Abstract
Flavonoids are a class of plant and fungus secondary metabolites that serve functional roles in protecting against UV-induced oxidative stress, mediating auxin signaling, and promoting microbial defense. Flavonoids are extremely abundant in nature where their potent antioxidant capacity and very low toxicity makes them highly attractive as potential therapeutic agents. In terms of clinical applications, neither the Food and Drug Administration (FDA) nor the European Food Safety Authority (EFSA) has approved any health claims or drugs related to the use of flavonoids for therapeutic purposes. Quercetin is a common flavonol that has been shown to have potent antioxidant, anti-inflammatory, and anti-fibrotic activities both in vitro and in vivo in various tissues. Recently, the application of quercetin as a therapeutic has been gaining attention in the ocular surface scientific community in the study of dry eye, keratoconus, inflammation, and neovascularization of the cornea. This review will discuss the latest findings and the use of quercetin for the treatment of dystrophies of the ocular surface.
Impact statement
The eye represents a small portion of the human body, accounting for one decimal fraction of the anterior body surface. The cornea is an avascular, transparent tissue that acts as a primary barrier against mechanical and infectious damaging agents, protecting the internal structures of the eye. Corneal survival and function are affected by a number of factors including but not limited to injury, trauma, infection, genetics, and environment. Corneal injury, or trauma, often leads to loss of corneal transparency and even blindness. The concept of “curing” corneal opacity has been discussed in published form for over 200 years. Currently, full corneal transplant is the only treatment option. There is a strong interest in developing natural therapeutic products that come with minimum side effects. A novel antioxidant flavonoid, quercetin, has been gaining traction as a potential therapeutic to prevent the injured cornea. This review discusses the potential of this antioxidant.
Keywords: Quercetin, flavonoids, cornea, ocular surface, wound healing, anti-inflammatory, anti-fibrotic
Introduction
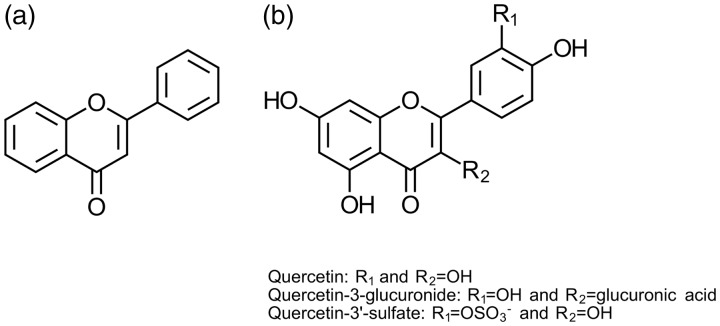
Flavonoids are abundant antioxidants found in nature in a variety of foods, such as green leafy vegetables, tea, berries, apples, and onions.1 The main structures of flavonoids are defined by a backbone of two fused phenyl rings and an oxygen-containing heterocyclic ring.2 Variations in the substituents of the rings give rise to the differences in flavonoid classes, as well as chemical reactivity and functionality (Figure 1(a)). Over six major classes of flavonoids have been defined based on substitution patterns of the aromatic rings with over 4000 individual flavonoid compounds identified.2,5
Figure 1.
(a) Backbone structure of commonly found flavonoids consisting of fused carbon rings and varying oxygen substituents. (b) Structure of quercetin and common metabolites found in circulation. Addition of a glucose-derived moiety at C-3 position gives rise to the quercetin-3-glucuronide form with a single hydroxyl group making up the aglycone form. Sulfation at the 3′- position represents the quercetin-3′-sulfate metabolite commonly found in plasma. Adapted from Harborne,2 Rossi et al.,3 and Cao et al.4
Numerous studies have shown protective effects of increased flavonoid uptake in animal models of disease6,7 and within the human population8–10 particularly in regards to cardiovascular and Alzheimer’s diseases. Despite studies suggesting positive effects of increased dietary intake of flavonoids, the potential health benefits of supplements are questioned by both the Food and Drug Administration (FDA) in the US, as well as the European Food Safety Authority (EFSA) in Europe due to the lack of a solid clinical trial demonstrating therapeutic benefits. Neither one of these organizations have approved any flavonoids as pharmaceutical drugs.
Quercetin is a member of the flavonol subclass that has received considerable attention by the scientific community in recent years (Figure 1(b)). Quercetin, like most flavonoids, is abundant in the human diet. Studies have measured an average daily consumption of quercetin of roughly 16–23 mg/day within human populations.11,12 Despite the broad marketing of quercetin as a beneficial supplement, the FDA and EFSA do not approve any clinical benefits to its consumption. However, quercetin is currently sold as a food supplement by all major pharmacy stores. Studies suggest that quercetin is rapidly cleared following oral consumption, undergoing extensive metabolism, and limiting systemic effects.13 Scientific studies, however, have been testing the benefits of quercetin with delivery to the target tissue using methods other than oral intake.14,15 In the treatment of pathologies affecting the ocular surface, the overwhelming delivery method is topical application via eye drops making it easily accessible and non-invasive for human use. This review will discuss the past and present studies that have investigated the bioactivity of quercetin in various ocular surface diseases and dystrophies.
Properties and bioavailability
Quercetin is biosynthesized in plants via the phenylpropanoid pathway. The complete pathway and biosynthesis details were summarized by Winkel-Shirley et al.16,17 in review articles. Quercetin, along with other flavonoids, function as potent antioxidants and serve to scavenge free radicals, bind transition metal ions, and inhibit lipid peroxidation.18,19 If not controlled, excessive reactive species can lead to protein oxidation and cell damage within the tissue or organ.
Quercetin’s oral bioavailability is still debatable with a number of contradictory studies as reviewed by Manach et al.20 The main debate is whether the quercetin aglycone or quercetin glucoside is driving the antioxidant characteristics of quercetin. Hollman et al.21 suggested that the absorption of the glucoside is better that the aglycone, due to the actions of the glucose transporter (SGLT-1). Quercetin glucosides are able to pass through the epithelial cell layer, but they have lower efficiency than the quercetin aglycone.22 In the same study, Murota and Terao22 suggested that quercetin absorption depends on the distribution of the sugar groups attached. Interestingly, studies suggest that quercetin aglycone or glucoside is not found in human plasma.23,24 On a study reported by Day et al.,23 the glucoside and aglycone form of quercetin were not present in human plasma following consumption of onions.23 However, two metabolites were found: quercetin 3′-sulfate and quercetin-3-glucuronide.23 Wittig et al.25, in a similar study only with fried onions tested, did not find quercetin aglycone or glucoside in the human plasma. Instead five different quercetin glucuronides were found.25 It has now been proposed that quercetin, as well as other flavonoids, do not need to be absorbed in order to have an effect. However, the degree to which quercetin can be absorbed following oral intake is still debatable. Overall, the effect of quercetin in vivo is still heavily questioned and more studies are necessary in order to identify and fully understand any potential benefits that quercetin might have to offer.
The ocular surface
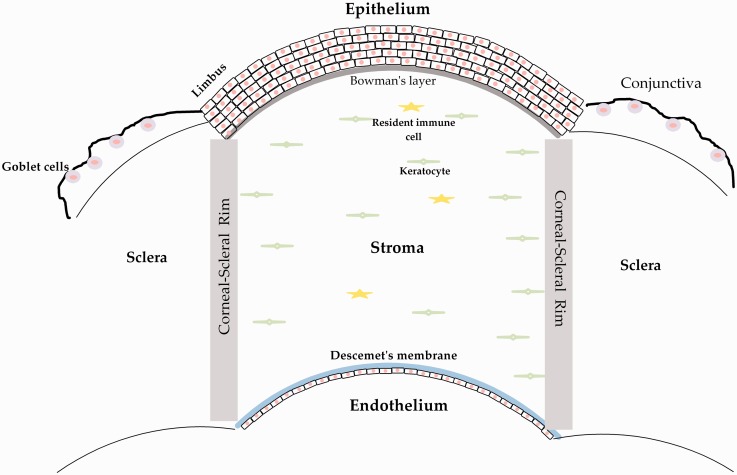
As the external portion of the eye, the ocular surface serves as a protective and functional barrier for the rest of the eye (Figure 2). Importantly, the cornea also plays a functional role in providing two-thirds of the refractive power of the eye, with the lens and retina providing the remaining one-third of the refractive power within the human eye. Furthermore, the conjunctiva is composed of goblet cells which function to secrete mucins providing lubrication for the anterior segment to reduce infection and barrier breakdown.28 The cornea is an avascular tissue with immune privilege which must be maintained in order for proper visual function. Ocular surface diseases that affect the structure or function of the cornea or conjunctiva can lead to corneal thinning, inflammation, neovascularization, and scarring. Excess reactive oxygen species (ROS) production due to mitochondrial dysfunction, UV-light, or hypoxia can promote neovascularization and inflammation leading to scarring and visual deficits (Figure 3). A number of pathologies affect the corneal surface and conjunctiva, including dry eye, allergies, microbial infection, keratoconus, chemical burns, Sjögren’s syndrome, and many more. Ocular surface diseases of the cornea or conjunctiva may result from prolonged inflammation or corneal defects leading to ocular irritation and reduced visual acuity. The common denominator of these diseases is that they can lead to partial or even complete vision loss, directly affecting quality of life. Unfortunately, to-date, we do not have a non-invasive treatment that can preserve corneal function and transparency. For cases that require surgical intervention, corneal transplantation remains the only solution.
Figure 2.
Cartoon representation of the anterior segment of the human eye highlighting the cornea, which is composed of five layers from anterior to posterior: (1) the stratified epithelium that secretes mucin and is continuously regenerated from stem cells found in the limbus, (2) the cell-free collagen matrix, Bowman’s layer, (3) the stroma, which makes up 90% of total corneal structure, and is composed of the collagen-secreting keratocyte and resident immune cells, (4) Descemet’s membrane, and (5) the single-layer endothelium which regulates fluid flux from the aqueous humor into the cornea. Based on ultrastructure of the cornea in earlier works.26,27
Figure 3.
Schematic of reactive-oxygen species (ROS)-mediated effects on cell function and signaling. UV-light, ischemia, hypoxia, and injury can promote ROS production leading to activation of VEGF-induced neovascularization, alterations in cellular metabolism favoring aerobic glycolysis, and canonical TGF-β signaling and myofibroblast differentiation. The ability of the cell to combat oxidative stress determines cell fate and cell differentiation. Within the ocular surface, particularly the avascular cornea, events that lead to neovascularization or inflammation can lead to severe and permanent tissue dysfunction
Given the problems and side effects a corneal transplant may have, scientists have been looking for an alternative for decades.29,30 Effective ocular drug delivery is dependent on many factors, including drug absorption, bioavailability, and retention on the anterior surface.31 Lipophilic drugs, in general, are associated with higher corneal epithelial permeability.32 Solubilization of these compounds into aqueous eye drops has been achieved with complexation to cyclodextrins, which contains a hydrophilic exterior that can interact with the aqueous milieu and internal hydrophobic moieties that stabilize the organic drug.33 Given the difficulties with bioavailability and degradation associated with oral consumption, topical application of quercetin or other flavonoids may prove more successful in the treatment of conditions that affect the ocular surface. Quercetin and other flavonoids have recently received an enormous amount of interest and several studies on the ocular surface have been performed in order to test their effects. The most significant studies are discussed below.
Anti-inflammatory properties
Numerous studies have shown that ROS levels regulate many cellular functions including activation and apoptosis of leukocytes,34–36 as well as expression of pro-inflammatory factors, such as vascular cell adhesion molecules (VCAMs), interleukins (IL), and vascular endothelial growth factor (VEGF), by epithelial and endothelial cells37–40 (Figure 3). Antioxidants have been reported to have inherent anti-inflammatory properties through the direct inhibition of ROS-promoted activation of NFκB signaling, which functions as a transcription factor promoting the expression of pro-inflammatory cytokines and chemokines.41,42 A number of studies have highlighted the anti-inflammatory properties of quercetin primarily via downregulation of NFκB both in vitro43,44 and in vivo.45,46 Various studies have identified potent anti-angiogenic properties of quercetin.47,48 A study by Ljubimov et al.49 found that inhibition of protein kinase CK2 (casein kinase 2) by quercetin (50 μM) resulted in reduced retinal neovascularization in a mouse model of oxygen-induced retinopathy with the direct regulation of expression of pro-angiogenic factors. Interestingly, quercetin’s role as an inhibitor of CK2 with rather broad specificity50 may give rise to the observed anti-angiogenic properties. CK2 has also been shown to play a role in proliferative and tumorigenic properties51–53 suggesting that the anti-cancer properties correlated with quercetin54,55 may also relate to its modulatory effects on CK2 activity.56
A study from Abengózar-Vela et al.57 showed the efficacy of quercetin as a therapeutic for corneal inflammation in vitro. The authors tested the anti-inflammatory and antioxidant effects of quercetin on human conjunctival and corneal epithelial cell lines. Results showed significant down regulation of IL-6 and IP-10 secretion, following quercetin stimulation, in a dose-dependent manner in both cell lines.57 Ultraviolet-B induced significant up-regulation of ROS, in vitro, and was significantly down-regulated by quercetin confirming its antioxidant efficacy.57
Reduced tear production, infection, injury, or allergy can compromise the immune privilege of the cornea and lead to ocular irritation, scarring, and reduced visual acuity. Quercetin has been found to have immunoregulatory properties when applied topically on the ocular surface in dry eye mouse models.58 In the most recent in vivo study, Oh et al.59 examined the effects of quercetin using an experimental dry eye mouse model; 0.5% quercetin eye drops resulted in significant tear volume increase and restoration of smooth corneal surfaces without detaching the corneal epithelium. Quercetin also increased goblet cell density59 suggesting that quercetin treatment may increase tear film production by directly modulating cell number.
Within the cornea, prolonged and uncontrolled pro-inflammatory processes are often associated with neovascularization within the cornea. Unlike the in vivo studies, in vitro studies investigating the effects of quercetin on the ocular surface did not appear until recently. One of the first studies was published by Donnini et al.60. In that article, the authors investigated the effects of quercetin and its main circulating conjugates (quercetin-3′-sulfate: Q3′S, and quercetin 3-glucuronide: Q3G) on cultured bovine endothelial cells.60 The authors correlated their findings with in vivo studies on the rabbit cornea following VEGF-induced angiogenesis. The results showed that Q3G and quercetin itself had no effect on quiescent endothelium, in vitro, while they inhibited endothelial function and angiogenesis in vivo.60 On the other hand, Q3′S significantly increased the growth of quiescent endothelial cells in vitro and had no effects on angiogenesis in vivo. The authors concluded that the ratio between Q3′S and Q3G is critical for the inhibition or activation of angiogenesis.60
Haynes et al.61 used computerized image analysis to evaluate quantitatively the ability of topically applied small molecules to reduce corneal neovascularization in a rat cornea.61 A large number of molecules were tested including quercetin, esculetin, prednisolone acetate, ketorolac, and others. The authors reported no significant effects on corneal neovascularization following treatment with 1% quercetin. While a small range of concentrations were tested during this study, it is important to take into account these observations when designing a quercetin-based ocular treatment.61
Metabolic regulator
Various studies have shown that quercetin is a regulator of systemic metabolism in physiological and pathological conditions.62–64 Notably, quercetin has been shown to exhibit anti-diabetic properties when ingested orally in streptozotocin (STZ)-induced Type 1 diabetic mouse model,65 as reviewed in Mukhopadhyay et al.66 In vivo studies investigating the effects of quercetin on the ocular surface date back to the late 1980s. However, not many studies have been reported since that time with regard to quercetin’s metabolic activity on the ocular surface. Lee et al.67 reported the distribution of ketone reductase activity in the rabbit cornea and its influence on ocular metabolism. The authors tested several inhibitors on ocular ketone reductase activity: quercetin, barbital, pyrazole, and dicoumarol.67 Quercetin was effective in inhibiting the ketone reductase activity in all the ocular tissues, being more so in the lens and the iris-ciliary than in the conjunctiva and corneal epithelium.67
Stoddard et al.68 investigated the bioavailability and efficacy of antioxidants in human corneal limbal epithelial cells (HCLE). Quercetin was the most potent at quenching ROS. On the other hand, quercetin was more slowly taken up by the cells than other compounds tested in the study.68 Even though this is an in vitro study, the availability of a topical ophthalmic formulation in vivo is relatively short due to frequent tear production and clearance (0.08–0.4 µL/min) depending on age.69 Therefore, any treatment needs to be taken up by corneal cells quickly if corneal diseases or dystrophies are to be treated topically and non-invasively.
A corneal thinning disease termed Keratoconus has been associated with increased susceptibility to ROS-induced apoptosis,70–73 altered cellular metabolism favoring aerobic glycolysis,74 and mitochondrial dysfunction75–77 within the corneal stroma. The specific metabolic effects of quercetin were investigated by our group in 2015 showing that quercetin upregulated the endogenous antioxidant pathway, the pentose phosphate pathway, in human keratoconus corneal cells.78 Our results showed that quercetin reduced lactate production by human keratoconus corneal stromal cells to levels similar to that of healthy controls favoring more favorable ATP production via the citric acid cycle.78,79 These studies suggest that quercetin may prove useful in targeting oxidative stress within the cornea in the context of Keratoconus by modulating energy production.
Anti-fibrotic
Scarring of the cornea is one of the most devastating outcomes following infection or trauma and is a major cause of blindness worldwide.80 The mechanisms of corneal wound healing are known to involve cytokine- and growth factor-mediated interactions within the epithelium and stroma in a cascade of events that result in increased matrix deposition and wound closure.81,82 Amongst other corneal diseases and dystrophies, corneal scarring occurs in a number of Keratoconus patients and is a leading cause of corneal transplantation in the US.83 In 2015, our lab investigated the anti-fibrotic effects of quercetin on human keratoconus corneal cells and concluded that it exhibited therapeutic benefit in vitro.78,79 Results showed that quercetin down-regulated key fibrotic markers, Collagen III and α-smooth muscle actin, by human keratoconus corneal stromal cells.79 We also showed that quercetin downregulated transforming growth factor-β2 (TGF-β2) in the presence and absence of excess lactate.79 It is well established that TGF-β signaling is one of the key players in corneal fibrosis, and various models have been proposed on how to inhibit its activity.81,84 Quercetin may be a successful therapeutic option for inhibiting corneal scarring by controlling TGF-β expression. Further studies are warranted to verify the anti-fibrotic effects of quercetin in vivo.
Gupta et al.85 examined the ability of inhibitors of arachidonic acid metabolism to influence the rate of epithelial closure in organ cultured rat corneas following 3-mm diameter central epithelial debridement. One of the inhibitors tested was quercetin at 100 μM concentration and was compared to indomethacin (1 μM), esculetin (100 μM), and flurbiprofen (1 μM).85 The authors observed a delayed epithelial wound healing rate following treatments with esculetin, as well as quercetin.85 While no further concentrations were tested, the role of these inhibitors is still to be investigated. This is the only organ culture study reported so far looking at the effects of quercetin on the anterior part of the eye.85
Proposed mechanism of action
Quercetin has been found to have pleiotropic bioactivity regulating inflammation, angiogenesis, cellular metabolism, and extracellular matrix deposition. As a potent antioxidant, quercetin has the ability to directly modulate ROS levels produced during normal cellular metabolism, as well as during pathological conditions, such as hypoxia, injury, or mitochondrial dysfunction. ROS regulate a number of signaling processes, in particular activation of NFκB, which as a transcription factor activates pro-inflammatory genes, including cytokines and chemokines that promote recruitment of inflammatory cells (Figure 3). Activation of pro-inflammatory genes within the corneal stroma or conjunctiva can lead to tissue damage due to prolonged inflammation and neovascularization. Evidence suggests that quercetin reduces ROS-induced activation of NFκB thereby reducing inflammation. Targeting oxidative stress is important in a number of tissues and may also be a novel target in the treatment of oxidative-stress-related corneal dystrophies, such as Keratoconus. Many studies have also shown that quercetin inhibits TGF-β signaling by regulating canonical SMAD2/3 phosphorylation suggesting that quercetin may serve as a potent anti-scarring agent. Further studies are required to determine if quercetin exhibits anti-fibrotic characteristics in preventing corneal scarring in vivo.
Future directions
Corneal defects are a leading cause of blindness worldwide, second only to cataracts.80 For decades, we and others have worked towards the goal of keeping the cornea and ocular surface transparent in order to ensure quality vision. To achieve this, several strategies have been employed ranging from eye drops to full corneal transplants.86–89 The data indicate that these strategies have succeeded in some occasions and failed in others. One of the most common observed failures has been the inability to “rescue” a chronic corneal scar or injury without the need of a corneal transplant. A study reporting global data from 2012 to 2013 showed a significant shortage of corneal tissue worldwide with only 1 out of 70 individuals in need of a corneal transplant were able to receive the surgery.90 However, we should acknowledge that successes have also been observed with corneal transplantation,91 and despite the long-term side effects, many people have regained their vision. Clearly, the global need to restore vision due to corneal blindness remains a driving force for alternative drug development.
Given the accelerated interest in flavonoids and recently in quercetin as a potent anti-inflammatory45,58 and anti-fibrotic79,92 agent, it is critical to accelerate our efforts towards more translational and clinical studies aimed at the development of non-invasive therapeutics for ocular surface diseases. A recent search of the ClinicalTrials.gov database (accessed on 19 May 2016) revealed that approximately 44 clinical trials are currently investigating the effects of quercetin or quercetin-included supplements on various diseases. Surprisingly, none of these studies are related to ocular pathologies. It is therefore clear that we need to increase our efforts towards the development of novel therapeutics for ocular surface diseases.
One of the main reasons quercetin as well as other flavonoids are attractive to pharmaceutical companies is that they come with a very low cost tag and extremely high safety profile.93,94 It is also possible that these natural compounds can be used in conjunction with other current chemotherapeutic drugs in order to enhance their effectiveness. These kinds of studies are almost non-existent and require in-depth pharmacokinetics and pharmacodynamics analyses.
The study of quercetin is complex because of the scarcity of data in both in vitro and in vivo studies, on short- and long-term effectiveness, as well as bioavailability. There is, however, great promise based on the studies reported that this flavonoid can help with the treatment of at least some ocular surface diseases. As we look forward to the future, there are several factors that need to be considered when designing and executing these studies: (1) the lack of mechanism by which quercetin exerts its beneficial effects, (2) uncertainty about its bioavailability in a pharmacological form, and (3) the complexity of the numerous ocular surface diseases within the human population.
Conclusions
Quercetin and flavonoids in general are considered safe compounds, since unwanted toxic effects in humans are not frequently encountered. In fact, they can be administered in humans at high concentrations without any major threats. Unfortunately, only few flavonoids have made it to the clinical setting in terms of disease treatment and/or prevention. Quercetin is a very promising flavonoid with significant in vitro and in vivo beneficial effects for the ocular surface. Clearly, there is a great need for more effective medical therapy, and this warrants continued investigation both at the cellular and clinical level.
Authors’ contributions
DK and TBM wrote the paper.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
We acknowledge the support of the National Institutes of Health (NEI) Grants 5R01EY023568 (DK), and 5R01EY020886 (DK). This work was supported by an unrestricted grant (DMEI) from Research to Prevent Blindness (New York, NY USA). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Herrmann K. Flavonols and flavones in food plants: A review†. Int J Food Sci Technol 1976; 11: 433–48. [Google Scholar]
- 2.Harborne JB. The flavonoids: Advances in research since 1980, New York: Springer, 1988. [Google Scholar]
- 3.Rossi M, Rickles LF, Halpin WA. The crystal and molecular structure of quercetin: A biologically active and naturally occurring flavonoid. Bioorganic Chem 1986; 14: 55–69. [Google Scholar]
- 4.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Rad Biol Med 1997; 22: 749–60. [DOI] [PubMed] [Google Scholar]
- 5.Vinson JA, Dabbagh YA, Serry MM, Jang J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J Agri Food Chem 1995; 43: 2800–2. [Google Scholar]
- 6.Aviram M, Dornfeld L, Kaplan M, Coleman R, Gaitini D, Nitecki S, Hofman A, Rosenblat M, Volkova N, Presser D, Attias J, Hayek T, Fuhrman B. Pomegranate juice flavonoids inhibit low-density lipoprotein oxidation and cardiovascular diseases: Studies in atherosclerotic mice and in humans. Drugs Under Exp Clin Res 2001; 28: 49–62. [PubMed] [Google Scholar]
- 7.Onozuka H, Nakajima A, Matsuzaki K, Shin R-W, Ogino K, Saigusa D, Tetsu N, Yokosuka A, Sashida Y, Mimaki Y, Yamakuni T, Ohizumi Y. Nobiletin, a citrus flavonoid, improves memory impairment and Aβ pathology in a transgenic mouse model of Alzheimer's disease. J Pharmacol Exp Ther 2008; 326: 739–44. [DOI] [PubMed] [Google Scholar]
- 8.Hertog MG, Feskens EJ, Kromhout D, Hollman P, Katan M. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993; 342: 1007–11. [DOI] [PubMed] [Google Scholar]
- 9.Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Int Med 1996; 125: 384–9. [DOI] [PubMed] [Google Scholar]
- 10.Cook N, Samman S. Flavonoids – Chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 1996; 7: 66–76. [Google Scholar]
- 11.Nishimuro H, Ohnishi H, Sato M, Ohnishi-Kameyama M, Matsunaga I, Naito S, Ippoushi K, Oike H, Nagata T, Akasaka H, Saitoh S, Shimamoto K, Kobori M. Estimated daily intake and seasonal food sources of quercetin in Japan. Nutrients 2015; 7: 2345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollman PCH, Katan MB. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem Toxicol 1999; 37: 937–42. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth K, Piskula M. Food content, processing, absorption and metabolism of onion flavonoids. Crit Rev Food Sci Nutr 2007; 47: 397–409. [DOI] [PubMed] [Google Scholar]
- 14.Casagrande R, Georgetti SR, Verri WA, Dorta DJ, dos Santos AC, Fonseca MJ. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J Photochem Photobiol B: Biol 2006; 84: 21–7. [DOI] [PubMed] [Google Scholar]
- 15.Vicentini FT, Simi TR, Del Ciampo JO, Wolga NO, Pitol DL, Iyomasa MM, Bentleya VLB, Fonsecaa MJV. Quercetin in w/o microemulsion: In vitro and in vivo skin penetration and efficacy against UVB-induced skin damages evaluated in vivo. Eur J Pharm Biopharm 2008; 69: 948–57. [DOI] [PubMed] [Google Scholar]
- 16.Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 2001; 126: 485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winkel-Shirley B. Biosynthesis of flavonoids and effects of stress. Curr Opinion Plant Biol 2002; 5: 218–23. [DOI] [PubMed] [Google Scholar]
- 18.Groot Hd, Rauen U. Tissue injury by reactive oxygen species and the protective effects of flavonoids. Fund Clin Pharmacol 1998; 12: 249–55. [DOI] [PubMed] [Google Scholar]
- 19.Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci 1997; 2: 152–9. [Google Scholar]
- 20.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 2005; 81: 230S–42. [DOI] [PubMed] [Google Scholar]
- 21.Hollman P, De Vries J, van Leeuwen SD, Mengelers M, Katan MB. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr 1995; 62: 1276–82. [DOI] [PubMed] [Google Scholar]
- 22.Murota K, Terao J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch Biochem Biophys 2003; 417: 12–7. [DOI] [PubMed] [Google Scholar]
- 23.Day AJ, Mellon F, Barron D, Sarrazin G, Morgan MR, Williamson G. Human metabolism of dietary flavonoids: Identification of plasma metabolites of quercetin. Free Rad Res 2001; 35: 941–52. [DOI] [PubMed] [Google Scholar]
- 24.Janisch KM, Williamson G, Needs P, Plumb GW. Properties of quercetin conjugates: Modulation of LDL oxidation and binding to human serum albumin. Free Rad Res 2004; 38: 877–84. [DOI] [PubMed] [Google Scholar]
- 25.Wittig J, Herderich M, Graefe EU, Veit M. Identification of quercetin glucuronides in human plasma by high-performance liquid chromatography–tandem mass spectrometry. J Chromatograph B 2001; 753: 237–43. [DOI] [PubMed] [Google Scholar]
- 26.Beuerman RW, Pedroza L. Ultrastructure of the human cornea. Microscopy Res Tech 1996; 33: 320–35. [DOI] [PubMed] [Google Scholar]
- 27.Stocker FW. The endothelium of the cornea and its clinical implications. Transac Am Ophthal Soc 1953; 51: 669–669. [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson JD, Wright JC. Conjunctival goblet cell densities in ocular surface disease. Arch Ophthalmol 1984; 102: 1049–51. [DOI] [PubMed] [Google Scholar]
- 29.Li F, Carlsson D, Lohmann C, Suuronen E, Vascotto S, Kobuch K, Sheardown H, Munger R, Nakamura M, Griffith M. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc Natl Acad Sci 2003; 100: 15346–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Gan L, Carlsson DJ, Fagerholm P, Lagali N, Watsky MA, Munger R, Hodge WG, Priest D, Griffith M. A simple, cross-linked collagen tissue substitute for corneal implantation. Investigative ophthalmology & visual science 2006; 47(5): 1869–75. [DOI] [PubMed] [Google Scholar]
- 31.Lee VH, Robinson JR. Topical ocular drug delivery: Recent developments and future challenges. J Ocular Pharmacol Therap 1986; 2: 67–108. [DOI] [PubMed] [Google Scholar]
- 32.Davies NM. Biopharmaceutical considerations in topical ocular drug delivery. Clin Exp Pharmacol Physiol 2000; 27: 558–62. [DOI] [PubMed] [Google Scholar]
- 33.Challa R, Ahuja A, Ali J, Khar R. Cyclodextrins in drug delivery: An updated review. Aaps Pharmscitech 2005; 6: E329–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finkel T. Signal transduction by reactive oxygen species in non-phagocytic cells. J Leukocyte Biol 1999; 65: 337–40. [DOI] [PubMed] [Google Scholar]
- 35.Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Rad Biol Med 2007; 42: 153–64. [DOI] [PubMed] [Google Scholar]
- 36.Fadeel B, Åhlin A, Henter J-I, Orrenius S, Hampton MB. Involvement of caspases in neutrophil apoptosis: Regulation by reactive oxygen species. Blood 1998; 92: 4808–18. [PubMed] [Google Scholar]
- 37.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett 2008; 266: 37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia C, Meng Q, Liu L-Z, Rojanasakul Y, Wang X-R, Jiang B-H. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res 2007; 67: 10823–30. [DOI] [PubMed] [Google Scholar]
- 39.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature molecular and cellular mechanisms. Hypertension 2003; 42: 1075–81. [DOI] [PubMed] [Google Scholar]
- 40.Hsu H-Y, Wen M-H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J Biol Chem 2002; 277: 22131–9. [DOI] [PubMed] [Google Scholar]
- 41.Sen CK, Packer L. Antioxidant and redox regulation of gene transcription. FASEB J 1996; 10: 709–20. [DOI] [PubMed] [Google Scholar]
- 42.Gloire G, Legrand-Poels S, Piette J. NF-κB activation by reactive oxygen species: Fifteen years later. Biochem Pharmacol 2006; 72: 1493–505. [DOI] [PubMed] [Google Scholar]
- 43.Min Y-D, Choi C-H, Bark H, Son H-Y, Park H-H, Lee S, Park J-W, Park E-K, Shin H-I, Kim S-H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res 2007; 56: 210–5. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz PA, Braune A, Hölzlwimmer G, Quintanilla-Fend L, Haller D. Quercetin inhibits TNF-induced NF-κB transcription factor recruitment to proinflammatory gene promoters in murine intestinal epithelial cells. J Nutr 2007; 137: 1208–15. [DOI] [PubMed] [Google Scholar]
- 45.Rogerio A, Kanashiro A, Fontanari C, Da Silva E, Lucisano-Valim Y, Soares E, Faccioli LH. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm Res 2007; 56: 402–8. [DOI] [PubMed] [Google Scholar]
- 46.Nickel T, Hanssen H, Sisic Z, Pfeiler S, Summo C, Schmauss D, Hoster E, Weis M. Immunoregulatory effects of the flavonol quercetin in vitro and in vivo. Eur J Nutr 2011; 50: 163–72. [DOI] [PubMed] [Google Scholar]
- 47.Tan WF, Lin LP, Li MH, Zhang YX, Tong YG, Xiao D, Ding J. Quercetin, a dietary-derived flavonoid, possesses antiangiogenic potential. Eur J Pharmacol 2003; 459: 255–62. [DOI] [PubMed] [Google Scholar]
- 48.Zhao D, Qin C, Fan X, Li Y, Gu B. Inhibitory effects of quercetin on angiogenesis in larval zebrafish and human umbilical vein endothelial cells. Eur J Pharmacol 2014; 723: 360–7. [DOI] [PubMed] [Google Scholar]
- 49.Ljubimov AV, Caballero S, Aoki AM, Pinna LA, Grant MB, Castellon R. Involvement of protein kinase CK2 in angiogenesis and retinal neovascularization. Invest Ophthal Visual Sci 2004; 45: 4583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sarno S, Ruzzene M, Frascella P, Pagano MA, Meggio F, Zambon A, Marco Mazzorana, Maira GD, Lucchini V, Pinna LA. Development and exploitation of CK2 inhibitors. Mol Cell Biochem 2005; 274: 69–76. [DOI] [PubMed] [Google Scholar]
- 51.Sarno S, Moro S, Meggio F, Zagotto G, Dal Ben D, Ghisellini P, Battistuttad R, Zanottid G, Pinna LA. Toward the rational design of protein kinase casein kinase-2 inhibitors. Pharmacol Ther 2002; 93: 159–68. [DOI] [PubMed] [Google Scholar]
- 52.Landesman-Bollag E, Romieu-Mourez R, Song DH, Sonenshein GE, Cardiff RD, Seldin DC. Protein kinase CK2 in mammary gland tumorigenesis. Oncogene 2001; 20: 3247–57. [DOI] [PubMed] [Google Scholar]
- 53.Duncan JS, Litchfield DW. Too much of a good thing: The role of protein kinase CK2 in tumorigenesis and prospects for therapeutic inhibition of CK2. Biochim Biophys Acta 2008; 1784: 33–47. [DOI] [PubMed] [Google Scholar]
- 54.ElAttar TM, Virji AS. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anti-Cancer Drugs 1999; 10: 187–94. [DOI] [PubMed] [Google Scholar]
- 55.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett 2008; 269: 315–25. [DOI] [PubMed] [Google Scholar]
- 56.Lolli G, Cozza G, Mazzorana M, Tibaldi E, Cesaro L, Donella-Deana A, Meggio F, Venerando A, Franchin C, Sarno S, Battistutta R, Pinna LA. Inhibition of protein kinase CK2 by flavonoids and tyrphostins. A structural insight. Biochemistry 2012; 51: 6097–107. [DOI] [PubMed] [Google Scholar]
- 57.Abengózar-Vela A, Calonge M, Stern ME, González-García MJ, Enríquez-De-Salamanca A. Quercetin and resveratrol decrease the inflammatory and oxidative responses in human ocular surface epithelial cells. Invest Ophthal Visual Sci 2015; 56: 2709–19. [DOI] [PubMed] [Google Scholar]
- 58.Abengózar-Vela A, Schaumburg CS, Stern ME, Calder VL, Calonge M, Enriquez-De-Salamanca A, Gonzalez M-JJ. An in vivo study of anti-inflammatory effect of Quercetin and Resveratrol polyphenols in a desiccating stress mouse model of dry eye. Invest Ophthal Visual Sci 2014; 55: 3654–3654. [Google Scholar]
- 59.Oh HN, Kim CE, Lee JH, Yang JW. Effects of quercetin in a mouse model of experimental dry eye. Cornea 2015; 34: 1130–6. [DOI] [PubMed] [Google Scholar]
- 60.Donnini S, Finetti F, Lusini L, Morbidelli L, Cheynier V, Barron D, Williamson G, Waltenberger J, Ziche M. Divergent effects of quercetin conjugates on angiogenesis. Br J Nutr 2006; 95: 1016–23.16611395 [Google Scholar]
- 61.Haynes W, Proia AD, Klintworth G. Effect of inhibitors of arachidonic acid metabolism on corneal neovascularization in the rat. Invest Ophthal Visual Sci 1989; 30: 1588–93. [PubMed] [Google Scholar]
- 62.Kobori M, Masumoto S, Akimoto Y, Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol Nutr Food Res 2011; 55: 530–40. [DOI] [PubMed] [Google Scholar]
- 63.Snyder SM, Zhao B, Luo T, Kaiser C, Cavender G. Consumption of quercetin and quercetin-containing apple and cherry extracts affects blood glucose concentration, hepatic metabolism, and gene expression patterns in obese C57BL/6J high fat-fed mice. J Nutr 2016; 146: 1001–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang M, Xie Z, Gao W, Pu L, Wei J, Guo C. Quercetin regulates hepatic cholesterol metabolism by promoting cholesterol-to-bile acid conversion and cholesterol efflux in rats. Nutr Res 2016; 36: 271–9. [DOI] [PubMed] [Google Scholar]
- 65.Kobori M, Masumoto S, Akimoto Y, Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol Nutr Food Res 2009; 53: 859–68. [DOI] [PubMed] [Google Scholar]
- 66.Mukhopadhyay P, Prajapati A. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – A review. RSC Adv 2015; 5: 97547–62. [Google Scholar]
- 67.Lee V, Chien D, Sasaki H. Ocular ketone reductase distribution and its role in the metabolism of ocularly applied levobunolol in the pigmented rabbit. J Pharmacol Exp Ther 1988; 246: 871–8. [PubMed] [Google Scholar]
- 68.Stoddard AR, Koetje LR, Mitchell AK, Schotanus MP, Ubels JL. Bioavailability of antioxidants applied to stratified human corneal epithelial cells. J Ocul Pharmacol Ther 2013; 29: 681–7. [DOI] [PubMed] [Google Scholar]
- 69.Mathers WD, Lane JA, Zimmerman MB. Tear film changes associated with normal aging. Cornea 1996; 15: 229–34. [DOI] [PubMed] [Google Scholar]
- 70.Chwa M, Atilano SR, Hertzog D, Zheng H, Langberg J, Kim DW, Kenney MC. Hypersensitive response to oxidative stress in keratoconus corneal fibroblasts. Invest Ophthal Visual Sci 2008; 49: 4361–9. [DOI] [PubMed] [Google Scholar]
- 71.Chwa M, Atilano SR, Reddy V, Jordan N, Kim DW, Kenney MC. Increased stress-induced generation of reactive oxygen species and apoptosis in human keratoconus fibroblasts. Invest Ophthal Visual Sci 2006; 47: 1902–10. [DOI] [PubMed] [Google Scholar]
- 72.Kenney MC, Brown DJ. The cascade hypothesis of keratoconus. Cont Lens Anterior Eye 2003; 26: 139–46. [DOI] [PubMed] [Google Scholar]
- 73.Kenney MC, Chwa M, Atilano SR, Tran A, Carballo M, Saghizadeh M, Vasiliou V, Adachi W, Brown DJ. Increased levels of catalase and cathepsin V/L2 but decreased TIMP-1 in keratoconus corneas: Evidence that oxidative stress plays a role in this disorder. Invest Ophthal Visual Sci 2005; 46: 823–32. [DOI] [PubMed] [Google Scholar]
- 74.Karamichos D, Hutcheon A, Rich C, Trinkaus-Randall V, Asara J, Zieske J. In vitro model suggests oxidative stress involved in keratoconus disease. Sci Rep 2014; 4: 4608–4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarker-Nag A, Hutcheon AE, Karamichos D. Mitochondrial Profile and Responses to TGF-β Ligands in Keratoconus. Curr Eye Res 2016; 41: 900–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Atilano SR, Coskun P, Chwa M, Jordan N, Reddy V, Le K, Wallace DC, Kenney MC. Accumulation of mitochondrial DNA damage in keratoconus corneas. Invest Ophthal Visual Sci 2005; 46: 1256–63. [DOI] [PubMed] [Google Scholar]
- 77.Abu-Amero KK, Azad TA, Kalantan H, Sultan T, Al-Muammar AM. Mitochondrial sequence changes in keratoconus patientsscreening mitochondrial genome in keratoconus patients. Invest Ophthal Visual Sci 2014; 55: 1706–10. [DOI] [PubMed] [Google Scholar]
- 78.McKay TB, Sarker-Nag A, Lyon D, Asara JM, Karamichos D. Quercetin modulates keratoconus metabolism in vitro. Cell Biochem Funct 2015; 33: 341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McKay T, Lyon D, Sarker-Nag A, Priyadarsini S, Asara J, Karamichos D. Quercetin attenuates lactate production and extracellular matrix secretion in keratoconus. Sci Rep 2015; 5: 9003–9003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: A global perspective. Bull World Health Org 2001; 79: 214–21. [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson SE, Mohan RR, Mohan RR, Ambrosio R, Hong JW, Lee JS. The corneal wound healing response: Cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res 2001; 20: 625–37. [DOI] [PubMed] [Google Scholar]
- 82.Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen 2009; 17: 153–62. [DOI] [PubMed] [Google Scholar]
- 83.Barr JT, Wilson BS, Gordon MO, Rah MJ, Riley C, Kollbaum PS, Zadnik K. Estimation of the incidence and factors predictive of corneal scarring in the Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study. Cornea 2006; 25: 16–25. [DOI] [PubMed] [Google Scholar]
- 84.Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-β receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol 1999; 179: 325–35. [DOI] [PubMed] [Google Scholar]
- 85.Gupta AG, Hirakata A, Proia AD. Effect of inhibitors of arachidonic acid metabolism on corneal reepithelialization in the rat. Exp Eye Res 1993; 56: 701–8. [DOI] [PubMed] [Google Scholar]
- 86.Majmudar PA, Forstot SL, Dennis RF, Nirankari VS, Damiano RE, Brenart R, Epstein RJ. Topical mitomycin-C for subepithelial fibrosis after refractive corneal surgery. Ophthalmology 2000; 107: 89–94. [DOI] [PubMed] [Google Scholar]
- 87.Lambiase A, Rama P, Bonini S, Caprioglio G, Aloe L. Topical treatment with nerve growth factor for corneal neurotrophic ulcers. N Engl J Med 1998; 338: 1174–80. [DOI] [PubMed] [Google Scholar]
- 88.Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet 2012; 379: 1749–61. [DOI] [PubMed] [Google Scholar]
- 89.Tsubota K, Satake Y, Kaido M, Shinozaki N, Shimmura S, Bissen-Miyajima H, Shimazaki J. Treatment of severe ocular-surface disorders with corneal epithelial stem-cell transplantation. N Engl J Med 1999; 340: 1697–703. [DOI] [PubMed] [Google Scholar]
- 90.Gain P, Jullienne R, He Z, Aldossary M, Acquart S, Cognasse F, Thuret G. Global survey of corneal transplantation and eye banking. JAMA Ophthal 2016; 134: 167–73. [DOI] [PubMed] [Google Scholar]
- 91.Garg P, Krishna P, Stratis A, Gopinathan U. The value of corneal transplantation in reducing blindness. Eye 2005; 19: 1106–14. [DOI] [PubMed] [Google Scholar]
- 92.Yoon JS, Chae MK, Jang SY, Lee SY, Lee EJ. Antifibrotic effects of quercetin in primary orbital fibroblasts and orbital fat tissue cultures of graves' orbitopathyantifibrotic effects of quercetin in GO. Invest Ophthal Visual Sci 2012; 53: 5921–9. [DOI] [PubMed] [Google Scholar]
- 93.Lu NT, Crespi CM, Liu NM, Vu JQ, Ahmadieh Y, Wu S, Lin S, McClune A, Durazo F, Saab S, Han S, Neiman DC, Beaven S, French SW. A phase I dose escalation study demonstrates quercetin safety and explores potential for bioflavonoid antivirals in patients with chronic hepatitis C. Phytother Res 2016; 30: 160–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valensi P, Le Devehat C, Richard J-L, Farez C, Khodabandehlou T, Rosenbloom RA, LeFante C. A multicenter, double-blind, safety study of QR-333 for the treatment of symptomatic diabetic peripheral neuropathy: A preliminary report. J Diab Complicat 2005; 19: 247–53. [DOI] [PubMed] [Google Scholar]