Abstract
In practice, clinicians generally consider anemia (circulating hemoglobin concentration < 120 g.l−1 in non-pregnant females and < 130 g.l−1 in males) as due to impaired hemoglobin synthesis or increased erythrocyte loss or destruction. Rarely is a rise in plasma volume relative to circulating total hemoglobin mass considered as a cause. But does this matter? We explored this issue in patients, measuring hemoglobin concentration, total hemoglobin mass (optimized carbon monoxide rebreathing method) and thereby calculating plasma volume in healthy volunteers, surgical patients, and those with inflammatory bowel disease, chronic liver disease or heart failure. We studied 109 participants. Hemoglobin mass correlated well with its concentration in the healthy, surgical and inflammatory bowel disease groups (r=0.687–0.871, P<0.001). However, they were poorly related in liver disease (r=0.410, P=0.11) and heart failure patients (r=0.312, P=0.16). Here, hemoglobin mass explained little of the variance in its concentration (adjusted R2=0.109 and 0.052; P=0.11 and 0.16), whilst plasma volume did (R2 change 0.724 and 0.805 in heart and liver disease respectively, P<0.0001). Exemplar patients with identical (normal or raised) total hemoglobin masses were diagnosed as profoundly anemic (or not) depending on differences in plasma volume that had not been measured or even considered as a cause. The traditional inference that anemia generally reflects hemoglobin deficiency may be misleading, potentially resulting in inappropriate tests and therapeutic interventions to address ‘hemoglobin deficiency’ not ‘plasma volume excess’. Measurement of total hemoglobin mass and plasma volume is now simple, cheap and safe, and its more routine use is advocated.
Introduction
Anemia is defined as a reduction in the circulating concentration of hemoglobin ([Hb]) to < 120 g.l−1 in non-pregnant females and < 130 g.l−1 in males.1
Such reductions can result from the destruction or loss of erythrocytes or a failure of their production, or from impaired hemoglobin synthesis. Given its diverse etiology, anemia is common, affecting over 1.6 billion people worldwide,2 and is associated with impaired functional capacity, reduced quality of life,3 and poorer outcome in diverse disease states.4–9
For these reasons, circulating hemoglobin concentrations are routinely measured in clinical practice. Once anemia is identified, the cause of impaired hemoglobin synthesis or erythrocytosis, or of increased red cell loss or destruction, is often sought and treatment (either of the underlying cause, or through the administration of packed donor red cells) initiated.
However, it is now becoming clear that this approach may be somewhat simplistic. The concentration of circulating hemoglobin will depend not just on the total circulating quantity of hemoglobin (total hemoglobin mass, (tHb-mass), but also on the volume of plasma (plasma volume, PV) in which it is suspended. In clinical practice these factors are not routinely considered separately, or measured, and even in experienced hands their estimation is not trivial. However, such assessment may be important if the drivers of an altered [Hb], and the appropriate therapeutic response, are to be truly understood. Thus, the circulating hemoglobin concentration in athletes matches that in sedentary individuals, meaning that a contribution of increased red cell oxygen carriage to elite performance was largely dismissed. Then, in 2001, Heinicke and colleagues demonstrated that tHb-mass was increased by some 35% in elite endurance athletes, [Hb] matching that in the untrained only because PV was expanded to a similar degree.10
Likewise, alterations in the relationship between plasma volume and tHb-mass might strongly influence [Hb] in disease states. Anemia is common in diseases such as cancer,11 inflammatory bowel disease (IBD),12 chronic heart failure (CHF),8 chronic kidney disease (CKD)13 and chronic liver disease (CLD).14 Traditionally, this has been considered the result of a reduced tHb-mass. But a low [Hb] might also be found when hemoglobin synthesis, erythrocytosis and tHb-mass are all entirely normal (or even high), if PV is disproportionately expanded. This can occur through disease-related changes in global water balance or distribution of body water. Thus, patients with IBD might face enteric blood loss, suppressed hemoglobin synthesis or anemia of chronic inflammation; CLD patients may likewise lose blood, have an expanded circulating PV due to hyperaldosteronism, or face fluid shifts as a result of raised portal venous pressure or hypoalbuminemia; and patients with chronic heart failure may suffer an increase in circulating volume due to factors including increased renin-angiotensin-aldosterone axis activity.15 In contrast, contractions in PV caused by pharmacotherapy may mask a fall in tHb-mass by maintaining [Hb].16
The extent to which this is true has not been addressed, largely due to historical methodological limitations: circulating red cell volume (RCV, ml) or PV has generally been determined through radiolabeling of red blood cells (RBCs) or albumin, respectively.17 Such techniques are costly and time consuming, and not without risk, and are thus not routinely deployed unless in special circumstances (this method is recommended for disease diagnosis by the Polycythemia Vera Study Group).18
Such barriers may be overcome through the use of an ‘optimized carbon monoxide (CO) rebreathing’ (oCOR) method:19 inhaled CO binds avidly to circulating hemoglobin, and the concentration of the resultant carboxyhemoglobin (COHb) complex can be readily measured. Knowing the quantity of absorbed CO, tHb-mass can be measured and, knowing [Hb], PV calculated. The method is cheap, simple and safe to use, but has only rarely been applied in the clinical setting.
Thus, the relative contributions of PV and tHb-mass to measured [Hb] across disease states have not been described. Herein we sought to address this issue.
Methods
This was a prospective, observational clinical study. We studied five groups: healthy volunteers (HV), preoperative patients awaiting major surgery and those suffering IBD, as well as patients in whom alterations in PV might more commonly occur (those with CLD or CHF). Each participant was studied on 1 occasion between February 2015 and May 2016.
Optimized Carbon Monoxide Rebreathing Method (oCOR)
The use of CO to determine tHb-mass was first proposed in the late 1800s, with refined techniques being published 100 years later.20 In 2005, Schmidt and Prommer reported a simpler and faster technique (described in detail below) which also required less blood sampling.19 It was applied almost exclusively, however, in the fields of athletic physiology, and thus failed to come to the attention of the bulk of the broader clinical/medical community.
tHb-mass was determined using the validated oCOR method described in detail by Schmidt and Prommer.19 In brief, COHb concentration in blood was measured before and after 2 min rebreathing a known CO volume (0.5 to 1.0 ml.kg−1 in this study depending on sex). Each participant was seated for 15 min to allow stabilization of PV, after which a mouthpiece connected them via a container of ‘soda lime’~10g (carbon dioxide scrubber) to a spirometer (Spico-CO Respirations-Applikator, Blood Tec, Germany) and a 3 liter anesthetic bag pre-filled with 100% oxygen. The patient exhaled to residual volume, breathed in the CO dose via the spirometer, held their breath for 10s, then continued normal breathing into the closed circuit via the spirometer for 1 min 50s. The participant then exhaled to residual volume, this exhaled volume being collected and analyzed to quantify the CO not absorbed into the bloodstream. Disconnected from the mouthpiece, participants finally fully exhaled to residual volume into a CO gas analyzer (Dräger Pac 7000, Drägerwerk AG & Co. KGaA, Germany) before and at 4 min after CO rebreathing, in order to determine the CO concentration exhaled after disconnecting the patient from the spirometer, that will also have not been absorbed into the blood.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics (Version 23.0 for Apple Macintosh, Chicago, IL, USA). Values are presented as mean ± standard deviation (SD), unless otherwise stated. Median and interquartile range (IQR) are reported when variables were not normally distributed. Categorical variables are presented as frequency (%). Pearson’s correlation coefficient assessed the relationship between [Hb] and tHb-mass, allowing adjustment for PV (ml). Linear regression assessed the proportion of variance in tHb-mass explained by [Hb], allowing adjustment for PV (ml). In both correlation and regression analyses [Hb] and tHb-mass are expressed in g.l−1 and grams, respectively, and PV in mls. This is also the case elsewhere, unless otherwise stated.
Differences across sub-groups were assessed by one-way analysis of variance (ANOVA), prior to which the assumption of normality was tested by the Levene’s test for homogeneity of variances. Where homogeneity of variance was verified, being the case for [Hb], hematocrit (Hct), tHb-mass (g), PV (%) and weight, a one-way ANOVA was performed, with post hoc comparisons by Gabriel’s test due to the slightly different sample sizes across subgroups. When homogeneity of variance was violated, as was the case for PV (ml & ml.kg−1), age and tHb-mass (g.kg−1), a Welch ANOVA21 was used with post hoc comparisons made by the Games-Howell test, as this does not rely on the assumption of equal variances.22
All tests were two-sided with statistical significance being accepted as a P-value of <0.05.
Power calculation
The power calculation was based on the study by Hinrichs et al.23 and was performed using G*3 Power version 3.1.9.2.24 According to Hinrichs and colleagues, the relationship (expressed as Pearson’s correlation coefficient) between [Hb] and tHb-mass was r=0.59, P<0.05. Based on this, using a two-tailed correlation: bivariate normal model, we calculated that 21 patients would provide 80% power at the 5% significance level to detect a correlation of at least r=0.59 between [Hb] and tHb-mass. Given that 5 groups were studied, a total of 105 participants were required.
Ethical approval
Ethical approval was granted by the London - Camden and Kings Cross Research Ethics Committee (REC reference: 13/LO/1902). Written informed consent was obtained from all participants.
Results
One hundred and nine participants (61% male: mean (IQR) age 52 (36–64) years) consented to take part in the study. The Online Supplementary Figure S1 shows a Consolidated Standards of Reporting Trials (CONSORT) flow diagram indicating included and excluded patients and sub-groups. Sixteen patients were tested at Southampton General Hospital, 90 at University College London Hospital (UCLH; including HV) and 3 at the Royal Free Hospital (RFH). Surgical specialties are shown in the Online Supplementary Table S1, with patient characteristics and etiology of disease for IBD patients in the Online Supplementary Table S2. Patient characteristics, medications and etiology of CLD and CHF are shown in the Online Supplementary Table S3 and Online Supplementary Table S4, respectively. There were no differences in weight between sub-groups. HV (n=21) were younger compared to all patient groups (P<0.0001) with CHF patients (n=22) being older than IBD (n=21, P=0.001), surgical (n=28, P=0.008) and CLD patients (n=16, P=0.002). Figure 1A–D shows hematological variables and Figure 2A,B displays PVs across different sub-groups.
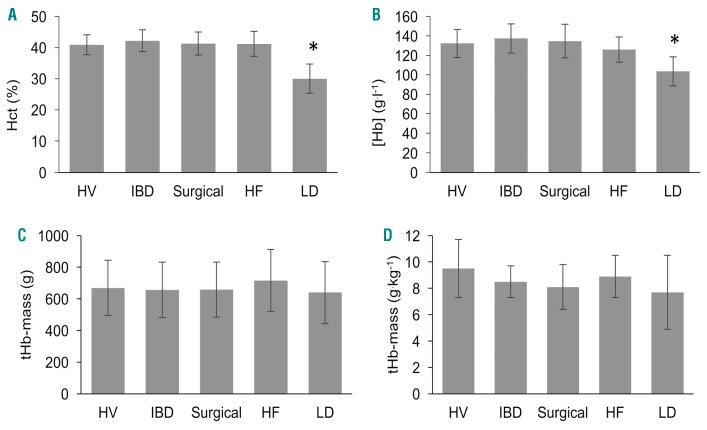
Figure 1.
Hematological variables in healthy volunteers and patient sub-groups. Hct (%), hematocrit percentage (A); [Hb], hemoglobin concentration (B); tHb-mass, total hemoglobin mass (g & g.kg−1) (C & D). *P<0.0001 for Hct and [Hb] in LD patients compared to all other groups. No differences in tHb-mass (g or g.kg−1) between groups. Data expressed as mean (± standard deviation error bars). HV: healthy volunteers; IBD: inflammatory bowel disease; HF: heart failure; LD: liver disease.
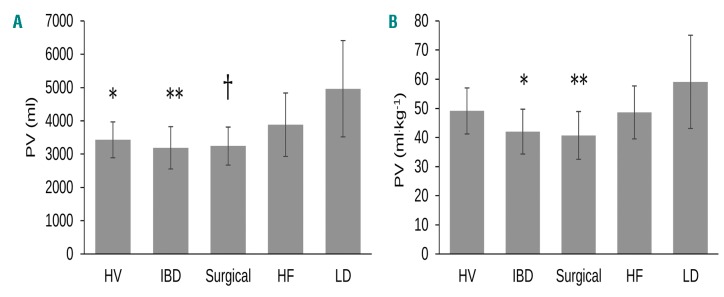
Figure 2.
Plasma volume in healthy volunteers and patient sub-groups. Plasma volume (ml & ml.kg−1) (A & B). (A) PV (ml) between LD and HV, *P=0.006, PV (ml) between LD and IBD, **P=0.002 and PV (ml) between LD and surgical patients, †P=0.003; (B) PV (ml kg−1) between LD and IBD, *P=0.008, PV between LD and surgical patients, **P=0.004. Data expressed as mean (± standard deviation error bars). HV: healthy volunteers; IBD: inflammatory bowel disease; HF: heart failure; LD: liver disease; PV: plasma volume.
Hemoglobin concentration
Hemoglobin concentration was 128.4 ± 18.1 g.l−1 in the subjects overall. Across sub-groups, CLD patients had lower [Hb] when compared to HV and other disease groups (P<0.0001, Figure 1B). Anemia prevalence (n=34 [39%]) varied across disease sub-groups (CLD 15 [94%], CHF 12 [55%], IBD 2 [9%], surgical 5 [18%]). Amongst HV, 5 (24%, all female) were anemic.
Total hemoglobin mass
Mean ± SD tHb-mass was 669 ± 181 grams (8.5 ± 1.9 g.kg−1 body mass) in subjects overall, with no statistically significant differences across sub-groups (Figure 1C,D). tHb-mass was higher in males than females: (758 ± 152 vs. 533 ± 132 g, P<0.0001; and 8.9 ± 1.8 vs. 7.9 ± 2.0 g.kg−1, P=0.006, respectively).
Plasma volume
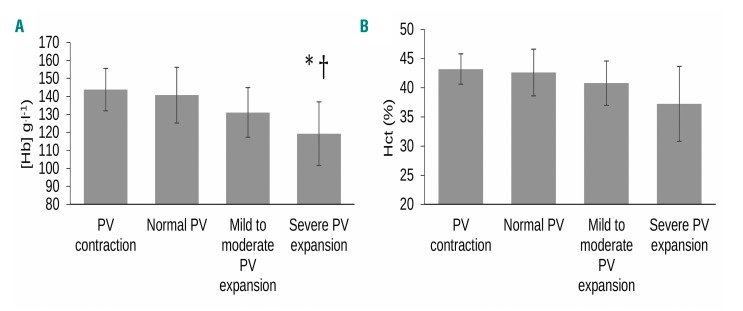
Mean ± SD PV was 3667 ± 1020 ml (47.1 ± 11.3 ml.kg−1) in subjects overall. PV was higher in males than females (4040 ± 1038 vs. 3093 ± 672 ml, respectively, P<0.0001), but not when weight adjusted (47.6 ± 11.3 vs. 46.2 ± 11.5 ml.kg−1, P=0.556). It also differed across disease groups, being expanded and more varied in CLD (4965 ± 1447 ml) compared to HV (3429 ± 538 ml, P=0.006), IBD (3202 ± 653 ml, P=0.002) and surgical patients (3297 ± 590 ml, P=0.003), but not CHF (3883 ± 953 ml, P=0.100), see Figure 2A. Adjusted for body weight, PV was similarly expanded in CLD (59.1 ± 16.0 ml.kg−1) when compared to IBD (42.5 ± 8.3 ml.kg−1, P=0.008) and surgical patients (41.3 ± 7.8 ml.kg−1, P=0.004), but again was similar to that in CHF patients (48.6 ± 9.2 ml.kg−1, P=0.172) or HV (49.1 ± 7.9 ml.kgl−1, P=0.191), see Figure 2B. Hemoglobin concentration was influenced by the degree of PV expansion (Figure 3A), being lower in patients with severe PV expansion (n= 46, 119.0 ± 17.7 g.l−1) than mild to moderate PV expansion (n=36, 131.1 ± 13.8 g.l−1, P=0.005), and normal PV (n=24, 140.7 ± 15.5 g.l−1, P<0.0001), but not PV contraction (n=3, 143.8 ± 11.8 g.l−1, P=0.139).
Figure 3.
Hemoglobin concentration and hematocrit in all patients categorized by plasma volume status. Plasma volume contraction (n=3) was classified as > minus 8% from expected norms. Normal PV (n=24) was classified as derived PV within ± 8% of the expected normal volume on an individual level. Mild to moderate volume expansion (n=36) was considered >8% to <25% deviation from expected norms, and severe PV expansion (n= 46) as >25% of the expected normal volume. *P=0.005 for [Hb] in severe PV expansion vs. mild to moderate, †P<0.0001 for [Hb] in severe PV expansion vs. normal PV. Data expressed as mean (± standard deviation error bars). PV: plasma volume; [Hb]: hemoglobin concentration (g.l−1); Hct (%): hematocrit percentage.
Relationships between hemoglobin concentration and total hemoglobin mass
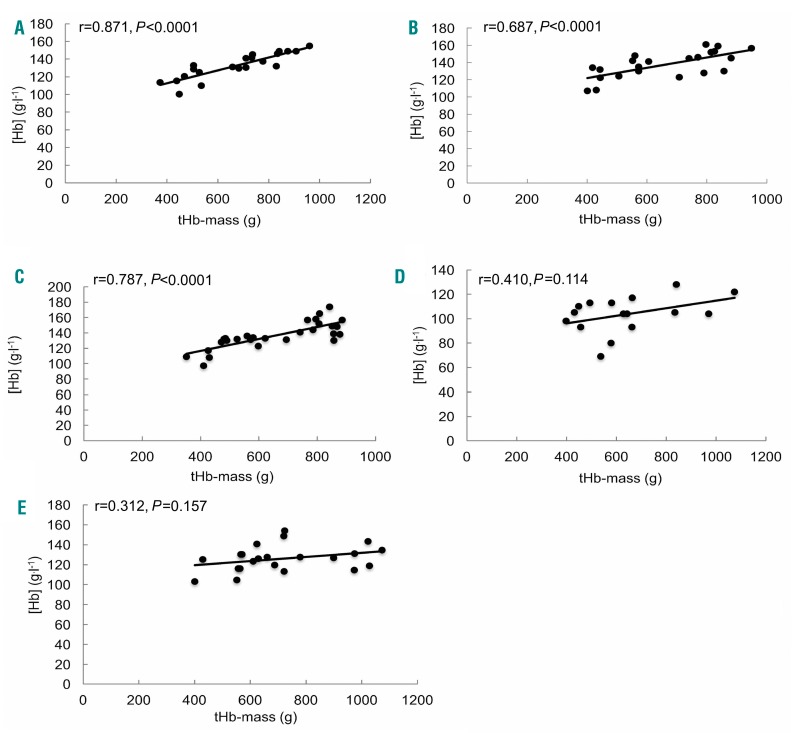
In the study cohort as a whole, [Hb] (g.l−1) correlated with tHb-mass (g) (r=0.500, P<0.0001, n=109), this being true in both males (r=0.452, P<0.0001) and females (r=0.462, P<0.0001). Whilst true in HV, IBD and surgical patients (r=0.871, P<0.0001; r=0.687, P<0.0001; and r=0.763, P<0.0001, respectively; Figure 4A–E) this was not the case in patients with CLD or CHF (r=0.410, P=0.114 and r=0.312, P=0.157, respectively).
Figure 4.
Unadjusted relationship between hemoglobin concentration and total hemoglobin mass. Healthy controls (A, n=21), patients with IBD (B, n=22), surgical patients (C, n=28), liver disease (D, n=16) and HF (E, n=22). tHb-mass (g): total hemoglobin mass; [Hb] (g.l−1): hemoglobin concentration.
Whilst consistently statistically significant, the strength of the relationship between [Hb] and tHb-mass weakened as PV rose, with r-values falling from 0.94 to 0.91, 0.86 and 0.57 for those with low (n=3), normal (n=24), mild-moderately expanded (n=36) and severely expanded (n=46) PV, respectively, (P=0.216 for the 3 with low PV, and P<0.0001 for the others). Sub-group analysis is hampered by small numbers, but data are presented in the Online Supplementary Table S5 for completeness.
Total hemoglobin mass, plasma volume and hemoglobin concentration on an individual level
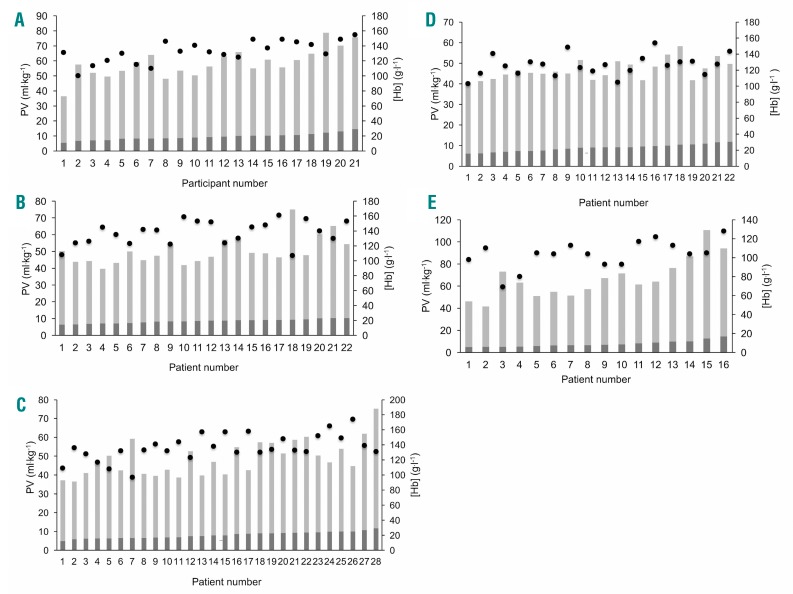
The data presented thus suggest that, at an individual level, [Hb] was not a good guide to tHb-mass, due to its being strongly influenced by PV. This is illustrated in Figure 5A–E, which shows data from individual participants ranked by weight-adjusted tHb-mass from smallest to largest with corresponding PV (ml.kg−1) and [Hb] (g.l−1) in HV (A), IBD (B), surgical (C), CLD (D) and CHF (E) patients, respectively. These show that patients who share a very similar tHb-mass may exhibit markedly different [Hb] due to differences in PV. For example, in patients with IBD (Figure 5B), patient numbers 17 and 18 have a very similar tHb-mass (9.2 g.kg−1 and 9.3 g.kg−1, respectively), yet 1 is defined as having a high normal [Hb] (161 g.l−1) and the other as being anemic ([Hb] 107 g.l−1), due to substantial differences in PV (37.2 ml.kg−1 vs. 65.7 ml.kg−1). Similarly, in CLD patients (Figure 5E), tHb-mass in patient numbers 2 and 3 are the same (5.2 g.kg−1) but the first is considered to be mildly anemic ([Hb] 110 g.l−1) while the second is deemed markedly anemic ([Hb] 69 g.l−1) due to a relatively raised PV in the latter (36.5 vs. 67.8 ml.kg−1, respectively).
Figure 5.
Individual participant data for total hemoglobin mass, plasma volume and hemoglobin concentration. Participants ranked by tHb-mass (g.kg−1) (dark gray bars) from smallest to largest with corresponding PV (ml.kg−1) (light gray bars) and [Hb] (g.l−1) (black circles) in healthy volunteers (A), inflammatory bowel disease (B), surgical (C), chronic heart failure (D), and chronic liver disease (E). PV: plasma volume; [Hb]: hemoglobin concentration.
Linear regression models
In the whole population, tHb-mass explained 25% of the variance in [Hb] (adjusted R2=0.250, P<0.0001). However, tHb-mass explained different amounts of the variance in [Hb] across patient groups, adjusted R2 for HV, surgical and IBD patients being 0.746, 0.565 and 0.446, respectively, (P<0.0001 in all cases). Of particular note, tHb-mass did not significantly explain variance in [Hb] in the 2 patient groups most likely to suffer expanded PV and shifts in fluid, CLD (adjusted R2=0.109, P=0.114) or CHF patients (adjusted R2=0.052, P=0.157). In keeping, PV independently accounted for a greater proportion of the variance in [Hb] in these groups (R2 change 0.724 in CHF and 0.805 in CLD) than in HV (0.192), surgical patients (0.374) or IBD patients (0.479; P<0.0001 in all cases).
Hematological variables by anemia status
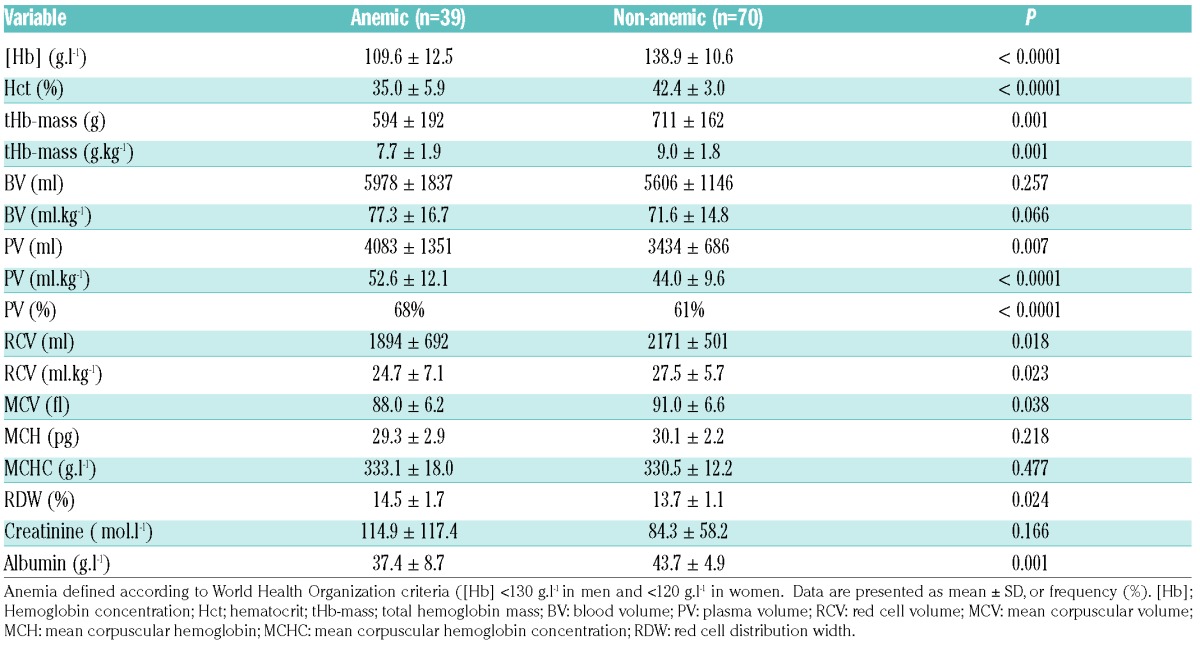
In the 39 anemic subjects (mean ± SD [Hb] 109.6 ± 12.5 g.l−1), when compared to the 70 non-anemic participants ([Hb] 138.9 ± 10.6 g.l−1), tHb-mass (g & g.kg−1) was significantly lower (594 ± 192 vs. 711 ± 162 g, P=0.001; 7.7 ± 1.9 vs. 9.0 ± 1.8 g.kg−1, P=0.001). However, PV (ml, and ml.kg−1) was also significantly higher in anemic subjects [(4083 ± 1351 vs. 3434 ± 686 ml, P=0.007; 52.6 ± 12.2 vs. 44.0 ± 9.6 ml.kg−1, P<0.0001 (Table 1)].
Table 1.
Hematological variables in anemic and non-anemic participants.
Discussion
We have studied the contribution of tHb-mass and PV to differences in the concentration of circulating hemoglobin. We have done so in HV and across a variety of disease states, selected to be more or less likely influenced by intravascular fluid status. By doing so, we have shown that: (i) variation in PV contributes significantly to variation in hemoglobin concentration, (ii) this contribution is greater in cases of CLD or heart failure, in other words diseases in which changes in total body water and in its distribution are more likely to occur, and (iii) perhaps most importantly, this may lead to some individuals being diagnosed as anemic whilst having a tHb-mass which is normal or even elevated. These findings are of direct clinical relevance. A basic medical education teaches that PV may be expanded in some disease states. But it is rarely, if ever, mentioned that this might lead to hemodilution of such a degree as to cause anemia. Thus, a diagnosis of anemia generally leads to investigation of a cause for reduced hemoglobin synthesis or increased erythrocyte destruction or loss. When such features are not identified, a diagnosis of ‘anemia of chronic disease’ is likely made. Rarely, if ever, is hemodilution considered as a cause, and measurement of tHb-mass or plasma volume performed. This is exemplified by the fact that no such measurements had been performed electively in the patients we studied. Such deficits in consideration or action may in part relate to the difficulty and expense of measuring such variables through traditional methods (radiolabeling of RBCs, for instance).
Thus, tHb-mass was generally strongly related to [Hb] (r≥0.687 and P<0.0001 in all cases); tHb-mass explained a good deal of the variance in [Hb] (adjusted R2 in HV, surgical and IBD patients being 0.746, 0.565 and 0.446, respectively, P<0.0001 in all cases); and PV independently accounted for only a small proportion of the variance in [Hb] over that due to tHb-mass (R2 change 0.192, 0.374 or 0.479 in HV, surgical and IBD patients, respectively). By contrast, in the 2 patient groups most likely to suffer expanded PV (CLD and CHF), tHb-mass did not correlate with [Hb] (r=0.410, P=0.114; r=0.312, P=0.157, respectively). Likewise, tHb-mass did not significantly explain variance in [Hb] (adjusted R2=0.109, P=0.114; adjusted R2=0.052, P=0.157, respectively), whilst PV independently accounted for a greater proportion of the variance in [Hb] over and above tHb-mass in these groups (R2 change 0.724 in CHF and 0.805 in CLD). Thus, [Hb] is strongly influenced by disease-related changes in PV. The relationship between [Hb] and tHb-mass weakened as PV rose (r-values falling from 0.94 and 0.91 in those with low or normal PV, to 0.57 amongst those in whom PV was severely expanded).
As a consequence, even amongst those in our small sample, we identified patients with identical and normal tHb-mass, in some of whom severe anemia would be diagnosed, likely triggering investigations focused upon failed erythrogenesis or increased red cell destruction. As specific exemplars of the phenomenon, 2 IBD patients had similar tHb-masses (9.2 and 9.3 g.kg−1), 1 having a high normal [Hb] (161 g.l−1) and the other being anemic ([Hb] 107 g.l−1), due to substantial differences in PV (37.2 ml.kg-1 vs. 65.7 ml.kg−1). Similarly, 2 CLD patients had the same tHb-mass (5.2 g.kg−1), 1 having mild anemia [Hb] (110 g.l−1), but the second being markedly anemic ([Hb] 69 g.l−1) due to a relatively raised PV in the latter (36.5 vs. 67.8 ml.kg−1, respectively).
Our findings are thus of real clinical importance, as a significantly low [Hb] can trigger a raft of (unwarranted) investigations (such as the assay of circulating hematinic factors) or treatments (such as the administration of packed RBCs), whilst denying the administration of agents to reduce PV, which might sometimes be required. Blood transfusion itself carries risks25 as well as a price in terms of healthcare costs. Meanwhile, in other circumstances, contraction in PV might offer false reassurance by maintaining [Hb] when tHb-mass is low.
Whilst tHb-mass is generally used as an index of oxygen carrying capacity and of circulating red cell mass, others have previously reported total circulating red blood cell volume (RCV) in this regard. In hematologically normal control subjects, Hct in the 20–50% range reportedly correlated well with RCV (determined by 51Chromuim (51Cr) labeling of RBCs: r=0.880, P<0.001).26 However, this relationship was disturbed when Hct fell outside this range, owing to wider variability in PV. Such data support those from other radiolabeling studies in suggesting that direct measurement of RCV (rather than the use of [Hb] or Hct) is required for the accurate diagnosis of polycythemia.27 Likewise, data derived from the same technique which we applied (oCOR) show PV to be expanded (variably, but along with increased RCV) in polycythemia rubra vera.28
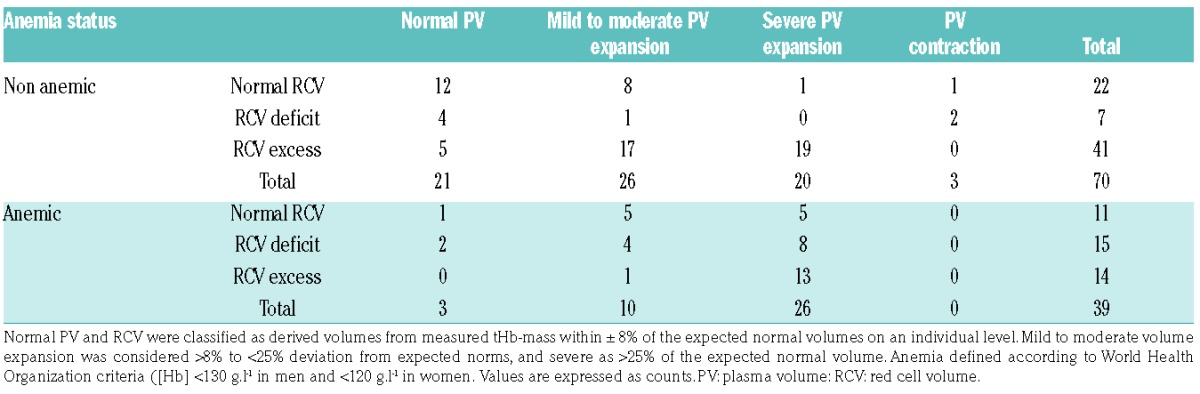
The focus of such studies differed from ours: namely, they sought to address the degree to which variation in PV altered the accuracy of the diagnosis of polycythemia, whilst we assessed the influence of variation in tHb-mass and PV on [Hb] per se and on the diagnosis of anemia. Nor have any studies in this field been comprehensive across disease states, or assessed tHb-mass (rather than RCV). Nonetheless RCV (by 51Cr labeling) has been shown to be similar in anemic and non-anemic CHF patients, suggesting that PV expansion accounted for this diagnosis.15 Indeed, this has been shown to occur (using I131-labeled albumin) in CHF due to systolic dysfunction, with poor correlation between [Hb] and RCV.29 Likewise, and using the same technique, Miller showed 19 of 32 patients hospitalized with decompensated CHF to be anemic, with only 4 of these having a true reduction in RCV.30 Using the oCOR method, we extend such observations (Table 2). Overall, 39 of the 109 participants were anemic, in only 2 (13.3%) of whom was this due to a reduced tHb-mass [352 g and 449 g] in the context of a normal PV. In the remaining 86.7%, reduced [Hb] was accounted for by PV expansion (n=4 mild to moderate, tHb-mass 494 ± 76 g, and n=8 severe, tHb-mass 539 ± 105 g). Interestingly, 14 of the 39 anemic patients (93%) had a relatively raised tHb-mass, with PV elevated to a greater degree (n=1 mild to moderate PV expansion (tHb-mass 610 g), n=13 severe PV expansion (tHb-mass 758 ± 220 g).
Table 2.
Anemia classification based on hemoglobin concentration, red cell volume and plasma volume in all patients (n=109).
The overall prevalence of anemia for our study participants (36%) is similar to that reported in previous studies in non-cardiac surgical patients (30.4%).9 Nine percent of IBD patients suffered anemia, which is less than has been reported across European Countries (24%).12 Some 54% of CHF patients suffered anemia in the study herein, somewhat more than has been previously reported in the Study of Anemia in a Heart Failure Population (STAMINA-HFP) Registry (34%),31 or in patients with advanced HF (30%),32 but in keeping with the data of others (55.6%33 to 61%).34 Of the CLD patients, 94% suffered anemia, a figure somewhat higher that that previously reported by some (50–75%),35,36 but in keeping with data in decompensated CLD (86%)37 or hepatitis C infection (75%).38
Amongst HV, 5 (24% of healthy volunteers) were anemic, a figure in keeping with global data relating to non-pregnant females (30%),2 and only slightly higher than the 16% reported in non-pregnant women aged 15–49 years from high income regions and 22% in menstruating women from central and eastern Europe.39 This may be related to volunteering bias in the current study whereby those who thought they might be anemic preferentially applied to participate.
The study herein utilized the optimized oCOR method, validated against 51Cr radiolabeling methodologies.40 An advantage of our study was its assessment of diverse patient groups. Sample sizes, whilst small, were appropriately powered to explore the relationship between tHb-mass and [Hb], albeit that, for administrative reasons, the sample size for patients with CLD (n=16) was below the 21 we originally sought, based on the study by Hinrichs and colleagues.23 Nonetheless, we were still able to demonstrate that PV changes in this group do indeed influence assessed [Hb] and anemia diagnoses.
Differing blood sample methods (capillary and venous) yield identical Δ%COHb (and thus tHb-mass) values.41 However, capillary [Hb] can be marginally higher than that in venous blood.42–45 The use of differing sampling techniques across testing sites may thus have contributed a little to variation in measured [Hb], although capillary blood [Hb] values were all corrected to venous conditions, and all blood samples were collected from the same anatomical site, and with patients in the same posture (seated). This factor does not therefore weaken the significance of our findings. Whilst the use of different blood gas machines and testing staff may have introduced error in the measurement of tHb-mass, we found a typical error (TE) of repeat tHb-mass measurements of 1.93% (95% confidence interval (CI) 1.3–3.4%: data not shown), values in keeping with other institutions using the oCOR method.19,46
Finally, the oCOR method is quick and simple, avoids the technical difficulties of working with radiolabeled compounds,40 offers an inconsequential burden for patients, is minimally invasive, and is safe even in patients with serious medical conditions and comorbidities such as stable coronary artery disease.47 This greatly widens the applicability of the oCOR test to measure tHb-mass and plasma volume in the clinical setting. To date, its clinical experimental application has been sparse, e.g., to demonstrate that low tHb-mass may account for impaired exertional performance in otherwise healthy diabetics.48 Whilst (rarely as of yet) applied to the measurement of red cell mass in patients with polycythemia rubra vera,28 it has yet to be utilized in routine clinical practice but might find great value, for example in the estimation of red cell mass and PV in cases of presumed excessive erythrocytosis. Our data might support wider use.
In conclusion, measured [Hb], and the diagnosis of anemia, can be strongly influenced by (or can largely depend upon) changes in PV. The scale of this impact may be greater in some diseases than others. Constraining investigation of anemia to the identification of causes of reduced Hb synthesis or of erythrocyte loss or destruction may be inappropriate for many. The concept of ‘anemia’ may thus need refining in clinical practice, and the oCOR method may support better and more appropriate assessments of the factors influencing circulating [Hb].
Supplementary Material
Acknowledgments
The authors would like to thank Siemens for supplying the RAPIDPoint 500 blood gas machine and associated consumables used during this study at the Southampton General Hospital. In addition, we would like to thank the NIHR/Wellcome Clinical Research Facility for allowing patients to be tested in this facility at University College London Hospital. Finally, we would like to thank Dr Nadine Wachsmuth for her assistance in training JO and JOMP in the optimized carbon monoxide rebreathing method at the Department of Sports Medicine/Sports Physiology, University of Bayreuth, Germany.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/9/1477
Funding
HM is funded in part by the NIHR University College London Hospitals Biomedical Research Centre, to whom we express our thanks. MPWG is funded in part by the NIHR Southampton Respiratory Biomedical Research Unit.
References
- 1.World Health Organisation. Haemoglobin concentration for the diagnosis of anaemia and assessment of severity 2011. http://www.who.int/vmnis/indicators/haemoglobin/en/ Accessed 28th September 2016.
- 2.World Health Organization. Worldwide prevalence of anaemia 1993–2005. 2008. http://apps.who.int/iris/bitstream/10665/43894/1/9789241596657_eng.pdf Accessed 5th October 2016.
- 3.Farag YM, Keithi-Reddy SR, Mittal BV, et al. Anemia, inflammation and health-related quality of life in chronic kidney disease patients. Clin Nephrol. 2011;75(6):524–533. [DOI] [PubMed] [Google Scholar]
- 4.Chambellan A, Chailleux E, Similowski T, Group AO. Prognostic value of the hematocrit in patients with severe COPD receiving long-term oxygen therapy. Chest. 2005; 128(3):1201–1208. [DOI] [PubMed] [Google Scholar]
- 5.Lipsic E, Asselbergs FW, van der Meer P, et al. Anaemia predicts cardiovascular events in patients with stable coronary artery disease. Neth Heart J. 2005;13(7–8):254–258. [PMC free article] [PubMed] [Google Scholar]
- 6.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003; 107(2):223–225. [DOI] [PubMed] [Google Scholar]
- 7.Caro JJ, Salas M, Ward A, Goss G. Anemia as an independent prognostic factor for survival in patients with cancer: a systemic, quantitative review. Cancer. 2001; 91(12):2214–2221. [PubMed] [Google Scholar]
- 8.Miceli A, Romeo F, Glauber M, de Siena PM, Caputo M, Angelini GD. Preoperative anemia increases mortality and postoperative morbidity after cardiac surgery. J Cardiothorac Surg. 2014;9:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011; 378(9800):1396–1407. [DOI] [PubMed] [Google Scholar]
- 10.Heinicke K, Wolfarth B, Winchenbach P, et al. Blood volume and hemoglobin mass in elite athletes of different disciplines. Int J Sports Med. 2001;22(7):504–512. [DOI] [PubMed] [Google Scholar]
- 11.Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:11S–26S. [DOI] [PubMed] [Google Scholar]
- 12.Filmann N, Rey J, Schneeweiss S, et al. Prevalence of anemia in inflammatory bowel diseases in european countries: a systematic review and individual patient data meta-analysis. Inflamm Bowel Dis. 2014;20(5):936–945. [DOI] [PubMed] [Google Scholar]
- 13.McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20(9):1501–1510. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15(37):4653–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adlbrecht C, Kommata S, Hulsmann M, et al. Chronic heart failure leads to an expanded plasma volume and pseudoanaemia, but does not lead to a reduction in the body’s red cell volume. Eur Heart J. 2008;29(19): 2343–2350. [DOI] [PubMed] [Google Scholar]
- 16.Feigenbaum MS, Welsch MA, Mitchell M, Vincent K, Braith RW, Pepine CJ. Contracted plasma and blood volume in chronic heart failure. J Am Coll Cardiol. 2000;35(1):51–55. [DOI] [PubMed] [Google Scholar]
- 17.The International Committee for Standardisation in Haematology (ICSH). Recommended methods for measurement of red-cell and plasma volume. J Nucl Med. 1980;21(8):793–800. [PubMed] [Google Scholar]
- 18.Pearson TC. Evaluation of diagnostic criteria in polycythemia vera. Semin Hematol. 2001;38(1 Suppl 2):21–24. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt W, Prommer N. The optimized CO-rebreathing method: a new tool to determine total haemoglobin mass routinely. Eur J Appl Physiol. 2005;95(5–6):486–495. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen JK. Blood and plasma volumes determined by carbon monoxide gas, 99mTc-labelled erythrocytes, 125I-albumin and the T 1824 technique. 1991;51(2):185–190. [DOI] [PubMed] [Google Scholar]
- 21.Welch BL. On the comparison of several mean values: an alternative approach. 1951;38(3/4):330–336. [Google Scholar]
- 22.Field A. Discovering statistics using SPSS. London: Sage Publications, 2005. [Google Scholar]
- 23.Hinrichs T, Franke J, Voss S, Bloch W, Schanzer W, Platen P. Total haemoglobin mass, iron status, and endurance capacity in elite field hockey players. J Strength Cond Res. 2010;24(3):629–638. [DOI] [PubMed] [Google Scholar]
- 24.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. [DOI] [PubMed] [Google Scholar]
- 25.Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011; 115(3):635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber H, Lewis SM, Szur L. The influence of anaemia, polycythaemia and splenomegaly on the relationship between venous haematocrit and red-cell volume. Br J Haematol. 1964; 10:567–575. [DOI] [PubMed] [Google Scholar]
- 27.Lorberboym M, Rahimi-Levene N, Lipszyc H, Kim CK. Analysis of red cell mass and plasma volume in patients with polycythemia. Arch Pathol Lab Med. 2005; 129(1):89–91. [DOI] [PubMed] [Google Scholar]
- 28.Ahlgrim C, Schumacher YO, Wrobel N, Waller CF, Pottgiesser T. Application of the optimized CO-rebreathing method for determination of hemoglobin mass in patients with polycythemia vera. Ann Hematol. 2014;93(7):1159–1165. [DOI] [PubMed] [Google Scholar]
- 29.Abramov D, Cohen RS, Katz SD, Mancini D, Maurer MS. Comparison of blood volume characteristics in anemic patients with low versus preserved left ventricular ejection fractions. Am J Cardiol. 2008;102(8):1069–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller WL, Mullan BP. Peripheral venous hemoglobin and red blood cell mass mismatch in volume overload systolic heart failure: implications for patient management. J Cardiovasc Transl Res. 2015; 8(7):404–410. [DOI] [PubMed] [Google Scholar]
- 31.Adams KF, Jr, Patterson JH, Oren RM, et al. Prospective assessment of the occurrence of anemia in patients with heart failure: results from the Study of Anemia in a Heart Failure Population (STAMINA-HFP) Registry. Am Heart J. 2009;157(5):926–932. [DOI] [PubMed] [Google Scholar]
- 32.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Borenstein J. Anemia is associated with worse symptoms, greater impairment in functional capacity and a significant increase in mortality in patients with advanced heart failure. J Am Coll Cardiol. 2002;39(11):1780–1786. [DOI] [PubMed] [Google Scholar]
- 33.Silverberg DS, Wexler D, Blum M, et al. The use of subcutaneous erythropoietin and intravenous iron for the treatment of the anemia of severe, resistant congestive heart failure improves cardiac and renal function and functional cardiac class, and markedly reduces hospitalizations. J Am Coll Cardiol. 2000;35(7):1737–1744. [DOI] [PubMed] [Google Scholar]
- 34.Androne AS, Katz SD, Lund L, et al. Hemodilution is common in patients with advanced heart failure. Circulation. 2003;107(2):226–229. [DOI] [PubMed] [Google Scholar]
- 35.Senzolo M, Burroughs AK. Haematological abnormalities in liver disease. In: Rodes J, Benhamou JP, Blei AT, Reichen J, Rizzetto M, eds. Textbook of Hepatology: From Basic Science to Clinical Practice. Oxford, UK: Blackwell Publishing Ltd; 2007. [Google Scholar]
- 36.Gonzalez-Casas R, Jones EA, Moreno-Otero R. Spectrum of anemia associated with chronic liver disease. World J Gastroenterol. 2009;15(37):4653–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar EH, Radhakrishnan A. Prevalence of anaemia in decompensated chronic liver disease. World J Med Sci. 2014;10(1):56–60. [Google Scholar]
- 38.McHutchison JG, Manns MP, Longo DL. Definition and management of anemia in patients infected with hepatitis C virus. Liver Int. 2006;26(4):389–398. [DOI] [PubMed] [Google Scholar]
- 39.Stevens GA, Finucane MM, De-Regil LM, et al. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1(1):e16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gore CJ, Hopkins WG, Burge CM. Errors of measurement for blood volume parameters: a meta-analysis. J Appl Physiol (1985). 2005;99(5):1745–1758. [DOI] [PubMed] [Google Scholar]
- 41.Garvican LA, Burge CM, Cox AJ, Clark SA, Martin DT, Gore CJ. Carbon monoxide uptake kinetics of arterial, venous and capillary blood during CO rebreathing. Exp Physiol. 2010;95(12):1156–1166. [DOI] [PubMed] [Google Scholar]
- 42.Patel A.J., Wesley R., Leitman S.F., Bryant B.J. Capillary versus venous haemoglobin determination in the assessment of healthy blood donors. Vox Sang. 2013;104(4):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shahshahani H.J., Meraat N., Mansouri F. Evaluation of the validity of a rapid method for measuring high and low haemoglobin levels in whole blood donors. Blood Transfus. 2013;11(3):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziemann M., Lizardo B., Geusendam G., Schlenke P. Reliability of capillary hemoglobin screening under routine conditions. Transfusion. 2011;51(12):2714–2719. [DOI] [PubMed] [Google Scholar]
- 45.Baart A.M., de Kort W.L., van den Hurk K., Pasker-de Jong P.C. Hemoglobin assessment: precision and practicability evaluated in the Netherlands-the HAPPEN study. Transfusion. 2016;56(8):1984–1993. [DOI] [PubMed] [Google Scholar]
- 46.Turner G, Richardson AJ, Maxwell NS, Pringle JS. Comparison of total haemoglobin mass measured with the optimized carbon monoxide rebreathing method across different Radiometer ABL-80 and OSM-3 hemoximeters. Physiol Meas. 2014;35(12): N41–9. [DOI] [PubMed] [Google Scholar]
- 47.Karlsen T., Leinan I.M., Aamot I.-L., Dalen H., Stoylen A. Safety of the CO-rebreathing method in patients with coronary artery disease. Med Sci Sports Exerc. 2016;48(1):33–38. [DOI] [PubMed] [Google Scholar]
- 48.Koponen AS, Peltonen JE, Paivinen MK, Aho JM, Hagglund HJ, Uusitalo AL, et al. Low total haemoglobin mass, blood volume and aerobic capacity in men with type 1 diabetes. Eur J Appl Physiol. 2013; 113(5):1181–1188. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.