Abstract
Vasculogenic mimicry (VM) is a non-classical mechanism recently described in many tumors, whereby cancer cells, rather than endothelial cells, form blood vessels. Transgelin is an actin-binding protein that has been implicated in multiple stages of cancer development. In this study, we investigated the role of transgelin in VM and assessed its effect on the expression of endothelial and angiogenesis-related genes during VM in MDA-MB-231 breast cancer cells. We confirmed the ability of MDA-MB-231 cells to undergo VM through a tube formation assay. Flow cytometry analysis revealed an increase in the expression of the endothelial-related markers VE-cadherin and CD34 in cells that underwent VM, compared with those growing in a monolayer, which was confirmed by immunocytochemistry. We employed siRNA to silence transgelin, and knockdown efficiency was determined by western blot analyses. Downregulation of transgelin suppressed cell proliferation and tube formation, but increased IL-8 levels in Matrigel cultures. RT-PCR analyses revealed that the expression of IL-8, VE-cadherin, and CD34 was unaffected by transgelin knockdown, indicating that increased IL-8 expression was not due to enhanced transcriptional activity. More importantly, the inhibition of IL-8/CXCR2 signaling also resulted in suppression of VM with increased IL-8 levels, confirming that increased IL-8 levels after transgelin knockdown was due to inhibition of IL-8 uptake. Our findings indicate that transgelin regulates VM by enhancing IL uptake. These observations are relevant to the future development of efficient antivascular agents.
Impact statement
Vasculogenic mimicry (VM) is an angiogenic-independent mechanism of blood vessel formation whereby aggressive tumor cells undergo formation of capillary-like structures. Thus, interventions aimed at angiogenesis might not target the entire tumor vasculature. A more holistic approach is therefore needed in the development of improved antivascular agents.
Transgelin, an actin-binding protein, has been associated with multiple stages of cancer development such as proliferation, migration and invasion, but little is known about its role in vasculogenic mimicry. We present here, an additional mechanism by which transgelin promotes malignancy by way of its association with the occurrence of VM. Although transgelin knockdown did not affect the transcript levels of most of the angiogenesis-related genes in this study, it was associated with the inhibition of the uptake of IL-8, accompanied by suppressed VM, indicating that transgelin is required for VM. These observations are relevant to the future development of efficient antivascular agents.
Keywords: Vasculogenic mimicry, transgelin, cancer stem cells, interleukin-8, endothelial markers, breast cancer
Introduction
Adequate blood supply is crucial for tumor growth and survival, as it ensures delivery of oxygen and nutrients, without which the tumor cannot grow beyond a certain size.1 Angiogenesis, the process by which blood vessels are formed from the pre-existing vasculature, is a conventional mechanism exploited by tumors to facilitate growth, and is a well-recognized hallmark of cancer.2 This process has been shown to play a role in growth and metastasis in malignancies such as breast cancer, which is the most commonly diagnosed cancer in women.3,4 Hence, targeting angiogenesis is considered a major therapeutic strategy for the treatment of tumors; however, recent studies have shown that angiogenesis-independent pathways such as vasculogenic mimicry (VM) are also employed by tumors to ensure adequate nourishment.5
Maniotis et al. were the first to describe VM in melanoma, and subsequent in vivo and in vitro studies reported this process in glioblastomas, breast, ovarian, and head and neck carcinomas.5,6 VM refers to the ability of tumor cells to adopt properties of endothelial cells and form mosaic structures that can provide a blood supply for the tumor in vivo. In three-dimensional cultures in vitro, this process is recapitulated by the formation of tubular structures and patterned networks. VM is usually observed in high-grade invasive tumors, is associated with poor prognosis, and might be partly responsible for resistance to antiangiogenic drugs.7–9 Indeed, a number of studies revealed that angiogenesis inhibitors did not significantly affect VM, thus underscoring the need for improved antivascular therapies that specifically target VM.6,10,11
Transgelin (also known as SM22α and TAGLN) is an early marker of smooth muscle cell differentiation and is an actin-binding protein that affects cytoskeletal dynamics by stabilizing actin filaments. The association between transgelin and the cytoskeleton accounts for its involvement in cancer-related processes such as proliferation, migration, invasion, and apoptosis. Transgelin-mediated effects have been implicated in the pathogenesis of colorectal, stomach, pancreas, prostate, kidney, and bladder tumors. Moreover, differential expression of transgelin has been associated with poor prognoses in certain cases. Importantly, Lee et al. showed that transgelin enhanced migration and invasion of cancer stem cells (CSCs).12–14 In addition, Rao et al. described transgelin as a potentially useful diagnostic marker, based on its differential expression in triple negative (TN) and non-TN breast cancers.15
A number of studies suggest that transgelin might have diverse roles in vessel formation. For example, co-expression and co-localization of transgelin and the chemokine receptor CXCR4, which is implicated in angiogenesis and breast cancer metastasis, have been reported.13,16,17 In contrast, Nair et al. found that transgelin repressed metalloproteinase (MMP)-9 expression, a pro-angiogenic factor and mediator of VM.9,18,19 Furthermore, transgelin-2, a cytoskeletal protein and a transgelin homolog that is also differentially expressed in some tumors, was shown to participate in lovastatin-induced antiangiogenic effects in endothelial cells.20,21 Although a large number of studies have addressed the role of transgelin in tumorigenesis, there is paucity of information regarding the impact of transgelin on angiogenesis and VM.
In this study, we investigated the effect of transgelin on VM and the expression of angiogenesis-related genes in breast cancer cells.
Materials and methods
Cell culture
Human breast cancer cell lines, MDA-MB 231 and MCF-7, were purchased from the Korean Cell Line Bank (Seoul, Korea). Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS; Gibco-Life Technologies, Carlsbad, CA), 100 U/mL penicillin, and 100 µg/mL streptomycin. Cells were incubated at 37°C in a humidified atmosphere and in the presence of 5% CO2. Cells plated in Matrigel-coated wells were recovered using Cell Recovery Solution (Corning, Tewksbury, MA) following the manufacturer’s recommendations.
Flow cytometry
The FACS Calibur was used for analyses of cell surface markers. Monolayer cultures were harvested by trypsinization, and cells grown on Matrigel were recovered using Cell Recovery solution (Corning), according to the manufacturer’s instructions. The cells were washed with PBS containing 2% FBS (wash buffer), and were then resuspended at a density of 106 cells/100 µL of PBS containing 1% FBS. The suspensions were incubated at 4°C in the dark for 30 min in the presence of indicated antibodies, using concentrations recommended by the manufacturers. Labeled cells were washed first with PBS containing 1% FBS and then with wash buffer. Monoclonal antibodies against human CD44 (FITC-labeled) and CD24 (PE-labeled) were obtained from BD Pharmingen (San Jose, CA). Antibodies against CD34 (FITC-labeled), VEGFR2 (Alexa Fluor 547-labeled), and VE-Cadherin (APC-conjugated) were purchased from Biolegend (San Diego, CA).
Tube formation assay
We coated μ-plate angiogenesis 96-well plates (Ibidi GmbH, Germany) with 10 µL/well of Corning Matrigel growth factor reduced (GFR) Basement Membrane Matrix, and incubated the plates for 30 min at 37°C, as per instructions provided by the manufacturer. Cells grown as monolayers were trypsinized, and re-plated at a density of 1.25 × 104 cells/well on Matrigel-coated wells. When 24-well plates were used, we coated each well with 150 µL of Matrigel and seeded 1.5 × 105 cells/well. For fluorescence imaging, cultures were washed with HBSS and cells were stained with Calcein AM (Corning), according to the manufacturer’s protocol. To assess quantitative differences among individual cell groups, we determined total tube length, using MetaMorph software (Molecular Devices, Sunnyvale, CA).
Immunocytochemical studies involving immunofluorescence and confocal microscopy
Approximately 10 h after plating, cultures in μ-plate angiogenesis 96-well plates were washed with cold PBS and fixed with cold methanol for 10 min. Cells were then permeabilized with 0.2% Triton-X 100, blocked with 5% BSA, and labeled with Alexa Fluor 594-VE-cadherin or FITC-CD34 antibodies. After 1 h of incubation, the cells were counterstained with DAPI for 20 min. Washing was performed three times for 5 min after each step. Images were acquired using a confocal laser scanning microscope, LSM 700 (Carl Zeiss, Jena, Germany).
Transfection
For small interfering RNA (siRNA) studies, we seeded cells one day before transfection in six-well plates at a density of 1.8 × 105 cells/well. Cells were then transfected with either scrambled (control) siRNA (UGGUUUACAUGUCGACUAA) or siRNA targeting transgelin (CCAAAAUCGAGAAGAAGUAUU) (Dharmacon, Lafayette, CO, USA), using Dharmafect 4 transfection reagent (Dharmacon), according to instructions provided by the manufacturer. Approximately 6 h after transfection, the culture medium was replaced with fresh medium. For RT-PCR analyses, total RNA was extracted 24 h after transfection. For all other assays, cells were cultured for 48 h and were then trypsinized and used in subsequent studies, or lysed as described below; lysates were subjected to further analyses.
Semi-quantitative RT-PCR
Total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA) and cDNA was synthesized from 1 µg of total RNA, using the ReverTra Ace® quantitative reverse transcription PCR Master Mix (Toyobo, Japan) in accordance with the manufacturers’ instructions. PCR primers were from Cosmogenetech (Seoul, Korea). Target regions were amplified using the primers listed in Table 1. PCR products were resolved on a 2% agarose gel and detected using EtBr with a BioRad Molecular Imager® GelDoc™ XR. For all RT-PCR analysis, β-actin was used as the loading control.
Table 1.
Primers for RT-PCR
| Name | Primer sequence (5′ → 3′) | Product size (bp) |
|---|---|---|
| β-actin | Forward: GGACTTCGAGC AAGAGATGG | 234 |
| Reverse: AGCACTGTGTT GGCGTACAG | ||
| IL-8 | Forward: CACCGGAAGG AACCATCTCACT | 322 |
| Reverse: TCAGCCCTCTTCA AAAACTTCTCC | ||
| Transgelin | Forward: GGTGGAGTGGATCATAGTGC | 243 |
| Reverse: ATGTCAGTCTTGATGACCCCA | ||
| VE-cadherin | Forward: GCACCAGTTTGGCCAATATA | 149 |
| Reverse: GGGTTTTTGCATAATAAG CAGG | ||
| VEGFR2 | Forward: CTGTAACAGATGAGAT GCTCCAAGG | 209 |
| Reverse: ACCAAAGGGGCA CGATTCCGTC |
Western blot analyses
Cultured cells were lysed in RIPA buffer (Sigma-Aldrich, St. Louis, MO) with a 1% protein inhibitor cocktail. Total protein levels were assessed using the Bradford assay. Aliquots (10 µg) were then subjected to electrophoresis on 12% SDS-polyacrylamide gels. Resolved proteins were transferred to polyvinylidene fluoride (PVDF) membranes, and membranes were blocked with 5% (w/v) non-fat dried milk. The membranes were then incubated with a 1:1000 dilution of mouse monoclonal anti-GAPDH (Santa Cruz Biotechnology, Dallas, TX) or a 1: 4000 dilution of rabbit polyclonal antitransgelin antibody (Abcam, Cambridge, MA) at 4°C overnight. Blots were incubated with goat antimouse or goat antirabbit horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX) for 1 h at room temperature. Blots were developed using an enhanced chemiluminescence detection kit (Bio-Rad, Hercules, CA, USA) to visualize immunoreactive bands. GAPDH was used as a loading control.
CCK-8 proliferation assay
Cell proliferation was determined using a cell counting kit (CCK-8, Dojindo, Japan) following the recommendations provided by the manufacturer. Briefly, cells were seeded on 96-well plates at a density of 3000 cells/well and allowed to adhere overnight. The cells were then transfected with control or targeted siRNAs or treated with the CXCR2 inhibitor, SB225002. At the indicated time points, cck-8 reagent was added (10 µL/well), and the plates were incubated in the dark for 2 h at 37°C. The optical densities were read at 450 nm using a 96-well plate reader. Assays were performed in replicates of six.
ELISA
Culture supernatants from cells grown on 24-well plates were collected 10 h after plating, and maintained at −80°C until further use. Interleukin-8 (IL-8) levels in the supernatants were quantified using the IL-8 Quantikine ELISA kit, (R&D, Minneapolis, MN) according to the manufacturer’s recommendations.
CXCR2 inhibition assay
SB225002 was obtained from Sigma-Aldrich and dissolved in dimethyl sulfoxide (DMSO, from Sigma-Aldrich). Cells were treated with SB225002s at the indicated final concentrations and with the specified incubation conditions. Cells or culture supernatants were harvested 10 h post-treatment for further analysis.
Statistical analyses
Data are presented as the mean ± standard deviation. All experiments were performed at least three independent times. A student’s t-test was performed to compare differences between groups. Statistical significance was set at P < 0.05. Data analysis was performed using the SigmaStat 3.5 statistical software package.
Results
MDA-MB-231 cells have a high proportion of cancer stem cells and are VM-competent
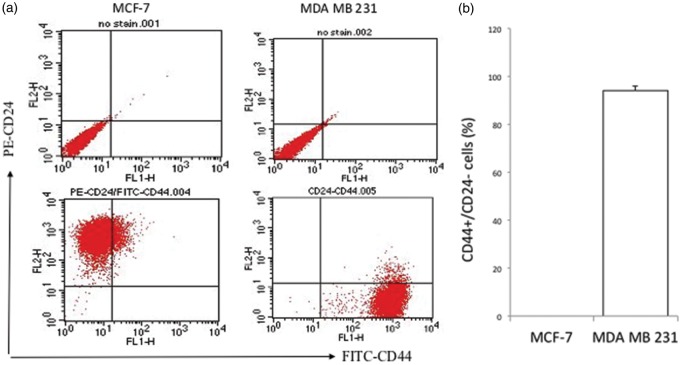
CSCs have been associated with the occurrence of VM.22–24 FACS analysis to assess the proportion of CD44+/CD24− CSCs revealed that greater than 90% (94.3% ± 1.8) of the MDA-MB-231 cell population was composed of CSCs; this high proportion of CSCs is a characteristic of basal cell lines. The observation that the majority of cells in the MDA-MB-231 cell line have stem-like properties was in agreement with previous reports.25,26 However, we did not detect a CD44+/CD24− subpopulation in MCF-7 cells; this was comparable to the findings of other studies that reported CSC proportions of 0–3% for this cell line. MCF-7 cell populations rather had a high proportion (approximately 99%) of CD24+ cells, consistent with its luminal characteristics (Figure 1(a) and (b)).25,26
Figure 1.
Characteristic differences between MCF-7 and MDA MB 231 cells. (a) Representative flow cytometry and (b) quantitative analyses showing the proportions of breast cancer stem cells (CD44+/CD24−) in MCF-7 and MDA-MB-231 cells. (c) Depicting differences in tube formation ability between MCF-7 and MDA-MB-231 breast cancer cells cultured under similar conditions (in monolayer and Matrigel cultures). MDA-MB-231 cells exhibit vasculogenic properties when cultured on Matrigel. Confocal microscopy images were acquired using an LSM 510 confocal microscope equipped with a 10 × objective and using 70 × magnification. Cells seeded on Matrigel-coated 24-well plates were stained with calcein AM approximately 10 h after plating. (A color version of this figure is available in the online journal.)
Next, we assessed tube formation in MDA-MB-231 and MCF-7 cell lines. Previous studies showed that the highly invasive MDA-MB-231 cells, but not the poorly invasive MCF-7 cells, form tube-like structures27; our studies confirmed these findings. Although MDA-MB-231 and MCF-7 cells were cultured under identical experimental conditions, it was observed that MDA-MB-231 cells developed capillary-like networks; however MCF-7 cells formed clusters on Matrigel surfaces (Figure 1(c)). These data suggest that the vasculogenic properties of breast cancer cells are due to their inherent phenotypic properties and possibly related to the stem-like nature of the cells, in agreement with findings in other tumors.23
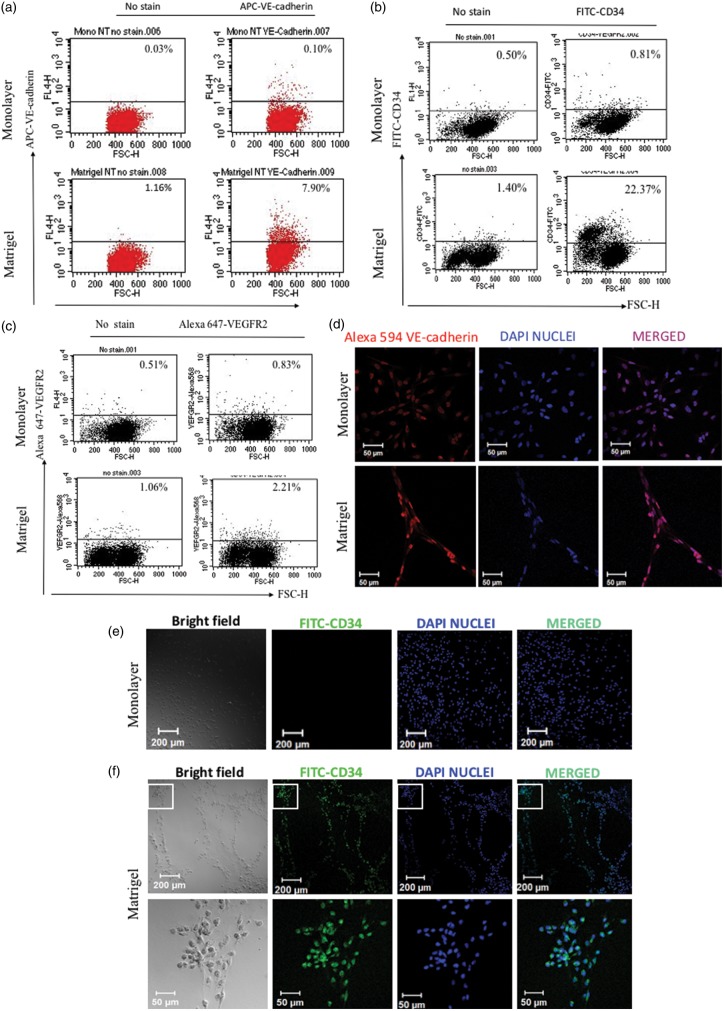
MDA-MB-231 cells express VE-cadherin in both monolayer and Matrigel cultures but express CD34 only in Matrigel cultures
Previous studies with ovarian CSCs have shown that VM is accompanied by the expression of VE-cadherin, an endothelial marker and CD34, an endothelial progenitor marker22; however, to our knowledge, no studies have determined whether this is also a feature of breast cancer cells. We assessed the expression of the endothelial markers VE-cadherin, CD34, and VEGFR2 in breast cancer cells grown as monolayers and compared the results to those obtained using cells grown on Matrigel that had undergone VM. FACS analyses revealed that there was increased cell surface expression of VE-cadherin (∼5%) and CD34 (∼20%), and a negligible increase of VEGFR2+ cells, following growth on Matrigel compared with monolayer cultures (Figure 2(a) to (c)). Further analyses by immunocytochemistry showed that VE-cadherin was expressed at the cytoplasmic level in both monolayer and Matrigel cultures, whereas CD34 was expressed only in Matrigel cultures (Figure 2(d) to (f)). Taken together, our results indicate that MDA-MB-231 cells have the ability to acquire increased endothelial characteristics during VM, and that CD34 and VE-cadherin might be required for this process.
Figure 2.
Analyses of VE-cadherin and CD34 expression in monolayer and Matrigel cultures. Flow cytometric analyses of (a) VE-cadherin, (b) CD34, and (c) VEGFR2 expressions in monolayer and Matrigel cultures. Immunocytochemical analyses of expression of (d) VE-cadherin, (e) and (f) CD34 in cells grown on monolayer and Matrigel cultures, respectively. Confocal images were acquired using an LSM 700 microscope equipped with a 10 × objective lens. Magnifications are indicated by the scale bar
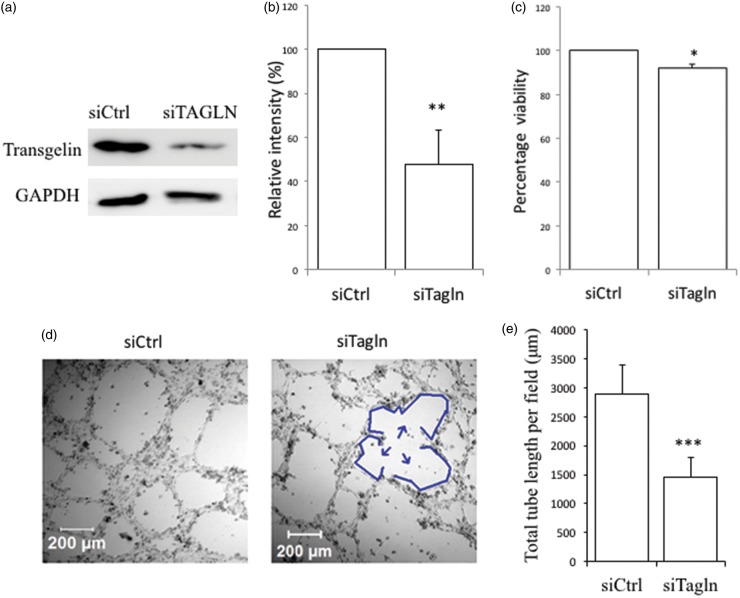
Proliferation and VM are suppressed by transgelin knockdown in MDA-MB-231 cells
Transgelin is a cytoskeletal protein implicated in a variety of cancer-related processes such as cell proliferation, migration, invasion, and metastasis.12 To investigate the role of transgelin in VM, we employed siRNA-based silencing approaches and confirmed efficient knockdown of transgelin through western blot and densitometry analyses (Figure 3(a) and (b)). We determined the effect of transgelin knockdown on proliferation and found a modest (∼10%) but statistically significant reduction in the growth of transgelin-depleted cells compared with that of control cell populations (P < 0.05, Figure 3(c)). More importantly, decreasing transgelin expression significantly reduced the ability of cancer cells to form tube-like structures (P < 0.001). Specifically, whereas control cells typically developed complete loops, transgelin silencing was associated with fragmentation of capillary-like networks formed by the cells (Figure 3(d) and (e)). Taken together, these data suggest that transgelin is required for both cell proliferation and tube formation.
Figure 3.
Transgelin knockdown decreases the proliferative and vasculogenic abilities of MDA-MB-231 cells. The efficiency of transgelin (Tagln) knockdown was assessed by (a) Western blot and (b) densitometry analyses. (c) Downregulation of transgelin reduces cellular proliferation. (d) Tube formation was inhibited by silencing transgelin. Cells were transfected as indicated and 48 h later, cells were seeded on 24-well Matrigel-coated plates. After approximately 10 h, images were acquired using a META 510 confocal microscope equipped with a 10 × objective lens and using 70 × magnification. Arrows indicate regions of fragmentation in network formation. (e) Quantified total tube lengths obtained from an average of four random fields per well (*P < 0.05, ** P < 0.01, *** P < 0.001). (A color version of this figure is available in the online journal.)
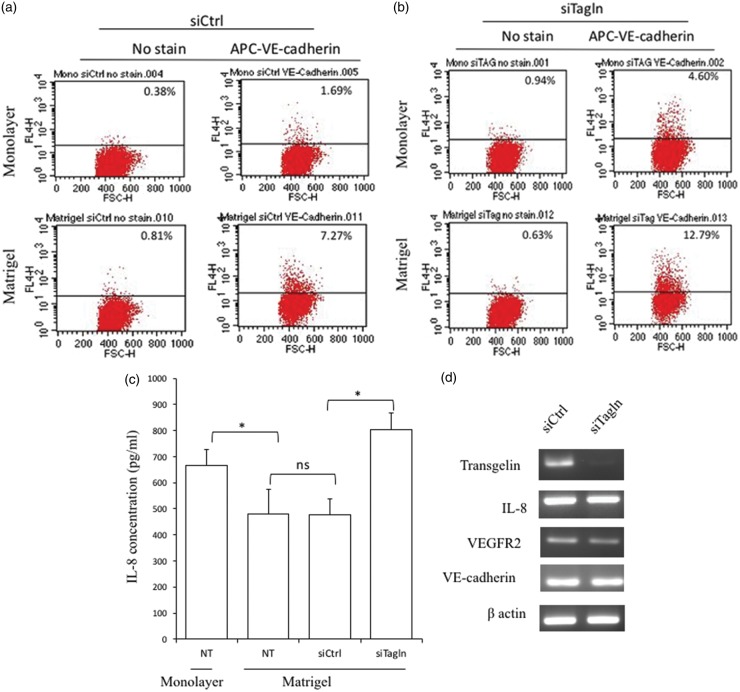
Effect of transgelin knockdown on the levels of angiogenesis-related and endothelial markers
Next, we investigated the effect of transgelin knockdown on the expression of VE-Cadherin in MDA-MB-231 cells grown as monolayers versus cells that had developed vascular networks in response to growth on Matrigel. Flow cytometry analyses revealed that VE-cadherin expression increased following growth on Matrigel regardless of transgelin expression (Figure 4(a) and (b)).
Figure 4.
Effect of transgelin knockdown on the levels of angiogenesis-related and endothelial markers. Flow cytometric analysis of VE-cadherin expression in monolayer and Matrigel cultures of (a) control siRNA-transfected cells (siCtrl) and (b) transgelin siRNA-transfected cells (siTagln). (c) Levels of IL-8 secreted by non-transfected cells grown as monolayers and on Matrigel as well as control siRNA-transfected and transgelin knockdown cells grown on Matrigel. Supernatants were harvested after a 10 h-incubation (*P < 0.05; n.s.: not statistically significant). (d) RT-PCR analysis of the effect of transgelin downregulation on the expression of IL-8 and endothelial markers. (A color version of this figure is available in the online journal.)
IL-8 (CXCL8) is a chemokine known to promote tumor angiogenesis and metastasis.28 A number of studies have linked tumor IL-8 expression to proliferation, migration, invasion, and angiogenesis.29 In addition, IL-8 signaling is known to affect MMP-2, which has been implicated in VM.9,18,19 Moreover, the highly invasive MDA-MB-231 cells are known to secrete IL-8.30,31 We determined the levels of IL-8 secreted by cells grown as monolayers and on Matrigel, and assessed the impact of transgelin on this process. ELISA data (Figure 4(c)) showed that IL-8 secretion was generally lower in cultures with a more prominent vascular network, in agreement with previous studies on ovarian cancer cells.22 Indeed, secretion of IL-8 by highly invasive MDA-MB-231 cells was significantly higher when cells were grown as monolayers compared with that in Matrigel cultures that typically develop VM (P = 0.045). In addition, transgelin knockdown, which decreased vasculogenic activity in cells grown on Matrigel, led to significantly higher IL-8 levels compared with that in control Matrigel cultures (P = 0.034) (Figure 4(c)). Thus, the data are consistent with a model, whereby IL-8 participates in transgelin-regulated autocrine signaling events required for VM.
Subsequently, we determined whether downregulation of transgelin affected the expression of IL-8 and endothelial markers at the mRNA level. We could not detect changes in the transcript levels of IL-8 and VE-cadherin by RT-PCR analysis, whereas we found VEGFR2 expression was slightly downregulated by knockdown of transgelin (Figure 4(d)), confirming that transgelin is not directly associated with the expression of IL-8 and VE-cadherin. We could not detect expression of CD34 in both the control (siCtrl) and transgelin knockdown (siTagln) monolayer samples, confirming that monolayer cultures of MDA-MB-231 cells do not express CD34 (data not shown). Taken together, our results indicate that MDA-MB-231 cells have the ability to acquire endothelial characteristics independent of transgelin during VM.
Blocking CXCR2 receptor signaling impairs proliferation and VM and leads to increased IL-8 levels
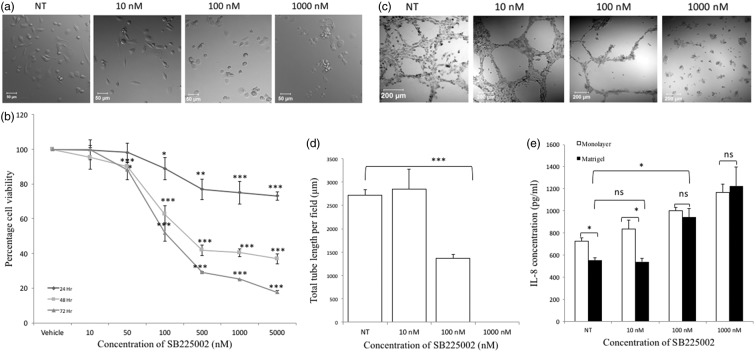
Apart from expressing IL-8, MDA-MB-231 cells have also been reported to express its cognate receptors CXCR1/CXCR2. In addition, autocrine IL-8/receptor signaling during the growth of TN breast cancer cells, including MDA-MB-231, is well established.32 CXCR2 is known to act as a scavenger for its ligands, and as such, blocking CXCR2 can lead to increased ligand levels.33 We hypothesized that decreased IL-8 levels during VM was due to enhanced IL-8/CXCR2 autocrine signaling. To verify that decreased levels in IL-8 during VM formation was due to uptake by MDA-MB-231 cells, we blocked IL-8 uptake through CXCR2 by treating the cells with SB225002, a selective non-peptide CXCR2 antagonist, with >150-fold selectivity for CXCR2 over CXCR1.28 Treatment with the CXCR2 inhibitor resulted in morphological changes to cells in monolayer culture, wherein cells lost their spindle-like morphology and became more rounded, in a dose-dependent manner (Figure 5(a)). Consistent with studies in other cancer cells, SB225002 inhibited proliferation of cells in a dose- and time-dependent manner (Figure 5(b)).34,35 As expected, SB225002 inhibited tube formation in a dose-dependent fashion, with a statistical significance of P < 0.001 at 100 nM; no tube formation was observed at a concentration of 1000 nM, and more importantly, this correlated with increased IL-8 levels (Figure 5(c) to (e)). The concentration of IL-8 in monolayer cultures also increased with increasing doses of the SB225002 suggesting a correlation between IL-8 uptake and normal cellular functions such as proliferation (Figure 5(e)). Altogether, the data confirmed an increase in IL-8 uptake during VM formation in an autocrine manner and that IL-8/CXCR2 signaling was necessary for tube formation, which correlated with increased IL-8 levels.
Figure 5.
The CXCR2 antagonist SB225002 decreases proliferation, impairs vaculogenic mimicry formation and blocks the uptake of IL-8 in cells in a dose-dependent fashion. (a) MDA-MB-231 cells change from being spindle-like to a rounded morphology with increasing concentrations of SB225002. (b) Proliferation in the cells decreased in a time and concentration-dependent manner. (c) SB225002 suppressed vasculogenic mimicry. (d) Quantified tube length showing the effect of SB2225002 on vasculogenic mimicry. (e) Levels of IL-8 in the monolayer and Matrigel cultures increased in a dose-dependent fashion (*P < 0.05; ** P < 0.01; ***P < 0.001; n.s.: not statistically significant)
Discussion
VM, the ability of tumor cells to undergo vessel formation de novo, has been described in multiple cancers (reviewed in Fan et al.6). Findings from previous studies show that VM is associated with aggressive tumors and poor patient prognoses. Breast tumors lacking expression of estrogen, progesterone, and HER2 receptors, commonly referred to as TN, are known for their high aggressiveness.9,36 Kaplan-Meier analyses have shown lower overall survival for TN breast cancer patients compared with non-TN groups.9,37 Importantly, VM was proportionately higher in TN breast cancers than in tumors classified as luminal types A and B. Similar to previous studies, our results show that TN MDA-MB-231 cells exhibit VM, whereas MCF-7 cells do not, possibly reflecting differences in the malignant properties of tumors from which these cells were derived.27 Our FACS analyses revealed that CSCs accounted for greater than 90% of the MDA-MB-231 population, in contrast to MCF-7 cells, which had 0% CSCs; this was comparable to results of other studies.25,26
The sharp contrast in CSC subpopulation content observed when we compared the two cell lines might also account for differences in vasculogenic properties, since CSCs, described as a tumor cell population characterized by chemoresistance, the ability to initiate tumors, and self-renewal, have been found to be involved in VM.6 For instance, Alvero et al. reported that the CSC subpopulation of ovarian tumor cells, but not non-stem cells, could contribute to tumor neovascularization through VM.22 In addition, studies on other forms of malignancies, including glioblastoma, also provided support for CSC-mediated VM.23,24 Thus, our data are consistent with findings from others pointing to a strong relationship between VM and CSCs.
Understanding both similarities and differences between VM and angiogenesis would be beneficial for the development of antivascular chemotherapeutic agents. Studies by Alvero et al. showed that ovarian cancer cells that had undergone VM expressed endothelial-related markers such as CD34 and VE-cadherin.22 Similarly, Liu et al. reported that holoclone cells that had VM capabilities expressed VE-cadherin, MMP-2, and MMP-9.9 Findings from the present study also showed that the endothelial progenitor marker CD34 as well as VE-cadherin were expressed by cells that had undergone VM.
Indeed, VE-cadherin, a cell–cell adhesion molecule originally found on endothelial cells, is also expressed by invasive melanoma and some breast cancer cells.38–40 Expression of VE-cadherin has also been implicated in VM in hepatocarcinoma cells wherein reduced expression (resulting from knockdown of TWIST) led to decreased VM.41
CD34 is an endothelial cell and hematopoietic stem cell progenitor marker implicated in lumen formation during angiogenesis. Previous work reported that CD34+ cells migrate to, proliferate, and participate in capillary formation and, with CD34− cells, enhance tube formation in three-dimensional matrices. These results suggested that administration of CD34+-enriched cells might significantly improve neovascularization.42,43 The first step in lumen formation involves endothelial cell–cell adhesion. Studies focused on the development of mouse aortas showed that CD34-sialomucins, along with moesin and F-actin, localize to endothelial cell-to-cell contact points, and that VE-cadherin is necessary for localization of CD34.44,45 These observations could account for the enhanced expression of CD34 and VE-cadherin during VM in our studies.
Transgelin is an actin-binding protein associated with cancer-related processes such as proliferation, migration, invasion, and metastasis. Differential transgelin expression has been associated with multiple tumors, but there are conflicting reports regarding its impact on disease progression. For example, some studies reported transgelin downregulation in colorectal and breast carcinomas.46,47 In contrast, others found positive correlations between transgelin expression and malignancy in hepatocarcinoma, breast, and colorectal cancers, among others. Lee et al. also demonstrated that transgelin expression was higher in CSCs and that it promoted migration and invasiveness, thus enhancing metastasis in hepatocarcinoma. In a recent study, transgelin was shown to be more highly expressed in TN breast cancer samples compared with that in luminal-type tumors, suggesting its association with malignancy.13,15,48 These findings, combined with the observation that VM was highest among TN cancer patients, suggest positive correlations between transgelin and VM in breast tumors. This possibility is supported by our observation that siRNA-mediated knockdown of transgelin inhibited the proliferative and vasculogenic properties of breast cancer cells.
Epithelial-to-mesenchymal transition (EMT) has been implicated in VM.24 Lin et al. reported that transgelin downregulation resulted in a reduction in the expression of some EMT markers such as vimentin and fibronectin-1.13,15,48 Thus, one of the possible mechanisms through which transgelin affects VM is via EMT. Future studies are required to elucidate the mechanisms through which transgelin is involved in VM, and to further evaluate these relationships in animal models of breast cancer and in human tumor samples.
IL-8 expression in breast cancer cells is strongly correlated with invasiveness and is inversely linked to estrogen receptor (ER) status. IL-8 is expressed and secreted by ER-negative MDA-MB-231 cells but is essentially undetectable in ER-positive MCF-7 cells. In addition, silencing of IL-8 in breast cancer cells inhibited invasion.35 MDA-MB-231 cells have also been reported to express the cognate receptors for IL-8 CXCR1/CXCR2.32
Our studies revealed decreased levels of IL-8 in Matrigel-cultured cells that underwent tube formation, when compared with that in monolayer cultures. In addition, silencing transgelin, which impaired VM in Matrigel cultures, increased IL-8 levels. Furthermore, inhibiting IL-8/CXCR2 signaling resulted in the inhibition of VM, which was accompanied by increased IL-8 levels in conditioned medium. Indeed IL-8/CXCR2 signaling has been reported to be involved in proliferation, invasion, and cancer progression.35 However, to the best of our knowledge, this is the first study reporting the effect of inhibiting IL-8/CXCR2 signaling on VM. Since IL-8 is known to have autocrine and paracrine signaling properties, our data provide strong evidence for the role of IL-8/CXCR2 autocrine signaling during VM.49
CXCR2 is a G-protein-coupled receptor that has been found to be associated with tumor proliferation, progression, and invasion. The selective non-peptide CXCR2 antagonist, SB225002, also known as (N-(2-hydroxy-4-nitrophenyl)-N′- (2-bromophenyl) urea), acts by inhibiting the binding of IL-8 to CXCR2.50,51 From our data, treatment with SB225002 inhibited proliferation and VM, which was correlated with increased IL-8 levels.
A number of signaling pathways, including the PI3K/Akt/mTOR, PLC/PKC, and MAPK/ERK axes, are activated following IL-8-mediated engagement of its receptors.28 Some of these pathways, such as PI3K/Akt and ERK have been associated with VM.24 Thus, the inhibition of CXCR2 likely affects VM through these signaling pathways. Additional studies are necessary to elucidate the mechanisms by which IL-8/CXCR2 contributes to tube formation in cancer cells.
In conclusion, our work shows that transgelin downregulation impairs the vasculogenic abilities of breast cancer cells and might interfere with IL-8 signaling during this process. We also report that MDA-MB-231 cells have the ability to acquire endothelial characteristics during tube formation and that this might be independent of transgelin expression. Few studies have investigated whether transgelin affects the vascular properties of tumor cells. Thus, our findings represent an additional mechanism, whereby transgelin contributes to the malignancy of cancer cells. Additional studies are necessary to dissect the molecular mechanisms whereby transgelin regulates VM; such studies have the potential to favorably impact the future development of effective antivascular cancer therapeutics.
Acknowledgements
The study was supported by a grant from the School of Life Sciences and Biotechnology for BK21 PLUS, Korea University and Korea University Grant. Work was also supported by a grant from KEIT, Korea #10047890. The authors would like to thank Ms. Hyun-Joo Park and Ms. Jee-Hyun Lee for technical assistance with confocal microscopy and flow cytometry, respectively.
Authors’ contributions
ARA, BR, MJK and CWK were involved in the study design. ARA conducted most of the experiments and drafted the manuscript. ARA and MJK were involved in data analysis and final revision of the manuscript. ARA and MJK contributed equally to the study.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Hillen F, Griffioen AW. Tumour vascularization: sprouting angiogenesis and beyond. Cancer Metastasis Rev 2007; 26: 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 3.Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol 2005; 23: 1782–90. [DOI] [PubMed] [Google Scholar]
- 4.Servick K. Breast cancer. Breast cancer: a world of differences. Science 2014; 343: 1452–3. [DOI] [PubMed] [Google Scholar]
- 5.Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe’er J, Trent JM, Meltzer PS, Hendrix MJ. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am J Path 1999; 155: 739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan YL, Zheng M, Tang YL, Liang XH. A new perspective of vasculogenic mimicry: EMT and cancer stem cells (Review). Oncol Lett 2013; 6: 1174–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat Rev Cancer 2003; 3: 411–21. [DOI] [PubMed] [Google Scholar]
- 8.Hendrix MJ, Seftor EA, Kirschmann DA, Seftor RE. Molecular biology of breast cancer metastasis. Molecular expression of vascular markers by aggressive breast cancer cells. Breast Cancer Res 2000; 2: 417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu TJ, Sun BC, Zhao XL, Zhao XM, Sun T, Gu Q, Yao Z, Dong XY, Zhao N, Liu N. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene 2013; 32: 544–53. [DOI] [PubMed] [Google Scholar]
- 10.Francescone R, Scully S, Bentley B, Yan W, Taylor SL, Oh D, Moral L, Shao R. Glioblastoma-derived tumor cells induce vasculogenic mimicry through Flk-1 protein activation. J Biol Chem 2012; 287: 24821–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Schaft DW, Seftor RE, Seftor EA, Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW, Hendrix MJ. Effects of angiogenesis inhibitors on vascular network formation by human endothelial and melanoma cells. J Natl Cancer Inst 2004; 96: 1473–7. [DOI] [PubMed] [Google Scholar]
- 12.Dvorakova M, Nenutil R, Bouchal P. Transgelins, cytoskeletal proteins implicated in different aspects of cancer development. Expert Rev Proteomics 2014; 11: 149–65. [DOI] [PubMed] [Google Scholar]
- 13.Lee EK, Han GY, Park HW, Song YJ, Kim CW. Transgelin promotes migration and invasion of cancer stem cells. J Proteome Res 2010; 9: 5108–17. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Zhang R, Zhang L, Sun Y, Yao W, Zhao A, Li J, Yuan Y. Upregulation of transgelin is an independent factor predictive of poor prognosis in patients with advanced pancreatic cancer. Cancer Sci 2013; 104: 423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rao D, Kimler BF, Nothnick WB, Davis MK, Fan F, Tawfik O. Transgelin: a potentially useful diagnostic marker differentially expressed in triple-negative and non-triple-negative breast cancers. Hum Pathol 2015; 46: 876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang Z, Brooks J, Willard M, Liang K, Yoon Y, Kang S, Shim H. CXCR4/CXCL12 axis promotes VEGF-mediated tumor angiogenesis through Akt signaling pathway. Biochem Biophys Res Commun 2007; 359: 716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nair RR, Solway J, Boyd DD. Expression cloning identifies transgelin (SM22) as a novel repressor of 92-kDa type IV collagenase (MMP-9) expression. J Biol Chem 2006; 281: 26424–36. [DOI] [PubMed] [Google Scholar]
- 18.Liu WB, Xu GL, Jia WD, Li JS, Ma JL, Chen K, Wang ZH, Ge YS, Ren WH, Yu JH, Wang W, Wang XJ. Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma. Med Oncol 2011; 28(Suppl 1): S228–38. [DOI] [PubMed] [Google Scholar]
- 19.Kirschmann DA, Seftor EA, Hardy KM, Seftor RE, Hendrix MJ. Molecular pathways: vasculogenic mimicry in tumor cells: diagnostic and therapeutic implications. Clin Cancer Res 2012; 18: 2726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Ye Y, Shen D, Jiang K, Zhang H, Sun W, Zhang J, Xu F, Cui Z, Wang S. Identification of transgelin-2 as a biomarker of colorectal cancer by laser capture microdissection and quantitative proteome analysis. Cancer Sci 2010; 101: 523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao Y, Li Y, Han J, Pan Y, Tie L, Li X. Transgelin 2 participates in lovastatin-induced anti-angiogenic effects in endothelial cells through a phosphorylated myosin light chain-related mechanism. PloS One 2012; 7: e46510–e46510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alvero AB, Fu HH, Holmberg J, Visintin I, Mor L, Marquina CC, Oidtman J, Silasi DA, Mor G. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells 2009; 27: 2405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ricci-Vitiani L, Pallini R, Biffoni M, Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G, Larocca LM, De Maria R. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature 2010; 468: 824–8. [DOI] [PubMed] [Google Scholar]
- 24.Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu D, Yu X, Tian Y. Advanced research on vasculogenic mimicry in cancer. J Cell Mol Med 2015; 19: 315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer MJ, Fleming JM, Ali MA, Pesesky MW, Ginsburg E, Vonderhaar BK. Dynamic regulation of CD24 and the invasive, CD44posCD24neg phenotype in breast cancer cell lines. Breast Cancer Res 2009; 11: R82–R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fillmore CM, Kuperwasser C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res 2008; 10: R25–R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basu GD, Liang WS, Stephan DA, Wegener LT, Conley CR, Pockaj BA, Mukherjee P. A novel role for cyclooxygenase-2 in regulating vascular channel formation by human breast cancer cells. Breast Cancer Res 2006; 8: R69–R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campbell LM, Maxwell PJ, Waugh DJ. Rationale and means to target pro-inflammatory interleukin-8 (CXCL8) signaling in cancer. Pharmaceuticals (Basel) 2013; 6: 929–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ning Y, Manegold PC, Hong YK, Zhang W, Pohl A, Lurje G, Winder T, Yang D, LaBonte MJ, Wilson PM, Ladner RD, Lenz HJ. Interleukin-8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer 2011; 128: 2038–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freund A, Chauveau C, Brouillet JP, Lucas A, Lacroix M, Licznar A, Vignon F, Lazennec G. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene 2003; 22: 256–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freund A, Jolivel V, Durand S, Kersual N, Chalbos D, Chavey C, Vignon F, Lazennec G. Mechanisms underlying differential expression of interleukin-8 in breast cancer cells. Oncogene 2004; 23: 6105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, Zhang Y, Mazumdar A, Hilsenbeck SG, Mills GB, Brown PH. Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res 2013; 73: 3470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma B, Nannuru KC, Varney ML, Singh RK. Host Cxcr2-dependent regulation of mammary tumor growth and metastasis. Clin Exp Metastasis 2015; 32: 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Vasconcellos JF, Laranjeira AB, Leal PC, Bhasin MK, Zenatti PP, Nunes RJ, Yunes RA, Nowill AE, Libermann TA, Zerbini LF, Yunes JA. SB225002 induces cell death and cell cycle arrest in acute lymphoblastic leukemia cells through the activation of GLIPR1. PLoS One 2015; 10: e0134783–e0134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh JK, Farnie G, Bundred NJ, Simoes BM, Shergill A, Landberg G, Howell SJ, Clarke RB. Targeting CXCR1/2 significantly reduces breast cancer stem cell activity and increases the efficacy of inhibiting HER2 via HER2-dependent and -independent mechanisms. Clin Cancer Res 2013; 19: 643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature 2000; 406: 747–52. [DOI] [PubMed] [Google Scholar]
- 37.Zhang D, Sun B, Zhao X, Ma Y, Ji R, Gu Q, Dong X, Li J, Liu F, Jia X, Leng X, Zhang C, Sun R, Chi J. Twist1 expression induced by sunitinib accelerates tumor cell vasculogenic mimicry by increasing the population of CD133+ cells in triple-negative breast cancer. Mol Cancer 2014; 13: 207–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bach TL, Barsigian C, Chalupowicz DG, Busler D, Yaen CH, Grant DS, Martinez J. VE-cadherin mediates endothelial cell capillary tube formation in fibrin and collagen gels. Exp Cell Res 1998; 238: 324–34. [DOI] [PubMed] [Google Scholar]
- 39.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, Schatteman GC, Seftor RE. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA 2001; 98: 8018–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labelle M, Schnittler HJ, Aust DE, Friedrich K, Baretton G, Vestweber D, Breier G. Vascular endothelial cadherin promotes breast cancer progression via transforming growth factor beta signaling. Cancer Res 2008; 68: 1388–97. [DOI] [PubMed] [Google Scholar]
- 41.Sun T, Zhao N, Zhao XL, Gu Q, Zhang SW, Che N, Wang XH, Du J, Liu YX, Sun BC. Expression and functional significance of Twist1 in hepatocellular carcinoma: its role in vasculogenic mimicry. Hepatology 2010; 51: 545–56. [DOI] [PubMed] [Google Scholar]
- 42.Sidney LE, Branch MJ, Dunphy SE, Dua HS, Hopkinson A. Concise review: evidence for CD34 as a common marker for diverse progenitors. Stem Cells 2014; 32: 1380–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rookmaaker MB, Verhaar MC, Loomans CJ, Verloop R, Peters E, Westerweel PE, Murohara T, Staal FJ, van Zonneveld AJ, Koolwijk P, Rabelink TJ, van Hinsbergh VW. CD34+ cells home, proliferate, and participate in capillary formation, and in combination with CD34- cells enhance tube formation in a 3-dimensional matrix. Arterioscler Thromb Vasc Biol 2005; 25: 1843–50. [DOI] [PubMed] [Google Scholar]
- 44.Nelson KS, Beitel GJ. More than a pipe dream: uncovering mechanisms of vascular lumen formation. Dev Cell 2009; 17: 435–7. [DOI] [PubMed] [Google Scholar]
- 45.Strilic B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell 2009; 17: 505–15. [DOI] [PubMed] [Google Scholar]
- 46.Zhao L, Wang H, Deng YJ, Wang S, Liu C, Jin H, Ding YQ. Transgelin as a suppressor is associated with poor prognosis in colorectal carcinoma patients. Mod Pathol 2009; 22: 786–96. [DOI] [PubMed] [Google Scholar]
- 47.Shields JM, Rogers-Graham K, Der CJ. Loss of transgelin in breast and colon tumors and in RIE-1 cells by Ras deregulation of gene expression through Raf-independent pathways. J Biol Chem 2002; 277: 9790–9. [DOI] [PubMed] [Google Scholar]
- 48.Lin Y, Buckhaults PJ, Lee JR, Xiong H, Farrell C, Podolsky RH, Schade RR, Dynan WS. Association of the actin-binding protein transgelin with lymph node metastasis in human colorectal cancer. Neoplasia 2009; 11: 864–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li A, Varney ML, Valasek J, Godfrey M, Dave BJ, Singh RK. Autocrine role of interleukin-8 in induction of endothelial cell proliferation, survival, migration and MMP-2 production and angiogenesis. Angiogenesis 2005; 8: 63–71. [DOI] [PubMed] [Google Scholar]
- 50.Du M, Qiu Q, Gruslin A, Gordon J, He M, Chan CC, Li D, Tsang BK. SB225002 promotes mitotic catastrophe in chemo-sensitive and -resistant ovarian cancer cells independent of p53 status in vitro. PLoS One 2013; 8: e54572–e54572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.White JR, Lee JM, Young PR, Hertzberg RP, Jurewicz AJ, Chaikin MA, Widdowson K, Foley JJ, Martin LD, Griswold DE, Sarau HM. Identification of a potent, selective non-peptide CXCR2 antagonist that inhibits interleukin-8-induced neutrophil migration. J Biol Chem 1998; 273: 10095–8. [DOI] [PubMed] [Google Scholar]