There is increasing evidence that evaluating minimal residual disease (MRD) at various time points during acute myeloid leukemia (AML) therapy retains a strong prognostic value.1–3 In particular, the persistence of MRD positivity before hematopoietic stem cell transplantation (HSCT) has been associated with poor outcome.4,5 Multicolor flow cytometry (MFC) is widely recognized as a reliable and widely applicable technique for MRD evaluation, whereas real-time quantitative polymerase chain reaction (RT-qPCR) targeting specific molecular alterations is applicable to a minority of AML patients.6–8 Many trials have also shown the feasibility of evaluating MRD by WT1 gene expression levels in up to 70% of AML subjects.9,10 In the study herein, we show that combined MFC and molecular (WT1-based) evaluation of pre-transplant MRD can accurately predict the risk of post-transplant relapse in AML patients.
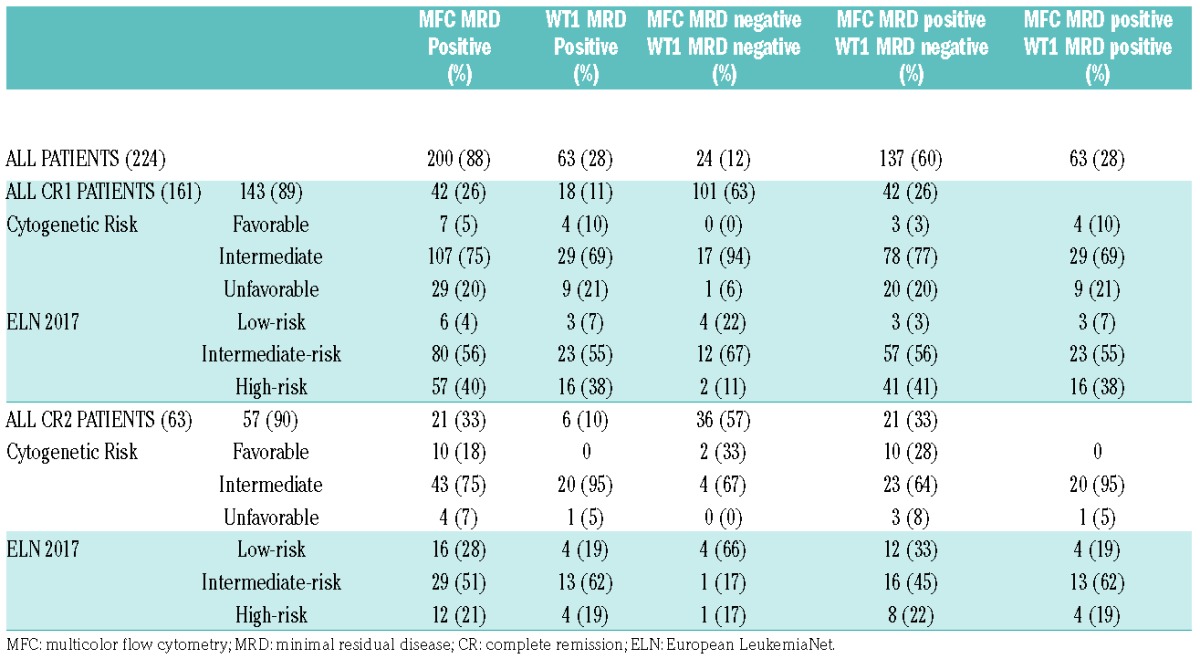
We retrospectively analyzed the outcome of 224 consecutive AML patients who were transplanted in our center in first or second complete remission (CR) between January 2004 and January 2014. In all patients, MRD was evaluated by MFC and WT1 gene expression on bone marrow samples before transplant. Patients without WT1 overexpression at diagnosis were not included in the study. The features of patients, including risk category according to European LeukemiaNet (ELN) 2017, are reported in Table 1. When immunophenotype at diagnosis was available, leukemia-associated immunophenotype (LAIP) was used to track residual leukemic cells. In the remaining patients the “different-from-normal” MFC approach was applied. The definition of MFC-based MRD (MFC MRD) positivity required the presence of no less than 250 clustered leukemic cells/106 total events (threshold of 2.5×10−4 residual leukemic cells). Bone marrow WT1 expression between 0 and 250 copies/Abl × 104 was assumed to be physiological (WT1 negative) on the basis of ELN experience. The cumulative incidence (CI) of relapse at various time points was calculated in competing risk analysis using the Fine and Gray subdistribution relative hazard method, counting non-relapse mortality (NRM) as a competing event.
Table 1.
Clinical features of patients and pre-HSCT MRD status.
After a median follow up of 64 months (95% CI 50.3 – 77.3 months), 62 patients relapsed (27.7%). Both WT1 and MFC MRD status had a significant impact on relapse probability. Patients with pre-transplant positive MRD by MFC or WT1 expression had a significantly higher incidence of relapse, accounting for 30% and 42%, respectively, as compared to 8.3% for MFC MRD negative patients and 21.3% for WT1 negative patients (P<0.003 and P<0.001, respectively).
A linear correlation between WT1 expression levels and relapse probability was not found. On the contrary, we found a trend for a higher relapse risk with increasing levels of MFC MRD. The incidence of relapse was 8.3% in patients with undetectable MFC MRD (2/24) compared to 17.4% (4/23) and 31.4% (27/86) in patients with MFC MRD in the range of 0.025–0.1% and 0.1–1%, respectively. For MFC MRD levels greater than 1% a linear correlation with relapse risk was no longer observed (31.9%, 29/91). All patients with undetectable MFC MRD were also WT1 negative.
WT1-based MRD (WT1 MRD) positive patients had the highest risk of relapse (negative predictive value (NPV): 79%; positive predictive value (PPV): 42%) whereas patients with undetectable MFC MRD had a very low relapse risk (NPV: 92%, PPV: 30%). The combined MRD evaluation was able to stratify patients into 3 groups: WT1 MRD negative (24/224, 12%), MFC MRD positive but WT1 MRD negative (150/224, 67%), and MFC MRD positive and WT1 MRD positive (50/224, 21%). These groups showed different relapse rates: 2/24 (8.3%) in MFC MRD negative patients, 35/150 (23.3%) in MFC MRD positive-WT1 negative patients, and 25/50 (50%) in patients who were positive for both MRD markers (P<0.0001). The combination allowed patients to benefit from both the high PPV of WT1 MRD and the high NPV of MFC MRD.
Multivariate logistic regression analysis showed combined MRD evaluation as the strongest independent predictor of relapse probability (P<0.0001). In competitive risk analysis, the 3-year CI of relapse was 25.9% (Figure 1). A lower CI of relapse was significantly associated with the occurrence of acute graft versus host disease (aGvHD) and negative MRD status before transplantation, measured by any method. MFC MRD negativity was the strongest predictor of a lower CI of relapse. Among MFC MRD positive patients, WT1 positive MRD status was capable of identifying patients with a significantly higher CI of relapse (P<0.0001). In multivariate analysis, the predictive value of combined MRD was found to be independent of induction therapy, donor type, conditioning regimen, disease status at HSCT, HSCT year and risk group. Combined MRD evaluation and the occurrence of aGvHD were the only predictors of a lower CI of relapse (P<0.001 and P=0.06, respectively).
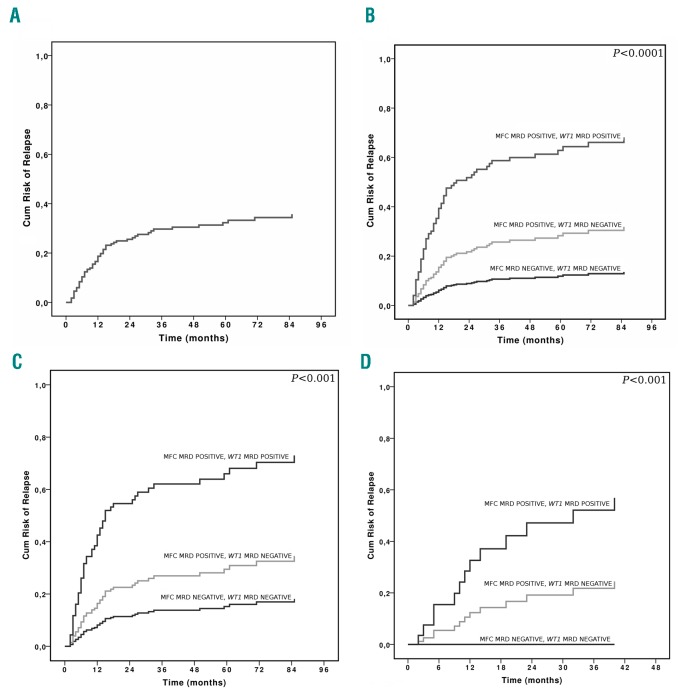
Figure 1.
CI of relapse according to combined MRD and disease status at transplantation. (A) CI of relapse in all patients. B) CI of relapse in all patients according to combined MRD. C) CI of relapse in patients transplanted in CR1 according to combined MRD, and D) CI of relapse in patients transplanted in CR2 according to combined MRD. Cum: cumulative; MFC: multicolor flow cytometry; MRD: minimal residual disease.
Of note, patients with negative MFC MRD in first or second CR undergoing HSCT had a similar cumulative relapse risk (Figure 1), with a 3-year CI of relapse of 8.4% and 5.6%, respectively (P=not significant [n.s.]). Similarly, MFC MRD positive/WT1 MRD negative patients had a 3-year CI of relapse of 17.3% and 18.5% for CR1 and CR2 patients, respectively (P=n.s.). Patients who were MRD positive by both methods, undergoing transplant in CR1 or CR2 had a similar, very high CI of relapse (Figure 1). One hundred and three patients died, 55 (53.4%) due to disease relapse.
Median overall survival (OS) was 74 months, and 3-year OS was 56.2% (Figure 2). The probability of long-term survival was significantly affected by HSCT year, conditioning intensity, MRD status before transplantation (evaluated by WT1 and combined modality), and type of donor, with better results observed for haploidentical (HAPLO) transplantation, followed by human leukocyte antigen (HLA)-identical transplant, when compared to unrelated donors (P<0.05, P<0.03, P<0.003 and P<0.001, respectively; data not shown).
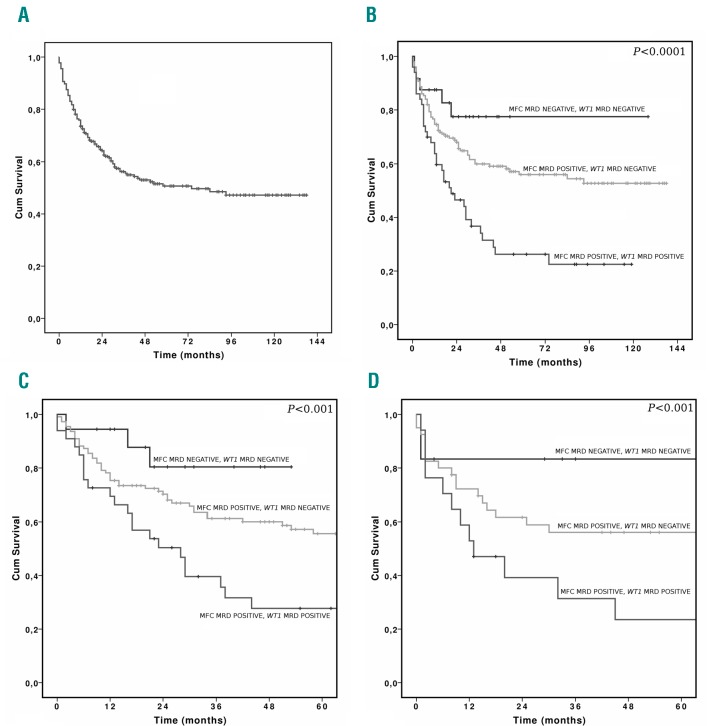
Figure 2.
Overall Survival (OS) according to combined MRD and disease status at transplantation. A) OS in all patients. B) OS in all patients according to combined MRD. C) OS in patients transplanted in CR1 according to combined MRD, and D) OS in patients transplanted in CR2 according to combined MRD. Cum: cumulative; MFC: multicolor flow cytometry; MRD: minimal residual disease.
The OS rate of MFC negative/WT1 negative, MFC positive/WT1 negative and MFC positive/WT1 positive was 73.5, 60 and 36.7%, respectively (Figure 2). Multivariate OS analysis revealed that the combined MRD evaluation and conditioning intensity were independent predictors of OS (P<0.001 and P<0.005, respectively), with a better outcome observed for patients receiving myeloablative conditioning. Better OS was associated with myeloablative conditioning (P=0.004) and negative combined MRD (P<0.001). The predictive value of MRD was found to be independent of induction schedules, donor type, disease status at transplantation and risk group.
In the study herein, patients with undetectable MFC MRD had a very favorable post-HSCT outcome, confirming recent reports by the Seattle group.5 Notably, a standardized and widely recognized cutoff to define MFC MRD positivity in AML is not available and different thresholds have been applied, ranging from any positive value to 0,1%.3,5,10,11 Furthermore, a linear correlation between MFC MRD level and relapse risk has not always been reported. In patients with NPM1 mutation or t(8;21), molecular analysis supported the accuracy of our MFC MRD data, since all MFC MRD negative patients also resulted negative for their specific marker, whereas a large proportion of MFC MRD positive patients were molecularly positive. Although a lower MFC threshold allowed for the identification of patients with a homogeneous very low CI of relapse, it may lack specificity in some clinical settings, such as in the case of regenerating marrow, or AML with monocytic differentiation, where, on occasion, the leukemic phenotype is not easily differentiated from that of normal cells. Moving the MFC positivity threshold to 0.1% enhances the specificity of our analysis, at the price of a parallel increase in the relapse rate of MFC MRD negative patients (false MRD negative patients). For these reasons, we integrated MFC information with molecular WT1-based MRD evaluation. Jacobsohn et al. showed that elevated WT1 gene expression in peripheral blood before HSCT in pediatric AML was able to predict relapse and poor long-term event-free survival (EFS).12 Candoni et al. recently reported on a series of FLT3 AML patients undergoing transplant, stating that the CI of relapse was lower in patients who were WT1-negative at the time of transplantation compared with those who were WT1-positive.13 To the best of our knowledge, no larger studies have explored the usefulness of integrating MFC and molecular tools in predicting post-transplant relapse. Our combined approach takes advantage of the high sensitivity of the MFC technique, with a lower cutoff and specific molecular analysis.
Our results confirm the high prognostic value of pre-HSCT MRD status for patients transplanted in both CR1 and CR2. The similar outcome between CR1 and CR2 patients outlines that the persistence of chemotherapy resistant leukemic cells in MRD positive patients is the most relevant indicator of outcome. The observation that the majority of patients receiving conventional therapy achieve only MRD positive CR highlights the strong need of new drugs for induction, consolidation and salvage treatment in order to achieve a pre-transplant MRD negative CR status.
We have previously demonstrated that, in patients with increasing WT1 levels during post-transplant follow up, pre-emptive donor lymphocyte infusions (DLI) improved survival as compared to patients who did not receive DLI.14,15 In this view, pre-transplant MRD positive patients might receive prophylactic DLI to reduce relapse risk and improve survival. An additional possibility could be prophylactic treatment with hypomethylating agents, histone deacetylase (HDAC) inhibitors or therapy with targeted drugs according to the mutational pattern at diagnosis.
In conclusion our combined cytofluorimetric and molecular MRD assessment improves the prognostic value of pre-transplant MRD evaluation, and might be useful for selecting the intensity of conditioning and driving post-transplant interventions.
Supplementary Material
Footnotes
Funding: this work was partially supported by IRCCS Azienda Ospedaliera Universitaria San Martino - IST Istituto Nazionale per la Ricerca sul Cancro, 5 per 1000 per la Ricerca Corrente.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017; 129(4):424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”¿ Am Soc Hematol Educ Program. 2014(1);222–233. [DOI] [PubMed] [Google Scholar]
- 3.Buccisano F, Maurillo L, Del Principe MI, et al. Prognostic and therapeutic implications of minimal residual disease detection in acute myeloid leukemia. Blood. 2012; 119(2):332–341. [DOI] [PubMed] [Google Scholar]
- 4.Bastos-Oreiro M, Perez-Corral A, Martínez-Laperche C, et al. Prognostic impact of minimal residual disease analysis by flow cytometry in patients with acute myeloid leukemia before and after allogeneic hemopoietic stem cell transplantation. Eur J Haematol. 2014; 93(3):239–246. [DOI] [PubMed] [Google Scholar]
- 5.Araki D, Wood BL, Othus M, et al. Allogeneic hematopoietic cell transplantation for acute myeloid leukemia: time to move toward a minimal residual disease-based definition of complete remission? J Clin Oncol. 2016; 34(4):329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimwade D, Jovanovic JV, Hills RK, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009; 27(22):3650–3658. [DOI] [PubMed] [Google Scholar]
- 7.Gorello P, Cazzaniga G, Alberti F, Dell’Oro MG, et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia. 2006; 20(6):1103–1108. [DOI] [PubMed] [Google Scholar]
- 8.Yin JA, O’Brien MA, Hills RK, et al. Minimal residual disease monitoring by quantitative RT-PCR in core binding factor AML allows risk stratification and predicts relapse: results of the United Kingdom MRC AML-15 trial. Blood. 2012; 120(14):2826–2835. [DOI] [PubMed] [Google Scholar]
- 9.Marani C, Clavio M, Grasso R, et al. Integrating post induction WT1 quantification and flow-cytometry results improves minimal residual disease stratification in acute myeloid leukemia. Leuk Res. 2013; 37(12):1606–1611. [DOI] [PubMed] [Google Scholar]
- 10.Cilloni D, Renneville A, Hermitte F, et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European Leukemia Net study. J Clin Oncol. 2009; 27(31):5195–5201. [DOI] [PubMed] [Google Scholar]
- 11.Terwijn M, van Putten WL, Kelder A, van der Velden VH, Brooimans RA, Pabst T, et al. High prognostic impact of flow cytometric minimal residual disease detection in acute myeloid leukemia: data from the HOVON/SAKK AML 42A study. J Clin Oncol. 2013; 31(31):3889–3897. [DOI] [PubMed] [Google Scholar]
- 12.Jacobsohn DA, Tse WT, Chaleff S, et al. High WT1 gene expression before haematopoietic stem cell transplant in children with acute myeloid leukaemia predicts poor event-free survival. Br J Haematol. 2009; 146(6):669–674. [DOI] [PubMed] [Google Scholar]
- 13.Candoni A, De Marchi F, Zanini F. Predictive value of pre transplantation molecular minimal residual disease assessment by WT1 gene expression in FLT3-positive acute myeloid leukemia. Exp Hematol 2017. February 1 [Epubahead of print] [DOI] [PubMed] [Google Scholar]
- 14.Pozzi S, Geroldi S, Tedone E, et al. Leukaemia relapse after allogeneic transplants for acute myeloid leukaemia: predictive role of WT1 expression. Br J Haematol. 2013; 160(4):503–509. [DOI] [PubMed] [Google Scholar]
- 15.Di Grazia C, Pozzi S, Geroldi S, Grasso R, Miglino M, Colombo N, et al. Wilms tumor 1 expression and pre-emptive immunotherapy in patients with acute myeloid leukemia undergoing an allogeneic hemopoietic stem cell transplantation. Biol Blood Marrow Transplant 2016; 22(7):1242–1246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.