitivity in acute lymphoblastic leukemia patients (n=359).
Next, the HLA-DRB1-HLA-DQA1-HLA-DQB1 haplotypes were independently inferred by using reference data from The Allele Frequency Net Database. The aim of inferring HLA-DQA1 alleles was to investigate the possible role of the structure of the HLA-DQ complex in the pathomechanism of AH. In contrast to the HLA-DR complex, the alpha chain is also polymorphic in the HLA-DQ heterodimer, therefore polymorphic amino acid positions in HLA-DQA1 may also influence the peptide preference of the HLA-DQ complexes and consequently the development of a specific immune response against asparaginase. Using data for haplotype frequencies in Caucasoid populations, and applying the aforementioned method, the estimation of extended haplotypes was possible in 72% of the patients (n=257; Online Supplementary Table S3). We compared the haplotype estimation results obtained by using the PHASE software to those extended haplotype data inferred by using The Allele Frequency Net Database. Results were in 100% agreement. Finally, among these 257 patients, 7 unique HLA-DQA1 alleles were inferred (Figure 1C).
Multivariate logistic regression analysis showed that HLA-DQA1*02:01 allele and HLA-DRB1*07:01–HLA-DQA1*02:01–HLA-DQB1*02:02 haplotype were positively and significantly associated with AH [P=3.45×10−5; OR=3.69 (1.99–6.84) and P=1.22×10−5; OR=5.00 (2.43–10.29), respectively] (Online Supplementary Table S3). These statistical associations were equivalent to the associations of HLA-DRB1*07:01 and HLA-DQB1*02:02 alleles, respectively; hence HLA-DQA1*02:01 exclusively and in all cases occurred together with HLA-DRB1*07:01.
Inference of polymorphic amino acid positions in HLA class II alleles
A total of 84 polymorphic amino acid positions were identified from 4-digit typing results of HLA-DRB1 and HLA-DQB1 genes and from inferred HLA-DQA1 allelic data using dbMHC database. In order to further investigate the possible sequence features that confer a greater risk to AH, multivariate logistic regression was performed to test for associations between polymorphic amino acid positions in HLA class II alleles and AH. In our patients with leukemia, a total of 27 positions associated significantly (Table 3).
Table 3.
Polymorphic amino acid positions in HLA-DRB1, HLA-DQB1 and HLA-DQA1 associated with E. coli asparaginase hypersensitivity in acute lymphoblastic leukemia patients (n=257).
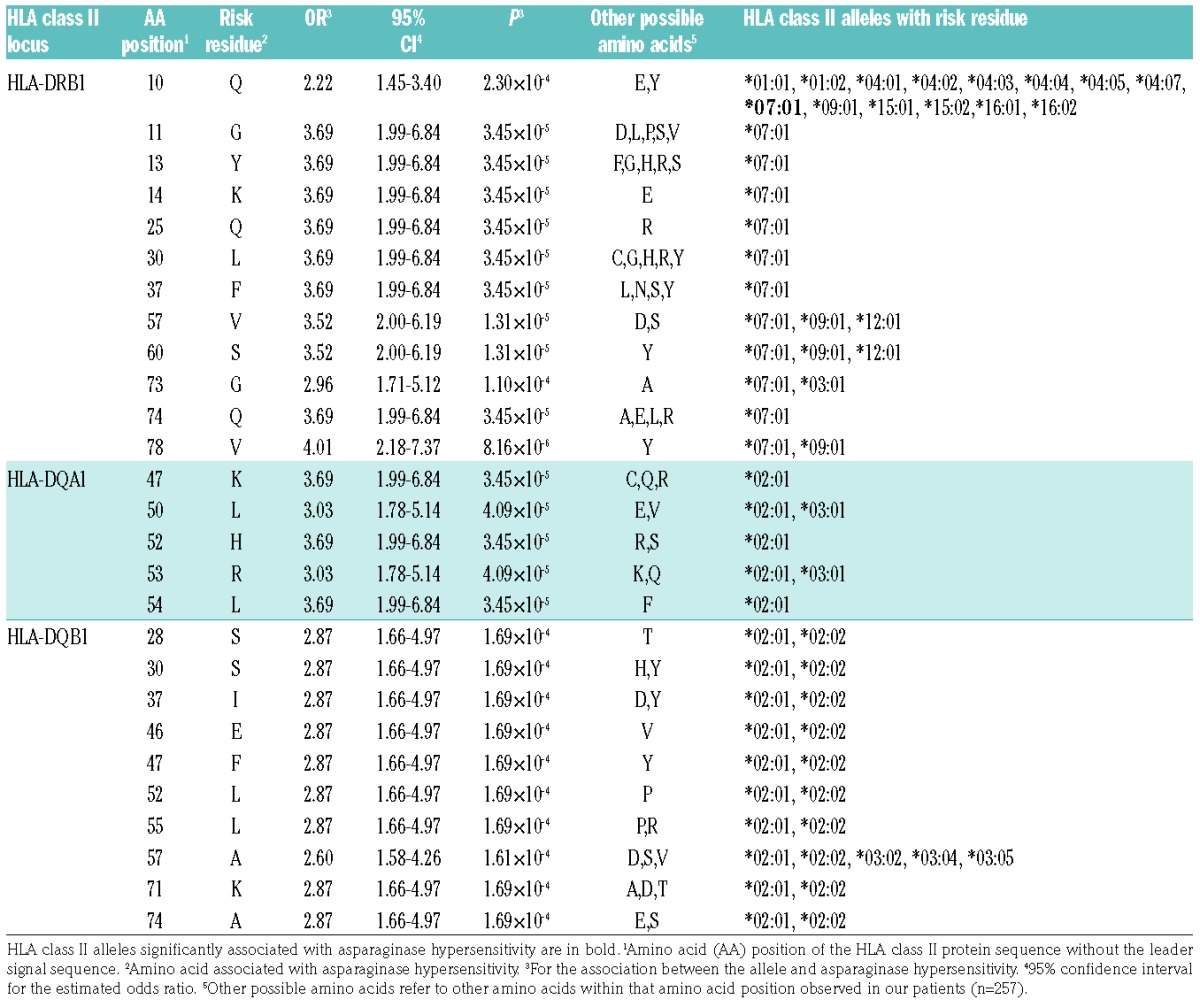
A valine at position 78 in HLA-DRB1 showed the strongest association with AH [P=8.16×10−6; OR=4.01 (2.18–7.37)] (Table 3). Out of the 27 risk amino acids, 7 and 3 were uniquely coded by HLA-DRB1*07:01 and HLA-DQA1*02:01, respectively [P=3.45×10−5; OR=3.69 (1.99–6.84)] (Table 3). All positions with risk amino acids in HLA-DQB1 belonged to at least two alleles and were, to a lesser extent, associated with hypersensitivity [P=1.69×10−4; OR=2.87 (1.66–4.97)] (Table 3).
Interestingly, while all amino acids conferring risk to AH were present in HLA-DQB1*02:02; the other HLA-DQB1 allele linked to HLA-DRB1*07:01, HLA-DQB1*03:03 possessed no risk amino acid at any risk position. These results confirm the important role of the HLA-DRB1*07:01–HLA-DQA1*02:01–HLA-DQB1*02:02 haplotype in the increased risk of AH.
Discussion
The aim of our study was to investigate the association of the variations in the HLA class II region with E. coli AH in pediatric ALL patients. By analyzing 359 Hungarian patients, we found that HLA-DRB1*07:01 and HLA-DQB1*02:02 were associated with an increased risk of developing AH. Haplotype reconstruction also revealed that the HLA-DRB1*07:01–HLA-DQA1*02:01–HLA-DQB1*02:02 haplotype positively and significantly associated with an increased risk of AH.
Previously, in 2014, a study by Fernandez et al. of 1870 pediatric ALL patients of European ancestry revealed the association of HLA-DRB1*07:01 allele with AH.9 Later, the same group confirmed the role of this allele in an ethnically diverse pediatric population. In our study, we also confirmed these findings, but after haplotype reconstruction we also found that only the HLA-DRB1*07:01–HLA-DQB1*02:02 haplotype associated significantly, and the HLA-DRB1*07:01–HLA-DQB1*03:03 haplotype did not reach statistical significance, possibly because of the lower allele frequency (0.038 vs. 0.091 for the HLA-DRB1*07:01–HLA-DQB1*02:02 haplotype). However, further investigations are warranted to elucidate the actual causality. The importance of the HLA-DQB1*02:02 allele in the hypersensitivity was also shown by the BN-BMLA method, which showed that if both HLA-DRB1 and HLA-DQB1 alleles were included in the Bayesian Network only HLA-DQB1*02:02 allele had direct strong relevance to AH with a high posterior probability (0.95). The situation is the same with the HLA-DQA1*02:01 allele as with the HLA-DRB1*07:01 allele, because they are in complete linkage disequilibrium with each other. This may suggest that, in our population, the HLA-DQB1*02:02 allele also seems to be an important genetic risk factor for AH in this region.
However, when the amino acid results are evaluated, there is some controversy in this statement. It is well known that proteins coded by the HLA-D, or also known as the MHC class II genes play a critical role in the immune response to extracellular antigens. Antigen presenting cells present processed antigens, or epitopes bound in a pocket formed by the MHC II chains to T-cell receptors on the surface of helper T cells, resulting in cytokine secretion by T cells leading to, among others, differentiation of B cells into antibody-secreting plasma cells. According to the theory, the stronger the MHC II protein binds an epitope, the stronger the immune response is. The strength of the binding is determined by the 3D structure of the MHC II protein which depends on its amino acid composition. Table 2 shows the amino acids associated with the AH and the different HLA class II alleles coding for these variants. Regarding E. coli asparaginase epitopes, Fernandez et al. predicted with bioinformatic methods that the HLA-DRB1*07:01 is a high binding allele which could be the reason for its association with AH. In our results, several amino acids within the HLA-DQB1 protein were also associated with increased AH, and all of them were present in HLA-DQB1*02:02. But they were also present in HLA-DQB1*02:01 which was not associated with AH. It means that from these amino acid results it cannot be predicted why amino acids in HLA-DQB1*02:02 were associated with AH. This may mean that although the presence of HLA-DQB1*02:02 allele may be decisive in the development of AH, the presence of the HLA-DRB1*07:01 and/or HLA-DQA1*02:01 alleles may also be necessary for the increased risk, raising the possibility that it is the extended haplotype rather than the individual alleles that is important in this respect.
There was another interesting finding in the present study. A significantly smaller proportion of T-cell ALL patients carried the HLA-DQB1*02:02 allele than did pre-B-cell ALL patients (6.5%; vs. 19.2% of patients with T-cell and pre-B-cell ALL, respectively). This may suggest two interpretations. Either the allele increases the risk to pre-B-cell ALL or decreases the risk to T-cell ALL. Earlier the HLA-DQB1*02:02 allele was found to be associated with increased risk of celiac disease indicating its autoimmunogenic potential.21 Although we did not determine its allele frequency in a healthy control population, its frequency data in The Allele Frequency Net Database in Caucasian populations were around the value found in the pre-B-cell ALL patients in our study. Thus, in contrast its role in celiac disease, the HLA-DQB1*02:02 allele might decrease the risk of the development of T-cell ALL. Furthermore, because this allele is always on the HLA-DRB1*07:01–HLA-DQA1*02:01–HLA-DQB1*02:02 haplotype, this could mean that this haplotype might provide some protection against development of T-cell ALL but not against pre-B-cell ALL. Naturally, subjects from the same population must be compared to test this assumption.
This study has some limitations. First, patients who died during the chemotherapy due to therapy-resistant progressive disease, or due to infections or toxicities of therapy are under-represented in our ALL population. Furthermore, data about hypersensitivity reactions to E. coli asparaginase was collected retrospectively from the patients’ files. This does not allow for meticulous documentation or fine grading of hypersensitivity reactions.
In some cases the inference of HLA-DQA1 alleles could not be precisely carried out. For further analyses with HLA-DQA1 alleles, data from undetermined haplotypes were merged into one of the possible HLA-DQA1 allele categories (Online Supplementary Table S4). In most cases, exon 2 of the involved alleles was identical at the amino acid sequence level and no haplotype was found with the other HLA-DQA1 allele in The Allele Frequency Net Database either. In the case of HLA-DRB1*13:01–HLA-DQA1*01:02/*01:03–HLA-DQB1*06:03 haplotype, the HLA-DQA1 allele was regarded as HLA-DQA1*01:03 because it was more likely based on the overall Caucasian haplotype frequency data. To increase the reliability of our inference results, a strict algorithm was applied, which led to the over-representation of common haplotypes.
There are, however, several strengths to this study. First, we studied a relatively large and homogenous European population (Hungarian). Second, we used NGS technology to determine the HLA-DQB1 and HLA-DQB1 HLA types, which is more accurate than the SNP-based imputation used in other studies. In addition, the nucleotide sequence-based HLA typing, in contrast to the costly and laborious direct experimental HLA typing, allows polymorphic HLA amino acid positions from the HLA alleles to be inferred. Third, ours is the first study to evaluate the role of HLA haplotypes in AH, and our results indicated that the HLA haplotype-based risk prediction might be more precise than the single allele-based risk calculation.
In conclusion, in our study, we found that the HLA-DRB1*07:01–HLA-DQA1*02:01–HLA-DQB1*02:02 haplotype was associated with a high risk of E. coli AH in pediatric ALL patients. Based on our results, besides the previously described HLA-DRB1*07:01 allele, the HLA-DQB1*02:02 allele might also be important in the development of AH. This needs further investigation.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/9/1578
References
- 1.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–1218. [DOI] [PubMed] [Google Scholar]
- 2.Rizzari C, Conter V, Stary J, et al. Optimizing asparaginase therapy for acute lymphoblastic leukemia. Curr Opin Oncol. 2013;25 Suppl 1:S1–9. [DOI] [PubMed] [Google Scholar]
- 3.Pieters R, Hunger SP, Boos J, et al. L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer. 2011;117(2):238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016;17(6):e231–239. [DOI] [PubMed] [Google Scholar]
- 5.Chen SH, Pei D, Yang W, et al. Genetic variations in GRIA1 on chromosome 5q33 related to asparaginase hypersensitivity. Clin Pharmacol Ther. 2010;88(2):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fernandez CA, Smith C, Yang W, et al. Genome-wide analysis links NFATC2 with asparaginase hypersensitivity. Blood. 2015;126(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajic V, Debeljak M, Goricar K, Jazbec J. Polymorphisms in GRIA1 gene are a risk factor for asparaginase hypersensitivity during the treatment of childhood acute lymphoblastic leukemia. Leuk Lymphoma. 2015:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Kutszegi N, Semsei AF, Gezsi A, et al. Subgroups of Paediatric Acute Lymphoblastic Leukaemia Might Differ Significantly in Genetic Predisposition to Asparaginase Hypersensitivity. PLoS One. 2015;10(10):e0140136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez CA, Smith C, Yang W, et al. HLA-DRB1*07:01 is associated with a higher risk of asparaginase allergies. Blood. 2014;124(8):1266–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao H, Wang Y, Zhang W, et al. A short-read multiplex sequencing method for reliable, cost-effective and high-throughput genotyping in large-scale studies. Hum Mutat. 2013;34(12):1715–1720. [DOI] [PubMed] [Google Scholar]
- 11.Stephens M, Scheet P. Accounting for decay of linkage disequilibrium in haplo-type inference and missing-data imputation. Am J Hum Genet. 2005;76(3):449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonzalez-Galarza FF, Takeshita LY, Santos EJ, et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015;43(Database issue):D784–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hullám G, Antal P, Szalai C, Falus A. Evaluation of a Bayesian model-based approach in GA studies. In: Sašo D, Pierre G, Juho R, eds. Proceedings of the third International Workshop on Machine Learning in Systems Biology. Proceedings of Machine Learning Research: PMLR, 2009:30–43. [Google Scholar]
- 15.Antal P, Hullam G, Gézsi A, Millinghoffer A. Learning complex bayesian network features for classification. In: Studený M, Vomlel J, editors. Proceedings of the Third European Workshop on Probabilistic Graphical Models; 2006 September 12−15; Prague: Czech Republic: Action M Agency; 2006. p. 9–16. [Google Scholar]
- 16.Antal P, Millinghoffer A, Hullám G, et al. Bayesian, systems-based, multilevel analysis of associations for complex phenotypes: from interpretation to decisions. In: Sinoquet C, Mourad R, eds. Probabilistic Graphical Models for Genetics, Genomics and Postgenomics. Oxford: Oxford University Press, 2014:318–352. [Google Scholar]
- 17.Antal P, Millinghoffer A, Hullám G, Szalai C, Falus A. A Bayesian View of Challenges in Feature Selection: Feature Aggregation, Multiple Targets, Redundancy and Interaction. In: Yvan S, Huan L, Iñaki I, Louis W, Yves Van de P, eds. Proceedings of the Workshop on New Challenges for Feature Selection in Data Mining and Knowledge Discovery at ECML/PKDD 2008. Proceedings of Machine Learning Research: PMLR, 2008:74–89. [Google Scholar]
- 18.Ungvari I, Hullam G, Antal P, et al. Evaluation of a partial genome screening of two asthma susceptibility regions using bayesian network based bayesian multilevel analysis of relevance. PLoS One. 2012;7(3):e33573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lautner-Csorba O, Gezsi A, Erdelyi DJ, et al. Roles of genetic polymorphisms in the folate pathway in childhood acute lym-phoblastic leukemia evaluated by Bayesian relevance and effect size analysis. PLoS One. 2013;8(8):e69843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lautner-Csorba O, Gezsi A, Semsei AF, et al. Candidate gene association study in pediatric acute lymphoblastic leukemia evaluated by Bayesian network based Bayesian multilevel analysis of relevance. BMC Med Genomics. 2012;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dieli-Crimi R, Cenit MC, Nunez C. The genetics of celiac disease: A comprehensive review of clinical implications. J Autoimmun. 2015;64:26–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.