Abstract
Non-alcoholic steatohepatitis (NASH) affects 8–10 million people in the US and up to 75% of obese individuals. Despite this, there are no approved oral therapeutics to treat NASH and therefore the need for novel approaches exists. The estrogen receptor β (ER-β)-selective agonist, β-LGND2, inhibits body weight and white adipose tissue, and increases metabolism, resulting in higher energy expenditure and thermogenesis. Due to favorable effects of β-LGND2 on obesity, we hypothesized that β-LGND2 will prevent NASH directly by reducing lipid accumulation in the liver or indirectly by favorably changing body composition. Male C57BL/6 mice fed with high fat diet (HFD) for 10 weeks or methionine choline-deficient diet for four weeks and treated with vehicle exhibited altered liver weights by twofold and increased serum transaminases by 2–6-folds. These changes were not observed in β-LGND2-treated animals. Infiltration of inflammatory cells and collagen deposits, an indication of fibrosis, were observed in the liver of mice fed with HFD for 10 weeks, which were effectively blocked by β-LGND2. Gene expression studies in the liver indicate that pregnane X receptor target genes were significantly increased by HFD, and the increase was inhibited by β-LGND2. On the other hand, metabolomics indicate that bile acid metabolites were significantly increased by β-LGND2. These studies demonstrate that an ER-β agonist might provide therapeutic benefits in NASH by directly modulating the function of xenobiotic and bile acid receptors in the liver, which have important functions in the liver, and indirectly, as demonstrated before, by inhibiting adiposity.
Impact statement
Over 75–90% of those classified as clinically obese suffer from co-morbidities, the most common of which is non-alcoholic steatohepatitis (NASH). While there are currently no effective treatment approaches for NASH, data presented here provide preliminary evidence that an estrogen receptor β-selective ligand could have the potential to reduce lipid accumulation and inflammation, and protect liver from NASH.
Keywords: Non-alcoholic steatohepatitis, non-alcoholic fatty liver disease, obesity, liver cirrhosis, inflammation, metabolic diseases, estrogen receptor β
Introduction
Non-alcoholic fatty liver disease (NAFLD), which affects 27–34% of the general U.S. population and about 75–90% of the morbidly obese population, is characterized principally by microvesicular steatosis that occurs in the absence of alcohol consumption.1,2 NAFLD is a broader descriptor for conditions that include non-alcoholic fatty liver and non-alcoholic steatohepatitis (NASH). Approximately 10 million people in the U.S. have progressed to NASH and 600,000 people to cirrhosis presenting this as the largest risk factor for developing liver cancer.2 Approximately, 30–50% of the patients with NASH progress to fibrosis in five years.3,4 NASH progresses into fibrosis and cirrhosis with five- and 10-year survival rates estimated at 67 and 59%, respectively.5 Although the progression of NASH is typically slow, the accompanying incidence of fibrosis and cirrhosis is high and the prognosis is unfavorable for these patients. The primary risk factors for NASH are obesity and metabolic disease. NASH is characterized histologically by cytological ballooning, Mallory’s hyaline (inclusion bodies found in liver cells), scattered inflammation, and pericellular fibrosis.6 Biochemically, patients with NASH demonstrate an increase in serum transaminases (alanine transaminase [ALT] and aspartate transaminase [AST]), triglycerides, and fatty acids, and develop insulin resistance. Although no approved or effective treatments for NASH are available, some alternatives such as dietary supplements and liver transplant are recommended.
The functions of estrogens are mediated by two estrogen receptors (ER), ER-α and ER-β. While ER-α promotes the actions of estradiol in breast, uterus, and bone, ER-β plays a role in diverse tissues, including the prostate, heart, and lungs.7–10 Although the isoforms responsible for the function in several tissues have been elucidated, the isoform that is responsible for the effects on metabolic diseases and obesity is still unclear.11–13 Evidence supporting the protective actions of estrogens during adipogenesis and lipid homeostasis also arise from comparisons drawn between pre- and postmenopausal women.14,15 Premenopausal women are protected from fat accumulation and body weight gain, while postmenopausal women exhibit weight gain, fat accumulation, liver inflammation, oxidative stress, and steatosis.16–18
The advantage of using ER-β-selective agonists to treat NASH or NAFLD is to monetize the beneficial effects of ER-β without the accompanying ER-α-associated side effects principally in the breast, prostate, and uterus. Earlier studies from our group showed that one of the ER-β-selective agonists belonging to the isoquinolinone scaffold, β-LGND2, prevented high fat diet (HFD) induced body weight and adipose tissue gain, without affecting food consumption by acting peripherally by converting white adipose tissue (WAT) to brown adipose tissue (BAT).19,20 This conversion of WAT to BAT by β-LGND2 resulted in an increase in thermogenesis and oxygen consumption, all culminating in weight loss. β-LGND2 was more effective than some of the commercial drugs used as comparator.20
Due to the favorable changes promoted by β-LGND2 on body weight and composition, we expected β-LGND2 to effectively reduce lipid accumulation in the liver of preclinical species. To test this hypothesis, we evaluated β-LGND2 in two preclinical models of fatty liver disease21,22 and performed studies to understand the underlying mechanisms of action. The results provide evidence that β-LGND2 has the potential to protect the liver from pathological changes promoted by modified dietary conditions.
Materials and methods
Animal studies were conducted in accordance with the University of Tennessee IACUC approved protocol. Animals were quarantined for three days before initiation of any experiment. Animals were maintained in a 12 h light:dark cycle. Animals were provided with food and water ad libitum. If unexpected mortalities in large numbers occurred in any single experiment, the information were brought to the attention of the Veterinarian and the IACUC committee and any remedial recommendations were immediately adopted.
HFD study
Male C57BL/six mice (n = 5/group) of four weeks of age were obtained from Harlan Laboratories (Harlan, IN). The animals were divided into groups and were fed with either regular rodent chow (normal diet [CON]) or HFD (Harlan, IN).19,20 The composition and characteristics of the diets are provided in Table 1. The study consisted of three groups, with one group received CON, while two groups received HFD. The CON group and one of the HFD-fed groups received vehicle (15% DMSO + 85% polyethylene glycol [PEG-300]), while the other HFD-fed group received β-LGND2 at 30 mg/kg/day from the initiation of the study. All treatments were delivered subcutaneously (s.c.). β-LGND2 (GTx, Inc., Memphis, TN) was selected from a library of ER-β-selective agonists, belonging to the isoquinolinone backbone.19,20 β-LGND2 binds and activates ER-β with a 100-fold selectivity over ER-α. Our earlier publication demonstrated that β-LGND2 reduced body weight and fat mass gain in mice fed with a HFD.19 β-LGND2 was synthesized internally and the purity and structural integrity were verified. The dose of 30 mg/kg/day s.c. was selected based on metabolism and pharmacokinetic (PK) properties of β-LGND2. The half-life of β-LGND2 in human and mice liver microsomes was 3 and 4.2 min, respectively (unpublished data). The PK properties represented as area under the curve of 30 mg/kg β-LGND2 in mice was ∼200 min µg/mL (unpublished data). Weekly body weight, food consumption, and body composition by MRI (EchoMRI, Houston, TX) were recorded. Body weight and body composition data from the experiments described in the manuscript were presented in our previous publication.20 CON diet-fed mice consumed 173 ± 12 g/seven animals/week, while the HFD-fed vehicle-treated and β-LGND2-treated mice consumed 105 ± 17 g/seven mice/week and 111 ± 8 g/seven mice/week, respectively. At the end of 10 weeks, the animals were sacrificed and blood and tissues were collected for gene expression, histology, and metabolomics. Animals were fasted for 12–16 h before sacrifice. Serum was separated from blood and ALT and AST were measured using kits (Sigma, St. Louis, MO).
Table 1.
Composition of diet used in the study
| Diet | Control | HFD | MCD |
|---|---|---|---|
| Vendor | Teklad | Teklad | Teklad |
| Catalog number | 7012 | 06414 | 90,262 |
| Fat (%) | 5.8 | 34.3 | 10 |
| Carbohydrate (%) | 44.3 | 27.3 | 64.3 |
| Protein (%) | 19.1 | 23.5 | 14.9 |
| Energy (kcal/g) | 3.1 | 5.1 | 4.1 |
| Other characteristics of the diet | Lacks methionine and choline; 40% sucrose | ||
| Pathological phenotype | None | Fat accumulation, metabolic phenotype | Inflammation, oxidative stress |
HFD: high fat diet; MCD: methionine and choline deficient diet.
Methionine and choline deficient diet (MCD) study
Male C57BL/six mice (n = 8/group), 8–10 weeks of age, were randomized based on body weight and fed with either CON or MCD (Teklad Diet 90262) and concurrently treated with vehicle or 30 mg/kg/day s.c. β-LGND2 from Day 1. The groups and treatments were similar to that described for the HFD study. The composition of MCD is provided in Table 1. The diet is deficient in amino acids methionine and choline. The MCD diet increases oxidative stress, inflammation, and lipid deposition, resulting in steatohepatitis and fibrosis. Typically, C57BL/6 mice fed on MCD diet loses 20% of body weight after three weeks, due to increased hepatic oxidative stress and steatosis. Species comparison indicated that the severity of steatosis was more in C57BL/6 mice.23 A disadvantage of MCD, although predominantly elicits the pathology associated with NASH, is that it lacks the metabolic profile, which is typical of human NASH. As MCD diet results in approximately 20% body weight loss that could result in mortality, we used eight animals/group in this study, while we used five animals/group in the HFD study, a diet that does not result in body weight loss. Body weight and food consumption were measured weekly and body composition by MRI was measured at the beginning and at the end of the study. At the end of four weeks, the animals were sacrificed and blood was collected for ALT measurement. Animals were fasted for 12–16 h. Serum was separated from blood and ALT was measured using kit (Sigma).
Histology
Tissues were immediately collected after sacrifice in 10% neutral-buffered formalin for histology studies. Tissues were paraffin embedded and the sections were stained with hematoxylin and Eosin (H&E) and Masson-Trichrome for collagen.24 Random fields (five/section) were chosen to count the number of inflammatory collagen stained cells and represented as average/group.
RNA sequencing
RNA was isolated using the Qiagen RNA isolation kit (Qiagen, Valencia, CA) and were further processed for RNA sequencing. Total RNA (1 µg) was enriched for Poly A RNA using the Ambion Dyna mRNA Micro Poly (A) kit according to manufacturer’s standard protocol (Thermo Fisher, Waltham, MA). The resulting enriched RNA samples were then used to prepare barcoded libraries for sequencing using the Ion Total RNAseq V2 kit (Thermo Fisher, Waltham, MA) and the Ion Xpress 1-32 Barcode kit (Thermo Fisher, Waltham, MA). Libraries were amplified for 15 cycles and then pooled using real-time PCR data. Following pooling, the library pools were purified on a Pippin Prep gel (Sage Science, Beverly, MA), quantified by real-time PCR and sequenced on an ion torrent proton sequencer. RNA sequencing studies were performed in UTHSC Molecular Resource Center.
We used gene set enrichment analysis (GSEA v2.0.13)25,26 to determine whether our rank-ordered gene list is enriched in genes from gene ontology, KEGG, transcription factor, or microRNA target gene sets. The gene list was ranked based on the shrunken log-based twofold change and the statistical significance of the enrichment score was determined by performing 1000 phenotype permutations and setting the enrichment statistics to classic. Other settings for GSEA preranked were left by the software default. These data are available through the Gene Expression Omnibus (GSE93154).
Metabolomics
Metabolomics studies were performed in the liver of mice that were fed with CON or HFD and treated with vehicle or β-LGND2 at Metabolon Inc. (Durham, NC) using standard protocols.27 Briefly, samples were prepared using the automated MicroLab STAR® system from Hamilton Company (Reno, NV). To remove protein, dissociate small molecules bound to protein or trapped in the precipitated protein matrix, and to recover chemically diverse metabolites, proteins were precipitated with methanol under vigorous shaking for 2 min (Glen Mills GenoGrinder 2000) followed by centrifugation. Samples were placed briefly on a TurboVap® (Zymark; Portland, OR) to remove the organic solvent. Several types of quality controls were analyzed in concert with the experimental samples: a pooled matrix sample generated by taking a small volume of each experimental sample (or alternatively, use of a pool of well-characterized human plasma) served as a technical replicate throughout the data set; extracted water samples served as process blanks; and a cocktail of QC standards that were carefully chosen not to interfere with the measurement of endogenous compounds were spiked into every analyzed sample, allowed instrument performance monitoring and aided chromatographic alignment. Overall process variability was determined by calculating the median RSD for all endogenous metabolites present in 100% of the pooled matrix samples.
All methods utilized a Waters ACQUITY ultra-performance liquid chromatography (UPLC) and a Thermo Scientific Q-Exactive high resolution/accurate mass spectrometer interfaced with a heated electrospray ionization (HESI-II) source and Orbitrap mass analyzer operated at 35,000 mass resolution. The sample extract was dried and then reconstituted in solvents compatible to each of the four methods. One aliquot was analyzed using acidic positive ion conditions, chromatographically optimized for more hydrophilic compounds. Another aliquot was also analyzed using acidic positive ion conditions; however, it was chromatographically optimized for more hydrophobic compounds. Another aliquot was analyzed using basic negative ion optimized conditions using a separate dedicated C18 column. The fourth aliquot was analyzed via negative ionization following elution from a HILIC column (Waters UPLC BEH Amide 2.1 mm × 150 mm, 1.7 µm) using a gradient consisting of water and acetonitrile with 10 mM ammonium formate, pH 10.8.
The informatics system consisted of four major components: the laboratory information management system, the data extraction and peak identification software, data processing tools for QC and compound identification, and a collection of information interpretation and visualization tools for use by data analysts. The hardware and software foundations for these informatics components were the LAN backbone and a database server running Oracle 10.2.0.1 Enterprise Edition. Peaks were quantified using area under the curve.
Two types of statistical analyses were performed: (1) significance tests and (2) classification analysis. Standard statistical analyses were performed in ArrayStudio (OmicsSoft, Durham, NC) on log transformed data. For those analyses not standard in ArrayStudio, the programs R (http://cran.r-project.org/) or JMP (SAS, Cary, NC) were used.
Principal components analysis is an unsupervised analysis that reduces the dimension of the data. Each principal component is a linear combination of every metabolite and the principal components are uncorrelated. The number of principal components is equal to the number of observations. The first principal component was computed by determining the coefficients of the metabolites that maximizes the variance of the linear combination. The second component finds the coefficients that maximize the variance with the condition that the second component is orthogonal to the first. The third component is orthogonal to the first two components and so on.
Statistics
Statistical analyses were performed using JMP Pro (SAS, Cary, NC) or sigma plot (Systat, San Jose, CA) software. Data were analyzed by one-way analysis of variance followed by Tukey’s test. Histology measurements were statistically analyzed using Chi-square test. RNA sequencing and metabolomics data were also analyzed for false discovery rate (FDR) and those genes or metabolites that had a FDR of q < 0.05 were taken into consideration for further analysis.
Results
β-LGND2 reversed the HFD- and MCD-dependent liver weight changes
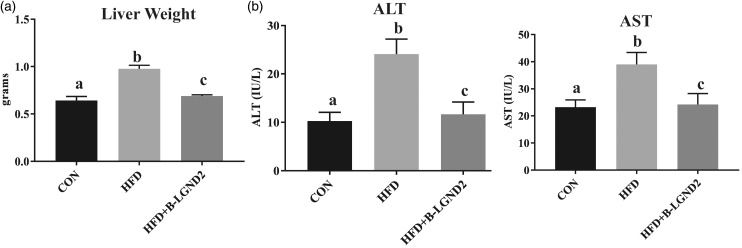
Mice fed with HFD exhibited an increase in liver weight compared to mice fed with CON (Figure 1(a)) and β-LGND2 prevented this increase in liver weight. Reflecting the change in liver weight, HFD also significantly increased the serum ALT and AST compared to CON (Figure 1(b)) and β-LGND2 prevented this increase and maintained at the CON level.
Figure 1.
β-LGND2 inhibits HFD-induced liver weight and serum transaminases. Male C57BL/six mice (n = 5/group) were maintained on either a rodent chow (normal diet [CON]) or high fat diet (HFD) and treated with vehicle or 30 mg/kg/day s.c. β-LGND2 for 10 weeks. Mice were sacrificed and liver weights were recorded (a). Blood was collected at sacrifice, serum separated, and serum transaminases were measured (b). Values are expressed as average ± SE. b > a and c at p < 0.05, while a and c are not statistically different
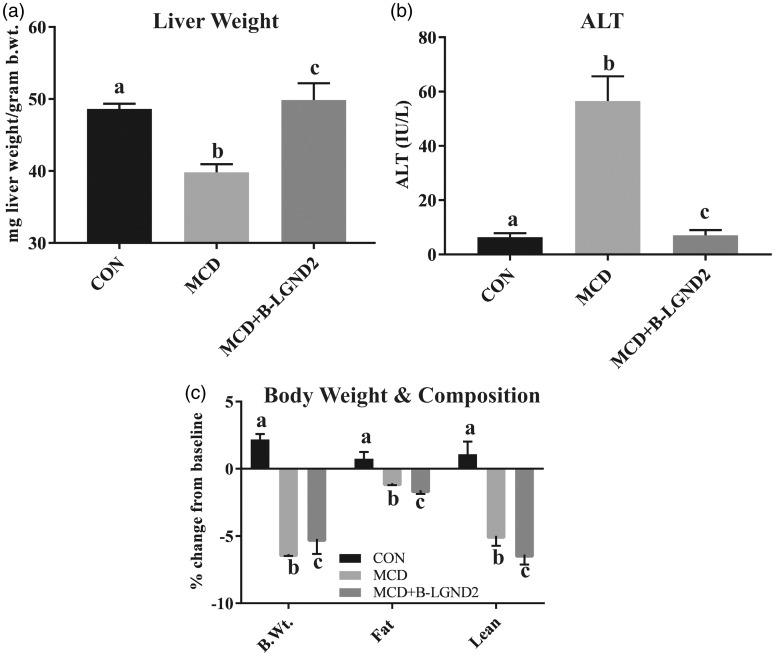
MCD diet induces NASH in 4–6 weeks.28,29 The MCD diet reproduces most of the histopathological features of NASH, including an increase in serum transaminases.21,22,30 However, the MCD does not reproduce the obesity and insulin resistance phenotypes that are characteristics of NASH in patients, rather reduces body weight.21,22 The MCD diet also decreases body weight, muscle mass, and fat mass. Mice fed with MCD demonstrated a reduction in liver weight (Figure 2(a)) and a 5–6-fold increase in serum ALT compared to mice fed with CON (Figure 2(b)). β-LGND2 prevented the decrease in liver weight and the increase in serum ALT and maintained them at the CON level. The absence of obesity in MCD-fed mice is shown in Figure 2(c).
Figure 2.
β-LGND2 reverses MCD-mediated liver weight and serum transaminases changes. Male C57BL/six mice (n = 8/group) were maintained on a normal diet (CON) or methionine choline deficient diet (MCD) and treated with vehicle or 30 mg/kg/day s.c. β-LGND2 for four weeks. Mice were sacrificed and liver weights were recorded (a). In panel a, b < a and c at p < 0.05, while a and c are not statistically different. Blood was collected at sacrifice, serum separated, and ALT was measured (b). In panel (b), b > a and c at p < 0.05, while a and c are not statistically different. Percent change from the beginning of the study in body weight, fat mass, and lean mass of the animals fed with CON or MCD and treated with β-LGND2 are provided in panel (c). In panel (c), b and c < a at p < 0.05, while b and c are not statistically different. Values are expressed as average ± SE. Liver weight was normalized to body weight (b.wt.)
These data collectively demonstrate that β-LGND2 prevented the pathological alterations in the liver that were promoted by various dietary conditions.
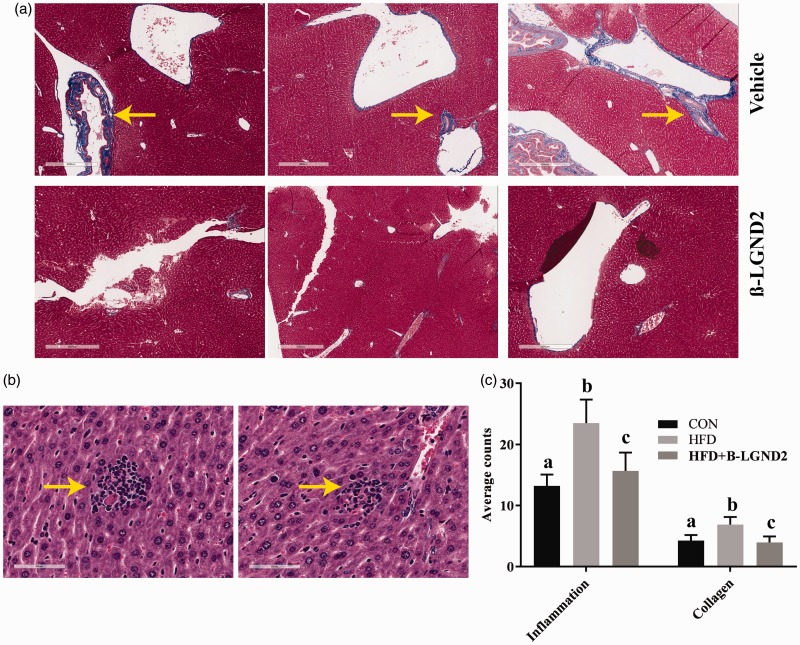
β-LGND2 partially prevented the inflammatory cell infiltration and fibrosis in liver of mice fed with HFD
Liver sections obtained from mice fed with CON or HFD and treated with vehicle or β-LGND2 were stained with Masson Trichrome to measure collagen deposition, an indicator of fibrosis, and hematoxylin and eosin. Histological analysis demonstrates an increase in fibrosis in the liver of HFD-fed mice, although the area covered by fibrosis was very minimal, which was reduced by β-LGND2 (Figure 3(a)). Although steatosis was observed in the liver of HFD-fed mice, it was very mild (less than 33% of the area31). Mononuclear inflammatory infiltration was also observed in the H&E stained liver sections of HFD-fed mice, while the infiltration was prevented by β-LGND2 (Figure 3(b)). While 6–7 regions of mononuclear inflammatory infiltration were observed in the liver of vehicle-treated HFD-fed mice, infiltration was observed in only 2–3 regions in the liver of β-LGND2-treated mice. Sections were quantified for inflammation and collagen and represented in Figure 3(c).
Figure 3.
β-LGND2 prevents liver pathology in HFD-fed mice. Male C57BL/six mice (n = 5/group) were maintained on a normal diet (CON) or high fat diet (HFD) and treated with vehicle or 30 mg/kg/day s.c. β-LGND2 for 10 weeks. Mice were sacrificed and liver from mice (n = 3/group) were fixed in formalin and stained with Masson trichrome stain (a) or hematoxylin and eosin (b). Arrows in (a) indicate collagen deposition and in (b) indicate inflammatory cells infiltration. (c) The sections were quantified and averages/group are represented as bar graph. b > a and c at p < 0.05, while a and c are not statistically different
RNA sequencing studies indicate that β-LGND2 inhibited pregnane X receptor (PXR) function
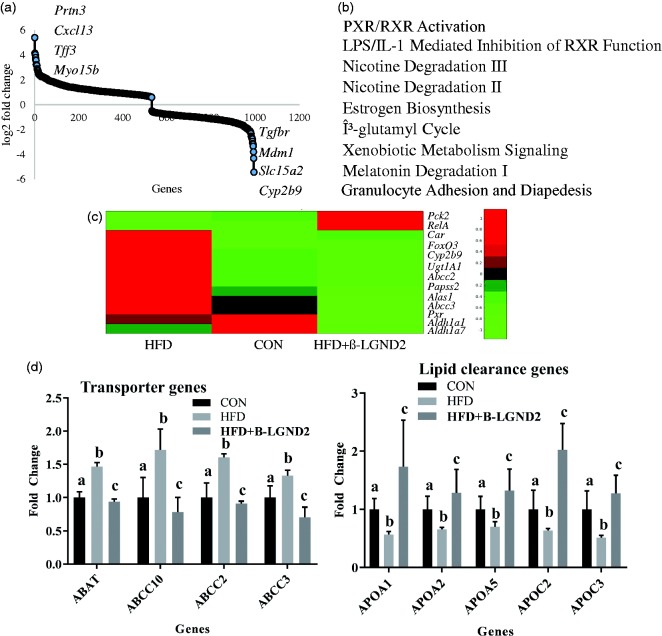
RNA was extracted from the liver (n = 3/group) of animals fed with CON or HFD and treated with vehicle or β-LGND2. RNA was sequenced to determine the genes that were differentially regulated by HFD and by β-LGND2. About 950 genes were found to be differentially regulated by β-LGND2 at q < 0.05 significance compared to vehicle-treated HFD-fed samples (Figure 4(a)). Ingenuity pathway analysis (IPA) indicated that PXR/RXR pathway genes were highly affected (though largely suppressed) in β-LGND2-treated samples (Figure 4(b)). Analysis of the genes in the PXR pathway that were altered by β-LGND2 indicated that about 20 genes were regulated by β-LGND2. Most, if not all, of the PXR-target genes were inhibited by β-LGND2 (Figure 4(c)) to the level that they were not significantly different from CON samples.
Figure 4.
β-LGND2 regulates distinct gene expression program in the liver of mice fed with HFD. Male C57BL/six mice (n = 5/group) were maintained on a normal diet (CON) or high fat diet (HFD) and treated with vehicle or 30 mg/kg/day s.c. β-LGND2 for 10 weeks. Mice were sacrificed and liver sections were snap frozen. RNA was isolated and expression of genes was measured by next-generation sequencing (n = 3/group). Genes that were statistically significantly (q < 0.05) regulated by β-LGND2 compared to vehicle-treated HFD-fed mice are represented in panel (a). (b) Ingenuity pathway analysis with statistically significant genes (q < 0.05) was performed to determine the pathways regulated by β-LGND2 in liver. (c) Genes that are differentially regulated by β-LGND2 (q < 0.05) and belonging to the PXR pathway are represented. (d) Genes (q < 0.05) that are important for lipogenesis (left) and clearance of lipids (right) are represented. In panel (d) left figure, b > a and c at p < 0.05, while a and c are not statistically different. In panel (d) right figure, b < a and c at p < 0.05, while a and c are not statistically different. Values are expressed as average ± SE
IPA also indicated that genes representing lipid and fatty acid metabolism were listed as top “disease and function-related” genes (Figure 4(d) left panel). About 83 genes belonging to these pathways were differentially regulated by β-LGND2 with most of the genes being down-regulated compared to HFD-vehicle-treated samples and statistically indifferent from the CON-fed animals. In addition, ∼100 inflammatory-response genes were differentially regulated in the β-LGND2-treated samples (mostly inhibited), indicating that anti-inflammatory effects could be at least one of the mechanisms by which β-LGND2 elicits its effects. Cardiovascular disease is another class that was enriched in the IPA analysis, agreeing with our previously published data and with alteration in the lipid metabolism pathways.9
One of the important classes of lipid transport proteins is the apolipoprotein (Figure 4(d) right panel). Expression of several apo class members was reduced in the liver of vehicle-treated HFD-fed mice, which were all increased significantly by β-LGND2. These results demonstrate that β-LGND2 has effects on multiple pathways all converging to protect the liver against the damage induced by enriched fatty food.
To confirm the histological findings, we searched for the expression of specific genes that contribute to fibrosis and inflammation (Figure 4 and supplementary table). Col27A1 and Col5A1, two genes belonging to the collagen class and were shown to be increased in cirrhosis,32 were up-regulated in the liver of vehicle-treated HFD-fed mice but not in the β-LGND2-treated HFD-fed mice. On the other hand, expression of inflammatory genes such as CxCl1, which are involved in neutrophil chemoattractant properties,33 were up-regulated in the liver of vehicle-treated HFD-fed mice but not in the β-LGND2-treated HFD-fed mice.
One of the genes, Cxcl12, shown to be a protector of liver from inflammation and injury,34,35 was up-regulated significantly by β-LGND2. Earlier studies have shown that administration of Cxcl12 analog elicited anti-inflammatory effects and protected liver from oxidative stress.35
Global metabolomics profiling indicates that β-LGND2 increased bile acid family metabolites
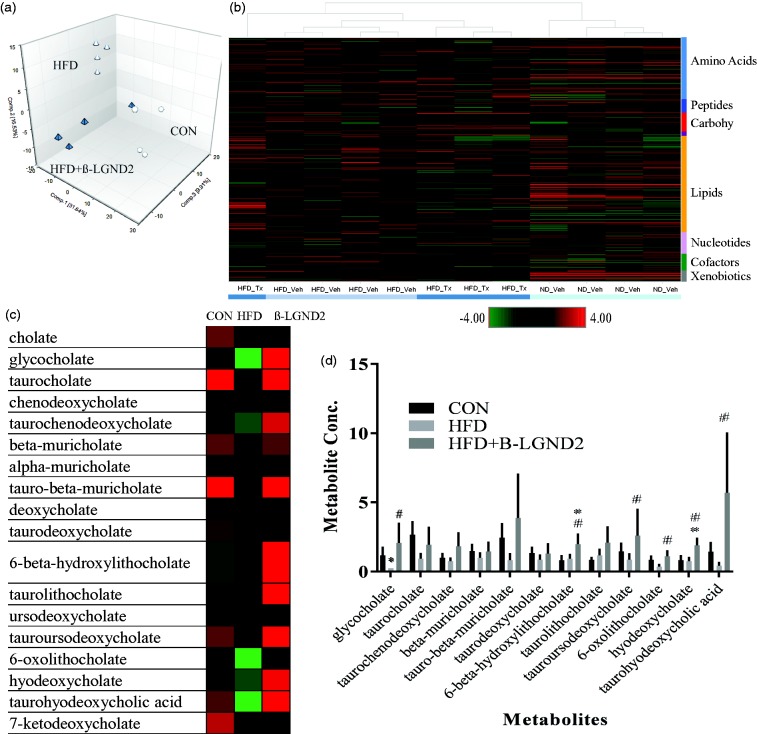
To further decipher the mechanism of action of β-LGND2, we performed global metabolomics profiling in the liver of mice fed with CON or HFD and treated with vehicle or β-LGND2 (Figure 5). HFD increased 77 and decreased 124 metabolites, compared to CON, while β-LGND2 increased 34 and decreased 81 metabolites, compared to HFD-fed vehicle-treated mice. Principal component analysis of the samples indicates that samples from β-LGND2-treated mice were clustered adjacent to samples from CON-fed mice (Figure 5(a)). Unsupervised hierarchical clustering of the samples clustered β-LGND2-treated samples between CON- and HFD-vehicle-treated samples (Figure 5(b)). Random forest classification using metabolites in the liver of the HFD-fed β-LGND2-treated and vehicle-treated groups resulted in a predictive accuracy of 88%.
Figure 5.
β-LGND2 regulates bile acid metabolites. Male C57BL/six mice (n = 5/group) were maintained on a normal diet (CON) or high fat diet (HFD) and treated with vehicle or 30 mg/kg/day s.c. β-LGND2 for 10 weeks. Mice were sacrificed and liver sections were snap frozen and metabolites were measured (n = 4/group). (a) Principal component analysis (PCA) showing the metabolites of individual samples. Circles represent CON-fed animals, white triangles represent HFD-fed vehicle-treated animals, while green triangles represent HFD-fed β-LGND2-treated animals. (b) Heat map (of metabolites q < 0.05) showing hierarchical clustering of individual samples. (c) Various bile acid metabolites expressed in the liver of mice maintained under different conditions. (d) Levels of bile acid metabolites are provided as bar graphs. Values are expressed as average ± SE. * indicates significance at q < 0.05 from CON-fed mice and # indicates significance at q < 0.05 from HFD vehicle-treated mice
Bile acids, one of the major pathways important for lipid homeostasis, were significantly regulated by HFD (Figure 5(c) and (d)). Cholic acid and its derivatives are the bile acids that modulate the function of farnesoid X receptor (FXR).36 Several cholic acid metabolites were up-regulated by β-LGND2 (some statistically significant, while others showing an increasing trend), all of which have effects on lipid homeostasis and cholesterol metabolism. One of the important target genes of FXR, Apoc2,37,38 was up-regulated in β-LGND2-treated samples (Figure 4(d)), providing evidence for potential FXR activation by β-LGND2.
Discussion
To our knowledge, the data provided here are the first evidence to demonstrate that an ER-β-selective agonist has robust hepatoprotective effects in models of NASH. ER-β-selective agonist, β-LGND2, prevented the NASH phenotype promoted by two mechanistically distinct inducers, the HFD and the MCD. It is important to point out that while HFD increased the liver weight, the MCD, which promotes NASH by a different mechanism, decreased the liver weight. Under both these conditions, β-LGND2 reversed the weight back to the level of the CON-fed mice, indicating that the liver alterations are not elicited only by blocking fat accumulation, which happened in the HFD condition, but also in the absence of fat accumulation as in MCD. This demonstrates that β-LGND2, in addition to effects on adipose and lipid homeostasis, promotes favorable changes by a pathway that is independent of lipid accumulation. We propose that this alternate pathway could affect oxidative damage or inflammation. RNA sequencing data corroborate this hypothesis with enrichment of genes belonging to xenobiotic metabolism signaling (e.g. Ugt2b28, Rela, Ugt1a6), NRF2-mediated oxidative stress response (e.g. Gsta, Gstm5), and mitochondrial dysfunction (e.g. Atpg1, Cox17). This work indicates that in addition to the primary effect on lipid accumulation, β-LGND2 also has effects on alternate pathways that could provide additional levels of protection against NASH or NAFLD.
Based on our previous results in models of obesity and metabolic diseases,19 we expected β-LGND2 to provide beneficial effects in preclinical models of NASH through control of lipid and triglycerides level or increase in metabolism pathways. However, the suppressed PXR and increased bile acid metabolites were serendipitous findings but congruent observations as there is no precedence for the interaction between ER-β and PXR or FXR or induction of bile acids by ER agonists. Several studies have earlier indicated the importance of PXR and FXR signaling in maintaining normal liver physiology and emergence of liver pathology.39 Activation of PXR and constitutive androstane receptor (CAR) may be beneficial for metabolic disorders such as type II diabetes but will result in an increase in triglycerides and incidence of NASH. Hence, inhibiting PXR is highly recommended for maintaining normal lipid levels and preventing NASH or NAFLD. Activation of PXR in mice caused hepatic fat accumulation due to increase in lipogenesis and fatty acid uptake.40,41 Several target genes of PXR such as Cyp2c9, Cyp3A4, Ugt1a1, which were inhibited by β-LGND2, are all implicated in the formation of NASH through activation of PXR.
Bile acids are important players in the maintenance of cholesterol levels, as they are immediate products of cholesterol catabolism.42 Bile acids function by binding to FXR and activating genes that are important for maintaining bile acid homeostasis.43,44 FXR is critical for liver and intestinal function. Whereas bile acids directly activate FXR, CAR and PXR regulate bile acid formation, thereby having an indirect effect on FXR function. Similar to PXR, CAR, whose expression was inhibited by β-LGND2, promotes lipid accumulation and hence inhibitors of CAR will be good therapeutics for NASH.45
Bile acids have also been shown to be important for the prevention of NASH.45,46 Studies have shown that knockdown of FXR in mouse models has resulted in hypercholesterolemia and development of NASH.47 These results show that FXR and PXR have opposing actions, which was reflected in our RNA sequencing studies where PXR-target genes were inhibited and FXR-target genes and ligands were up-regulated. This is not the first report showing a regulation of FXR and PXR in opposite directions by a chemical entity. Guggulsterone inhibits FXR and activates PXR, although directly, unlike β-LGND2.48,49
In order to ensure that β-LGND2 has no direct effect on PXR nor FXR, we performed transient transactivation studies by transfecting HepG2 cells with SHP-LUC and FXR or DR4-LUC and PXR, in the absence of ER-α or ER-β. β-LGND2 failed to induce or inhibit the expression of SHP-LUC or DR4-LUC (data not shown). This suggests that β-LGND2 did not have a direct effect on FXR or PXR promoter activity and the effects it elicited on PXR targets could be through an indirect mechanism of inducing ER-β-target genes, which in turn could have resulted in altering the function of PXR and FXR. Understanding the mechanism of ER-β regulation of PXR and FXR would further enhance the understanding of the dynamics and might provide additional therapeutic targets.
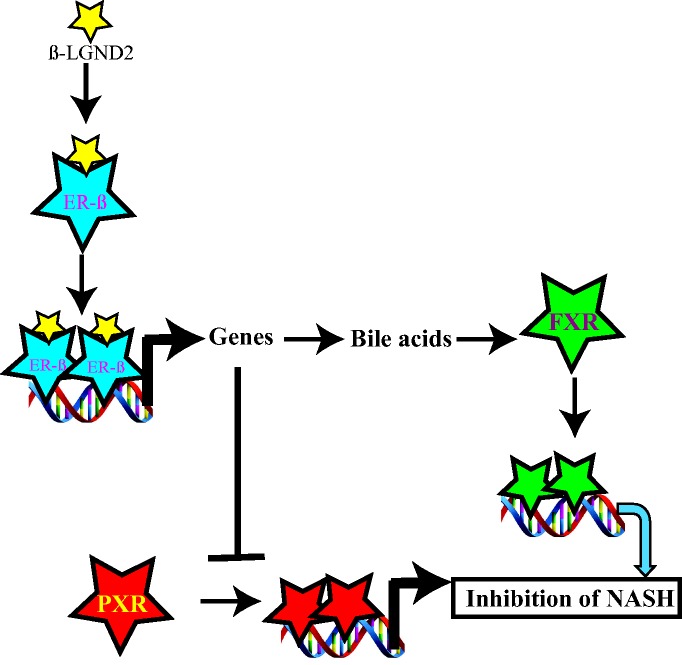
We have summarized these results in a model depicted in Figure 6. Activation of ER-β by β-LGND2 induced ER-β-target genes that could have resulted in an increase in bile acids. The bile acids bind to FXR to increase FXR-target genes, which in turn inhibited fat accumulation and incidence of NASH. On the other hand, the ER-β-target genes blocked PXR activation, inhibited PXR-target genes, and eventually reduced liver fat accumulation and liver damage.
Figure 6.
Model depicting the regulation of FXR and PXR by β-LGND2. FXR: farnesoid X receptor; PXR: pregnane X receptor
Collectively, these results provide firsthand evidence supporting the beneficial effects of ER-β-selective agonist, β-LGND2, in NASH, liver fibrosis, and overall in NAFLD. Currently, at least 5–6 different drugs are in clinical trials for the treatment of NASH. Several of these (obeticholic acid, PX104, and aramchol) are FXR agonists, while GFT505 is a PPAR-α/δ agonist and cenicriviroc is an immune modulator. However, these molecules do not have an effect on PXR. Since β-LGND2 regulates both FXR and PXR, albeit indirectly, it might be superior in reducing the NASH incidence than a FXR or PPAR agonist.
Supplementary Material
Acknowledgements
The authors thank Dr Daniel Johnson for his help in creating the heat maps for the manuscript. The authors also thank Ms Mayra Star for her help in maintenance and dosing of animals. The authors thank Drs Robert Getzenberg, Michael Mohler, and Diane Young of GTx for providing constructive comments on the manuscript. Part of the work was supported by NIH funding to DB (R01 (DK107535).
Authors’ contributions
SP, TT, DB performed experiments; DDM provided reagents; NQT analyzed data; RN conceived idea, performed data analysis, and wrote manuscript.
Declaration of conflicting interests
RN is a consultant to GTx, Inc.
Supplemental Material
Supplementary material for this paper can be found at http://journals.sagepub.com/doi/suppl/10.1177/1535370216688569.
References
- 1.Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis 2016; 20: 205–14. [DOI] [PubMed] [Google Scholar]
- 2.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011; 34: 274–85. [DOI] [PubMed] [Google Scholar]
- 3.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol 2003; 98: 2042–7. [DOI] [PubMed] [Google Scholar]
- 4.Fassio E, Alvarez E, Dominguez N, Landeira G, Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 2004; 40: 820–6. [DOI] [PubMed] [Google Scholar]
- 5.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD single topic conference. Hepatology 2003; 37: 1202–19. [DOI] [PubMed] [Google Scholar]
- 6.Larter CZ, Chitturi S, Heydet D, Farrell GC. A fresh look at NASH pathogenesis. Part 1: the metabolic movers. J Gastroenterol Hepatol 2010; 25: 672–90. [DOI] [PubMed] [Google Scholar]
- 7.Naves-Diaz M, Carrillo-Lopez N, Rodriguez-Rodriguez A, Braga S, Fernandez-Coto T, Lopez-Novoa JM, Lopez-Hernandez F, Cannata-Andia JB. Differential effects of 17beta-estradiol and raloxifene on bone and lipid metabolism in rats with chronic kidney disease and estrogen insufficiency. Menopause 2010; 17: 766–71. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi H, Pilbeam CC, Vargas SJ, Morse EE, Lorenzo JA, Raisz LG. Ovariectomy enhances and estrogen replacement inhibits the activity of bone marrow factors that stimulate prostaglandin production in cultured mouse calvariae. J Clin Invest 1995; 96: 539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedram A, Razandi M, Korach KS, Narayanan R, Dalton JT, Levin ER. ERbeta selective agonist inhibits angiotensin-induced cardiovascular pathology in female mice. Endocrinology 2013; 154: 4352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey P, Barros RP, Warner M, Strom A, Gustafsson JA. Insight into the mechanisms of action of estrogen receptor beta in the breast, prostate, colon, and CNS. J Mol Endocrinol 2013; 51: T61–74. [DOI] [PubMed] [Google Scholar]
- 11.Barros RP, Machado UF, Gustafsson JA. Estrogen receptors: new players in diabetes mellitus. Trends Mol Med 2006; 12: 425–31. [DOI] [PubMed] [Google Scholar]
- 12.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foryst-Ludwig A, Clemenz M, Hohmann S, Hartge M, Sprang C, Frost N, Krikov M, Bhanot S, Barros R, Morani A, Gustafsson JA, Unger T, Kintscher U. Metabolic actions of estrogen receptor beta (ERbeta) are mediated by a negative cross-talk with PPARgamma. PLoS Genet 2008; 4: e1000108–e1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allende-Vigo MZ. Women and the metabolic syndrome: an overview of its peculiarities. P R Health Sci J 2008; 27: 190–5. [PubMed] [Google Scholar]
- 15.Jeffery E, Wing A, Holtrup B, Sebo Z, Kaplan JL, Saavedra-Pena R, Church CD, Colman L, Berry R, Rodeheffer MS. The adipose tissue microenvironment regulates depot-specific adipogenesis in obesity. Cell Metab 2016; 24: 142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klair JS, Yang JD, Abdelmalek MF, Guy CD, Gill RM, Yates K, Unalp-Arida A, Lavine JE, Clark JM, Diehl AM, Suzuki A. Nonalcoholic Steatohepatitis Clinical Research N. A longer duration of estrogen deficiency increases fibrosis risk among postmenopausal women with nonalcoholic fatty liver disease. Hepatology 2016; 64: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab 2003; 88: 2404–11. [DOI] [PubMed] [Google Scholar]
- 18.Brady CW. Liver disease in menopause. World J Gastroenterol 2015; 21: 7613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yepuru M, Eswaraka J, Kearbey JD, Barrett CM, Raghow S, Veverka KA, Miller DD, Dalton JT, Narayanan R. Estrogen receptor-{beta}-selective ligands alleviate high-fat diet- and ovariectomy-induced obesity in mice. J Biol Chem 2010; 285: 31292–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ponnusamy S, Tran QT, Harvey I, Smallwood HS, Thiyagarajan T, Banerjee S, Johnson DL, Dalton JT, Sullivan RD, Miller DD, Bridges D, Narayanan R. Pharmacologic activation of estrogen receptor beta increases mitochondrial function, energy expenditure, and brown adipose tissue. FASEB J 2017; 31: 266–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanches SC, Ramalho LN, Augusto MJ, da Silva DM, Ramalho FS. Nonalcoholic steatohepatitis: a search for factual animal models. Biomed Res Int 2015; 2015: 574832–574832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol 2012; 18: 2300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, Kirsch RE, Hall Pde L. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol 2003; 18: 1272–82. [DOI] [PubMed] [Google Scholar]
- 24.Goldner J. A modification of the masson trichrome technique for routine laboratory purposes. Am J Pathol 1938; 14: 237–43. [PMC free article] [PubMed] [Google Scholar]
- 25.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 2003; 34: 267–73. [DOI] [PubMed] [Google Scholar]
- 26.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 2005; 102: 15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 2009; 81: 6656–67. [DOI] [PubMed] [Google Scholar]
- 28.Cong WN, Tao RY, Tian JY, Liu GT, Ye F. The establishment of a novel non-alcoholic steatohepatitis model accompanied with obesity and insulin resistance in mice. Life Sci 2008; 82: 983–90. [DOI] [PubMed] [Google Scholar]
- 29.Leclercq IA, Lebrun VA, Starkel P, Horsmans YJ. Intrahepatic insulin resistance in a murine model of steatohepatitis: effect of PPARgamma agonist pioglitazone. Lab Invest 2007; 87: 56–65. [DOI] [PubMed] [Google Scholar]
- 30.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol 2004; 40: 47–51. [DOI] [PubMed] [Google Scholar]
- 31.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis Clinical Research N. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005; 41: 1313–21. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez-Antolin G, Almohalla-Alvarez C, Bueno P, Almansa R, Iglesias V, Rico L, Ortega A, Munoz-Conejero E, Garcia-Pajares F, Bermejo-Martin JF. Evidence of active pro-fibrotic response in blood of patients with cirrhosis. PLoS One 2015; 10: e0137128–e0137128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li L, Xu L, Yan J, Zhen ZJ, Ji Y, Liu CQ, Lau WY, Zheng L, Xu J. CXCR2–CXCL1 axis is correlated with neutrophil infiltration and predicts a poor prognosis in hepatocellular carcinoma. J Exp Clin Cancer Res 2015; 34: 129–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saiman Y, Jiao J, Fiel MI, Friedman SL, Aloman C, Bansal MB. Inhibition of the CXCL12/CXCR4 chemokine axis with AMD3100, a CXCR4 small molecule inhibitor, worsens murine hepatic injury. Hepatol Res 2015; 45: 794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seemann S, Lupp A. Administration of a CXCL12 Analog in endotoxemia is associated with anti-inflammatory, anti-oxidative and cytoprotective effects in vivo. PLoS One 2015; 10: e0138389–e0138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forman BM, Goode E, Chen J, Oro AE, Bradley DJ, Perlmann T, Noonan DJ, Burka LT, McMorris T, Lamph WW, Evans RM, Weinberger C. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995; 81: 687–93. [DOI] [PubMed] [Google Scholar]
- 37.Kim MS, Shigenaga J, Moser A, Feingold K, Grunfeld C. Repression of farnesoid X receptor during the acute phase response. J Biol Chem 2003; 278: 8988–95. [DOI] [PubMed] [Google Scholar]
- 38.Hageman J, Herrema H, Groen AK, Kuipers F. A role of the bile salt receptor FXR in atherosclerosis. Arterioscler Thromb Vasc Biol 2010; 30: 1519–28. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Zhou J, Xie W. PXR and LXR in hepatic steatosis: a new dog and an old dog with new tricks. Mol Pharm 2008; 5: 60–6. [DOI] [PubMed] [Google Scholar]
- 40.Zhou J, Zhai Y, Mu Y, Gong H, Uppal H, Toma D, Ren S, Evans RM, Xie W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J Biol Chem 2006; 281: 15013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou J, Febbraio M, Wada T, Zhai Y, Kuruba R, He J, Lee JH, Khadem S, Ren S, Li S, Silverstein RL, Xie W. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 2008; 134: 556–67. [DOI] [PubMed] [Google Scholar]
- 42.Rao A, Kosters A, Mells JE, Zhang W, Setchell KD, Amanso AM, Wynn GM, Xu T, Keller BT, Yin H, Banton S, Jones DP, Wu H, Dawson PA, Karpen SJ. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci Transl Med 2016; 8: 357ra122–357ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu Y, Liu H, Zhang M, Guo GL. Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B 2016; 6: 409–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science 1999; 284: 1362–5. [DOI] [PubMed] [Google Scholar]
- 45.Maglich JM, Lobe DC, Moore JT. The nuclear receptor CAR (NR1I3) regulates serum triglyceride levels under conditions of metabolic stress. J Lipid Res 2009; 50: 439–45. [DOI] [PubMed] [Google Scholar]
- 46.Shirley JT, Cheng JB. Competition of leukotrienes and ICI-198,615 for [3H]LTD4 binding sites in guinea pig lung membranes suggests the involvement of two LTD4 receptor subtypes. J Pharmacol Exp Ther 1991; 258: 531–6. [PubMed] [Google Scholar]
- 47.Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther 2009; 328: 116–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Urizar NL, Liverman AB, Dodds DT, Silva FV, Ordentlich P, Yan Y, Gonzalez FJ, Heyman RA, Mangelsdorf DJ, Moore DD. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 2002; 296: 1703–6. [DOI] [PubMed] [Google Scholar]
- 49.Owsley E, Chiang JY. Guggulsterone antagonizes farnesoid X receptor induction of bile salt export pump but activates pregnane X receptor to inhibit cholesterol 7alpha-hydroxylase gene. Biochem Biophys Res Commun 2003; 304: 191–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.