Multiple myeloma (MM) is a malignant disorder of plasma cells with a heterogeneous clinical outcome that is affected by both numerical and structural chromosomal abnormalities, baseline characteristics (age, lactate dehydrogenase concentration, International Staging System score) and treatment regimen.1–3 MM is initiated by a number of genetic events, which sets a background upon which alterations associated with disease progression are superimposed.4,5 Deletion of the short arm of chromosome 17 (del17p) is an independent prognostic marker associated with poor clinical outcome, as identified by a number of studies, and more recently it has been incorporated into risk stratification models.2,3,6 It is detected in approximately 5–8% of newly diagnosed MM and in higher proportions of cases at relapse.4,7
It has been reported recently that there is a range in the distribution of cells with del17p in interphase fluorescence in situ hybridization (FISH) analyses.1,8,9 Experimentally the value for the number of positive cells in this test was established based on the background detection rate of false positive signals in cells lacking the abnormality. In clinical studies it is clear that del17p is present at different percentages within the plasma cell population. The impact of this variability on clinical outcomes has been uncertain with the importance having been addressed by only a few research groups.1,8,9 The Intergroup Francophone du Myeloma first described a cut-off level of 60% to be most powerful for del17p in FISH analysis.8
To address the importance of the cut-point for del17p, its relationship to risk status and mutation of TP53 we analyzed the clinical outcome of 747 newly diagnosed myeloma patients in the Total Therapy 3–5 trials. We analyzed the percentage of cells with del17p by inter-phase FISH analyses, correlating the results with gene expression profiling (GEP)-70 risk status and mutation within TP53 using a targeted sequencing panel.10–12 In our Total Therapy patients, interphase FISH analysis was carried out on bone marrow cell populations, and myeloma cells were identified using immunoglobulin light-chain antibodies (kappa or lambda).12 The percentage of del17p was based on 100-cell counts of light-chain isotype-positive myeloma cells.12 The therapeutic backbone for each TT trial has been tandem autologous stem cell transplantation given within the framework of induction, inter-therapy or consolidation and maintenance therapy.13–15 The TT3 trial (n=324) included low-risk (n=272) and high-risk (n=52) patients, the TT4 trial included low-risk patients (n=350) and the TT5 trial included high-risk patients (n=73). Among all the patients enrolled, 86% (n=643) were classified as having low-risk MM, and 14% (n=104) as having high-risk MM according to the GEP-70 risk score. Progression-free survival and overall survival were measured from the start of the protocol therapy; progression included relapse or death from any cause. Overall and progression-free survival curves were estimated with the Kaplan-Meier method and compared by the log-rank test.
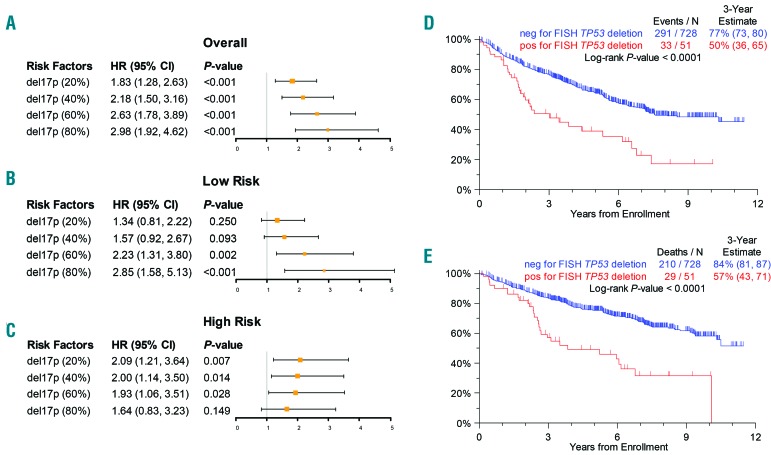
The presence of del17p in ≥20% of cells was detected in 10% (n=76) of all patients from the TT3-5 trials and was identified more than twice as frequently in high-risk MM patients than in low-risk patients (21% versus 8%). Overall the presence of del17p at this cut-point was associated with impaired clinical outcomes compared to cases lacking this abnormality: estimated 3-year progression-free survival 61% versus 76%; estimated 3-year overall survival: 67% versus 84%, respectively. In the overall population the hazard ratios for the prediction of both overall and progression-free survival increased at higher cut-points (Figure 1A).
Figure 1.
Survival data (A–C). Forest plots of overall survival hazard ratios for different cut points for (A) overall population; (B) low-risk patients; (C) high-risk patients. (D,E) Kaplan-Meier plots for survival of the overall population in TT3-5 using a 60% cut-point. (D) Progression-free survival. (E) Overall survival.
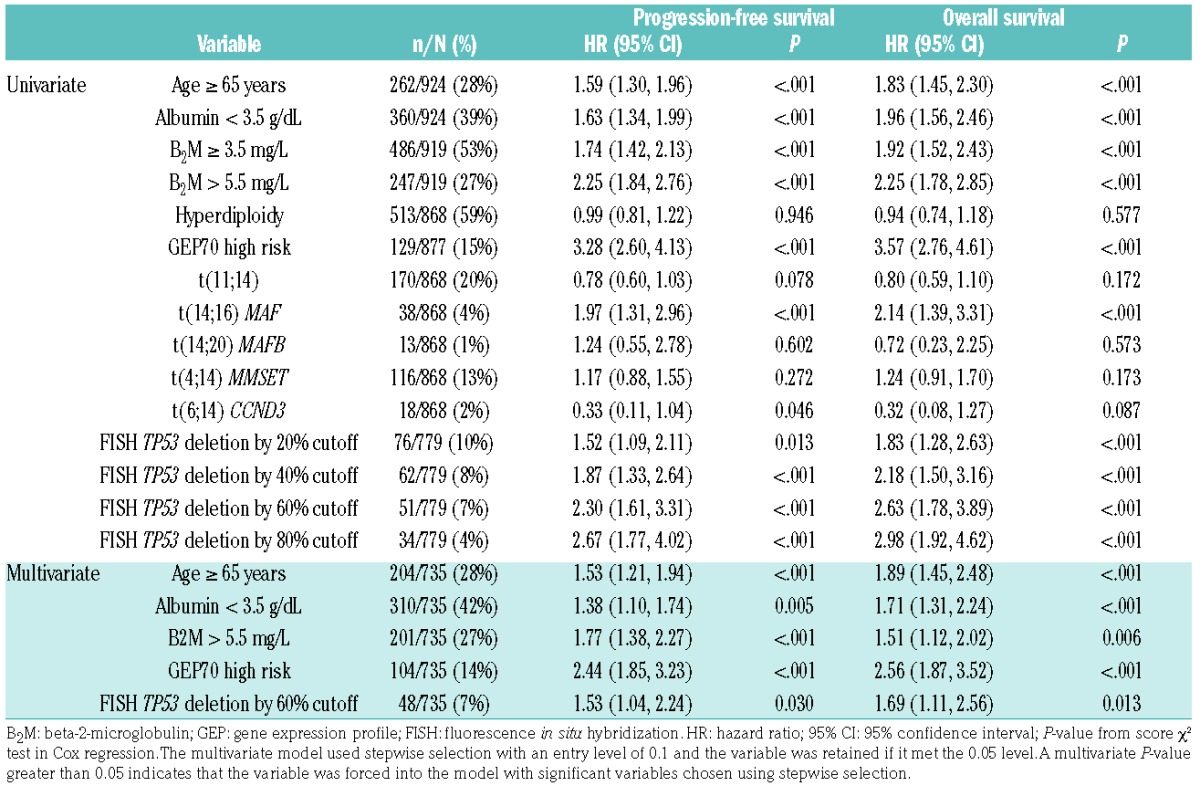
A stepwise Cox regression analysis of all cases was performed in order to determine the influence of confounding factors on the impact of percentage del17p levels on outcome. In addition to age ≥65 years, albumin concentration <3.5 g/dL, beta-2-microglobulin level >5.5 mg/L, and GEP-70 high-risk status, del17p always entered the final model whether the cut-point used was 20%, 40%, 60% or 80% (data not shown), suggesting that del17p is indeed an independent prognostic factor. In Table 1, we show the results when using all cut-points of del17p as input variables (along with the other clinical parameters), where the cut-point of 60% was selected as the optimum along with age ≥65 years, albumin concentration <3.5 g/dL, beta-2-microglobulin level >5.5 mg/L, and GEP-70 high-risk status. Using del17p at a 60% cut-point for the prediction of progression-free survival and overall survival had hazard ratios of 1.53 (95% confidence interval: 1.04–2.24; P=0.03) and 1.69 (95% confidence interval: 1.11–2.56; P=0.013), respectively (Figure 1D,E). We further compared outcome in the different trials in order to rule out any potential confounding factors from the treatment used, and no significant differences were seen.
Table 1.
Hazard ratios for overall and progression-free survival in univariate and multivariate analysis considering several percentages of del17p and different cytogenetic abnormalities and other confounding factors in Total Therapy 3, 4, and 5 patients.
GEP-70 risk status had an impact on the interpretation of the cut-point used. In low-risk patients no significant association with clinical outcome was seen at low cut-points (e.g. at 20% and 40%) (Figure 1B). In contrast, the presence of del17p in ≥60% cells was associated with significantly impaired outcome compared to cases with a lower percentage of del17p-positive cells, with 3-year overall survival rates of 73% versus 87%, respectively (P=0.002) and 3-year progression-free survival rates of 64% versus 81% (P=0.004). These findings were even more significant at a cut-point of 80%, the 3-year overall survival rates being 65% versus 87% (P=0.0003) and the 3-year progression-free survival rates being 56% versus 81% (P=0.0005), although the 80% cut-point did not identify a clinically useful number of patients. The prognostic importance of the cut-point was not seen in high-risk patients in whom a higher cut-point did not aid in the discriminatory value of the test (Figure 1C). In this analysis high-risk patients with del17p in ≥20% cells had a worse clinical outcome than patients without del17p: 3-year overall survival, 62% versus 26% (P=0.007) and 3-year progression-free survival, 45% versus 17% (P=0.07), and this was consistent across all cut-points (Figure 1C).
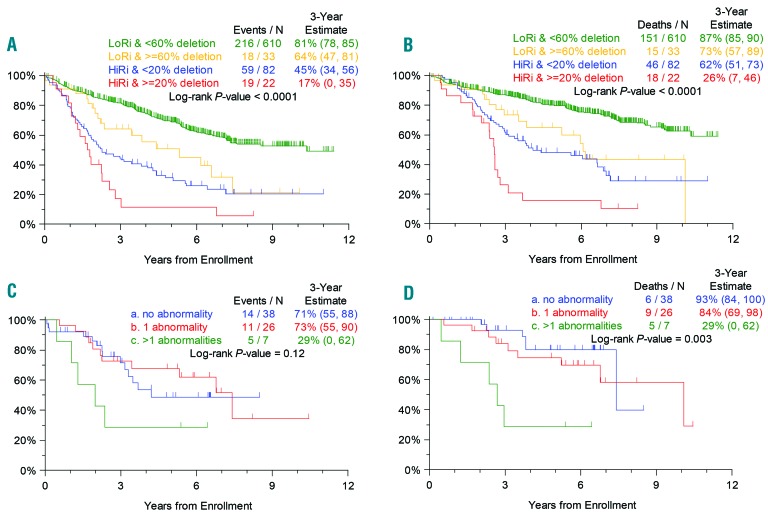
We went on to investigate whether integrating the percentage of del17p-positive cells with GEP-70 risk status could improve outcome prediction. In this analysis we were able to define three major groups that have distinct overall and progression-free survival rates (Figure 2A,B). These groups were defined as (i) low-risk MM with <60% del17p; (ii) high-risk MM with ≥20% del17p; and (iii) low-risk MM with ≥60% del17p plus high-risk MM with <20% del17p. The best outcomes were seen in low-risk patients with <60% del17p (3-year overall survival, 87%; 3-year progression free-survival, 81%) and the worst in high-risk patients with ≥20% del17p (3-year overall survival, 26%; 3-year progression-free survival, 17%). Interestingly, low-risk MM patients with ≥60% del17p and high-risk MM patients with <20% del17p formed an intermediate group and revealed no statistical difference in clinical outcome regarding overall and progression-free survival (Figure 2A,B).
Figure 2.
Survival data according to del17p status and GEP-70 risk group. (A,B). The interaction of del17p with GEP70 showing that the presence of del17p can split GEP70 high-risk patients into two groups introducing an ultra high risk group. (A) Progression-free survival. (B) Overall survival. (C,D). The interaction of del17p with mutation showing that homozygous inactivation of both alleles is a major driver of prognosis. (C) Progression-free survival. (D) Overall survival.
The biological issue being addressed in these analyses is the clinical prognostic value of inactivation of TP53. The relationship of the percentage of del17p-positive cells and mutation of the remaining allele has not been studied, nor has the prognostic value of bi-allelic inactivation of TP53. Using a subset of our patients with both TP53 mutation and interphase FISH data available (n=72), we performed a logistic regression analysis to investigate this issue. We found a significant correlation between the two events with the odds of having TP53 mutation increasing by 1.286-fold when there was a 10% increase in the percentage of cells carrying del17p (P=0.0387). We addressed the role of bi-allelic inactivation, (Figure 2C,D), showing that patients with homozygous deletion or both del17p and TP53 mutation have a significantly impaired outcome compared to those who have either del17p alone or TP53 mutation alone: 3-year overall survival 84% versus 29% (P=0.02) and 3-year progression-free survival 73% versus 29% (P=0.04), respectively.
In this study, we show that the presence of del17p is associated with adverse outcome and that the size of the clone carrying the abnormality is important. In addition, the genetic background upon which del17p is acquired is important, and the combination of GEP-70 risk status with the percentage of deletion provides a more precise prediction of outcome. In low-risk MM, a cut-point of 20% has no predictive value whereas the use of a cut-point of 60% identifies a group of patients with a significantly inferior outcome. In high-risk cases the 20% cut-point is clinically useful and is associated with adverse outcome, but there is a ceiling effect in this setting with cases positive above this level not being associated with significantly worse outcome. We provide a potential explanation to explain the relationship of the three variables (del17p, TP53 mutation, and GEP-70 risk status) we assessed. We show that there is an increased risk of TP53 mutation as the percentage of del17p-positive cells increases and that a major component of the adverse prognosis associated with these variables is associated with bi-allelic inactivation of TP53. In addition, we suggest that a significant component of the poor outcome of the GEP-70 high-risk group comes from its association with del17p and that this can be used to define an ultra high-risk subgroup within the overall GEP-70 group. We conclude that it is essential not only to report the presence of del17p in MM, but also to quantify the number of cells carrying the abnormality, taking account of bi-allelic inactivation (through homozygous deletion or concurrent mutation), and GEP-defined risk status if possible.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Merz M, Hielscher T, Seckinger A, et al. Baseline characteristics, chromosomal alterations, and treatment affecting prognosis of deletion 17p in newly diagnosed myeloma. Am J Hematol. 2016;91(11):e473–e477. [DOI] [PubMed] [Google Scholar]
- 2.Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015;33(26):2863–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moreau P, Cavo M, Sonneveld P, et al. Combination of International Scoring System 3, high lactate dehydrogenase, and t(4;14) and/or del(17p) identifies patients with multiple myeloma (MM) treated with front-line autologous stem-cell transplantation at high risk of early MM progression-related death. J Clin Oncol. 2014;32(20):2173–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinhold N, Ashby C, Rasche L, et al. Clonal selection and double-hit events involving tumor suppressor genes underlie relapse in myeloma. Blood. 2016;128(13):1735–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker BA, Wardell CP, Melchor L, et al. Intraclonal heterogeneity is a critical early event in the development of myeloma and precedes the development of clinical symptoms. Leukemia. 2014;28(2):384–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinhold N, Heuck CJ, Rosenthal A, et al. Clinical value of molecular subtyping multiple myeloma using gene expression profiling. Leukemia. 2016;30(2):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chavan SS, He J, Tytarenko R, et al. Bi-allelic inactivation is more prevalent at relapse in multiple myeloma, identifying RB1 as an independent prognostic marker. Blood Cancer J. 2017;7(2):e535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avet-Loiseau H, Attal M, Moreau P, et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood. 2007;109(8):3489–3495. [DOI] [PubMed] [Google Scholar]
- 9.An G, Li Z, Tai YT, et al. The impact of clone size on the prognostic value of chromosome aberrations by fluorescence in situ hybridization in multiple myeloma. Clin Cancer Res. 2015;21(9):2148–2156. [DOI] [PubMed] [Google Scholar]
- 10.Shaughnessy JD, Jr, Zhan F, Burington BE, et al. A validated gene expression model of high-risk multiple myeloma is defined by deregulated expression of genes mapping to chromosome 1. Blood. 2007;109(6):2276–2284. [DOI] [PubMed] [Google Scholar]
- 11.Zhan F, Huang Y, Colla S, et al. The molecular classification of multiple myeloma. Blood. 2006;108(6):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaughnessy J, Jr, Tian E, Sawyer J, et al. Prognostic impact of cytogenetic and interphase fluorescence in situ hybridization-defined chromosome 13 deletion in multiple myeloma: early results of total therapy II. Br J Haematol. 2003;120(1):44–52. [DOI] [PubMed] [Google Scholar]
- 13.van Rhee F, Szymonifka J, Anaissie E, et al. Total Therapy 3 for multiple myeloma: prognostic implications of cumulative dosing and premature discontinuation of VTD maintenance components, bortezomib, thalidomide, and dexamethasone, relevant to all phases of therapy. Blood. 2010;116(8):1220–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jethava Y, Mitchell A, Epstein J, et al. Adverse metaphase cytogenetics can be overcome by adding bortezomib and thalidomide to fractionated melphalan transplants. Clin Cancer Res. 2017;23(11):2685–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jethava Y, Mitchell A, Zangari M, et al. Dose-dense and less dose-intense Total Therapy 5 for gene expression profiling-defined high-risk multiple myeloma. Blood Cancer J. 2016;6(7):e453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.