Abstract
Acquired lymphedema is one of the most dreaded side effects of cancer treatment, such as surgical treatment or irradiation. However, due to the lack of appropriate animal models, there is no effective therapeutic method to cure acquired lymphedema. To develop a reproducible acquired lymphedema animal model, we devised a mouse hind limb model by removing a superficial inguinal lymph node, a popliteal lymph node, a deep inguinal lymph node, and the femoral lymphatic vessel. We measured the volume of lymphedematous leg and observed the change in level of hyaluronic acid (HA) and lymphangiogenic factors after injecting hyaluronidase. Our model showed the distinguishable swelling and the reliable symptoms compared to previously reported models. In the lymphedematous regions of our model, we confirmed that HA, a major component of extracellular matrix, accumulated to higher levels than in a normal mouse. This lymphedema volume was rapidly reduced by treating hyaluronidase. Following hyaluronidase injection, the lymphedematous region of our model resembled a normal hind limb. Our findings indicated that hyaluronidase promoted lymphangiogenesis on the lymphedematous limb. Based on hyaluronidase treatment in the lymphedematous region, this could potentially be a new therapeutic approach for acquired lymphedema mediated through the modification of the size of HA fragments.
Impact statement
In this manuscript, the essence of the work described in this manuscript involves the development of (1) a mouse limb model showing acquired lymphedema and (2) a potent therapeutic treatment using hyaluronidase to remedy acquired lymphedema in our model. In order to develop a reproducible acquired lymphedema animal model that reflects the most common symptoms experienced by lymphedema patients, we devised a mouse hind limb model by removing lymph nodes and lymphatics. Our model showed the distinguishable swelling and the reliable symptoms compared to previously reported models. In the lymphedematous regions of our model, we confirmed that hyaluronic acid (HA) accumulated to higher levels than in a normal mouse. This lymphedema volume was rapidly reduced by treating the lymphedematous leg with hyaluronidase, which also degraded high molecular weight HA to low molecular weight HA. Immunohistochemical analysis, quantitative real-time PCR analysis and lymphangioscintigraphy showed that hyaluronidase enhanced lymphangiogenesis in the lymphedematous limb.
Keywords: Acquired lymphedema, hyaluronic acid, hyaluronidase and lymphangiogenesis
Introduction
Acquired lymphedema is a set of pathological conditions characterized by the regional accumulation of excessive amounts of interstitial protein-rich fluid and tissue swelling caused by cancer treatments and by parasitic infections.1–3 Histopathologic symptoms in lymphedema include thickening of the basement membrane of lymphatic vessels; presence of adipocyte proliferation and hypertrophy; fragmentation and degeneration of elastic fibers; increased numbers of fibroblasts and inflammatory cells; and increased amounts of ground substance, pathological collagen fibers, and progressive tissue fibrosis.4,5 As one of the most serious side effects of treatments for cancer,6 approximately 20% of breast carcinoma survivors and 21.8% of gynecologic cancer survivors suffer from acquired lymphedema following surgical and/or radiation therapies.1,7,8 For lymphedema treatment, physical therapies are the standard treatment and drugs are not usually used for the long-term treatment of lymphedema. Therefore, the goals for the treatment of lymphedema are to temporarily control tissue swelling and to keep other problems from getting worse.
To understand the biological mechanisms of acquired lymphedema occurrence and therapy, a reproducible experimental model system is required. Despite many efforts in this area, however, therapeutic options for the alleviation of lymphedema still remain unsolved.1,9
In lymphedematous regions of patients, HA or hyaluronan, one of the major extracellular matrix (ECM) components, accumulates to levels that are significantly higher than in normal tissue fluids.10 HA is a non-sulfated glycosaminoglycan composed of the repeating polymeric disaccharides d-glucuronic acid and N-acetyl-d-glucosamine linked by a glucuronidic β bond.11 HA has various functions depending on the size of its fragments (molecular mass), as high molecular weight HA (HMWHA) is degraded into low molecular weight HA (LMWHA) by the hyaluronidase enzyme.12 Hyaluronidase hydrolyzes the hexosaminidic β (1–4) linkages between the N-acetyl-d-glucosamine and d-glucuronic acid residues in HA and releases HA fragments (LMWHA).
In this study, we developed a new lymphedema mouse model which presents with symptoms similar to those experienced by lymphedema patients. Using this mouse hind limb model, we confirmed that changes in HA molecular weight could be a key factor for lymphedema induction and treatment. We also demonstrated the alleviation effect of hyaluronidase as a potent therapeutic agent for lymphedema.
Results
A novel lymphedema mouse model
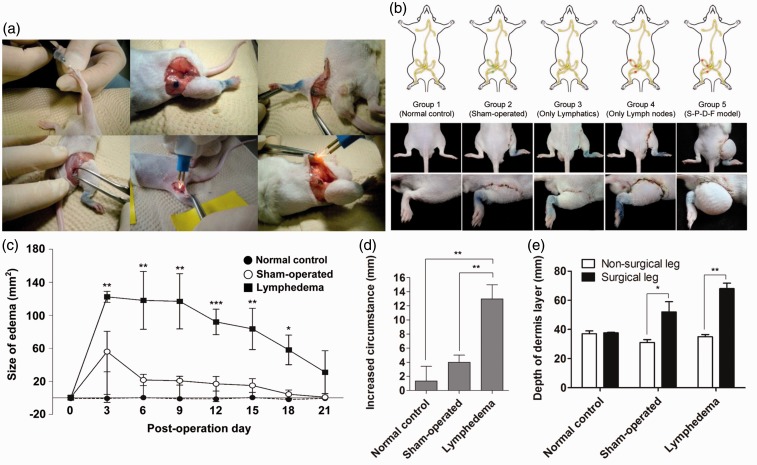
We designed a new protocol for the induction of lymphedema that cause symptoms similar to those observed in lymphedema patients. The development of acquired lymphedema in the right hind limb of mice was achieved by surgical removal of three lymph nodes, a superficial inguinal lymph node, a popliteal lymph node and a deep inguinal lymph node, and a femoral lymphatic vessel (SPDF removal group) (Figure 1(a)). To compare the severity of symptoms in our lymphedema-induced mouse model with the symptoms seen in other existing model systems, five groups of mice were prepared based on different surgical protocols: a normal control group, a sham-operated group, a lymphatics-only removal group, a lymph node-only removal group, and a SPDF removal group (Group 5). Our model showed the most dramatic enlargement of lymphedema compared to the other groups (Figure 1(b)). On day 7 postsurgery, the size of lymphedema-induced hind limbs was increased 118.03 ± 35.01% compared to the basal line of the non-operated limbs (Figure 1(c)). The volume of the edema in the lymphedema-induced mice increased approximately five times relative to the edema in sham-operated mice due to simple inflammatory damage (sham-operated group 21.5434 mm2 versus lymphedema group 118.0395 mm2). In this lymphedema mouse model, the lymphedematous enlargement of the right hind limb was maintained until 21 days postsurgery. The edema volumes were also measured by circumferential measurement, as described above (Figure 1(d)). Measurement by ultrasonography detected alteration of the depth of subcutaneous tissues in the lymphedematous limb (normal [left 36 ±2.45 mm/right 38 ± 0.82 mm], sham-operated [left 31 ±2.83 mm/right 38 ± 9.90 mm], and lymphedema groups [left 35 ± 2.16 mm/right 68 ± 5.35 mm] (Figure 1(e)).
Figure 1.
A novel acquired lymphedema mouse model. (a) Surgical procedure for inducing lymphedema. (b) Comparison of each surgical protocol and its associated symptoms. (c) Quantitative analysis of lymphedema size of each group (normal control, n = 5; sham operated, n = 10; and lymphedema groups, n = 8) followed over a time course. (d) Measurement of the increased circumference of the operated thigh compared with that of the contralateral thigh. (e) Ultrasonographic measurement of the depth of the dermis layer. *P < 0.05, **P < 0.01, ***P < 0.001. (A color version of this figure is available in the online journal.)
Change in the amount of HA in lymphedema following treatment with hyaluronidase
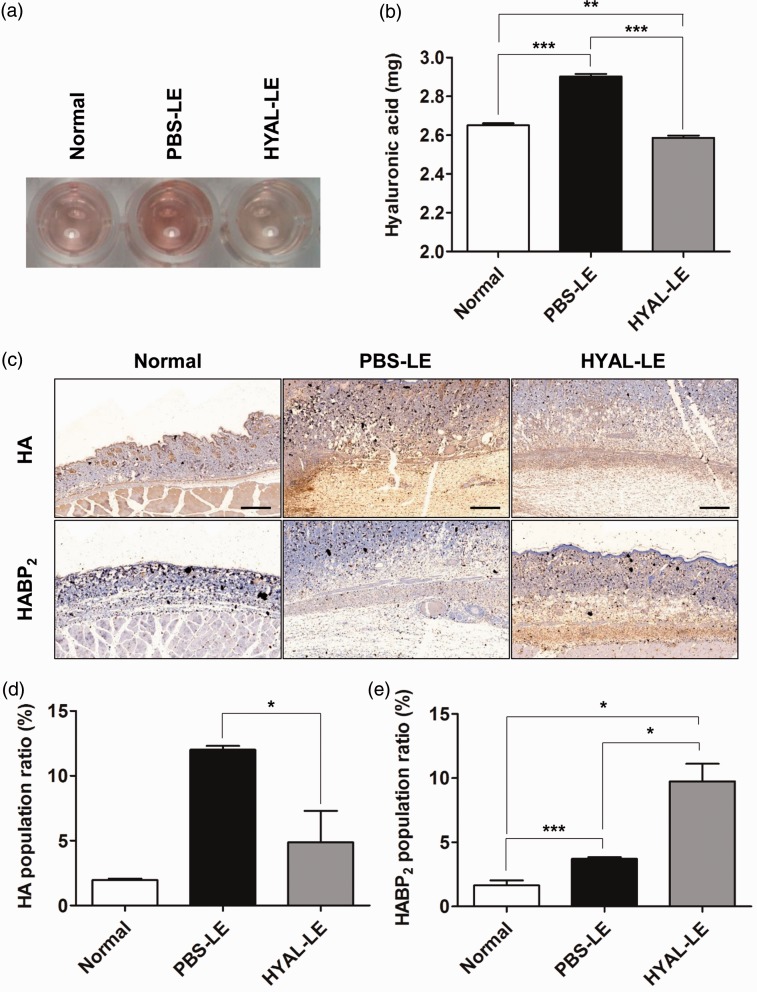
The concentration of HA in limbs from each group was evaluated by carbazole assay. The HA carbazole reaction assay was performed in a 96-well plate using the tissue samples prepared on day 7 postsurgery (Figure 2(a)). As in a previous clinical study of lymphedema patients,13 the HA content was increased in lymphedema-induced limb of mice. In the lymphedematous tissues prepared from PBS-treated lymphedema mice, the total concentration of HA increased 9.43% compared to that of the normal group (normal group 2.65 ± 0.21 mg/mL versus PBS-LE group 2.90 ± 0.03 mg/mL). After injection of hyaluronidase into the lymphedematous region to degrade HA, HA decreased 10.68% compared to the tissues prepared from PBS-treated lymphedema mice (HYAL-LE group 2.59 ± 0.02 mg/mL). (Figure 2(b)). Treatment with exogenous hyaluronidase in lymphedematous limbs reduced the total concentration of HA. In order to investigate HA localization and its content in tissues, we performed immunohistochemical analysis with anti-HA antibody. Immunohistochemical analysis showed that the distribution of HA in the HYAL-LE group was more greatly reduced after hyaluronidase injection than in the PBS-LE group (Figure 2(c), above). The PBS-LE group showed a distribution of HA that expanded into all subcutaneous and dermal layers. HABP2, an extracellular serine protease, was highly expressed in the HYAL-LE group compared to the PBS-LE group. This result indicated that LMWHA accumulated in the HYAL-LE group (Figure 2(c), below). Quantitative analysis of each stained area indicated that total HAs content decreased and LMWHA was enhanced (Figure 2(d) and (e)).
Figure 2.
Analysis of hyaluronic acid in the lymphedematous tissues. (a) A 96-well carbazole assay for the measurement of the concentration of prepared HA from the right hind limb of the normal, PBS-LE, and HYAL-LE groups. (b) Quantitative analysis by colorimetric method using serial dilutions of an HA standard. (c) Immunohistochemistry for hyaluronic acid (HA) and hyaluronic acid binding protein type 2 (HABP2). (d) Localizing area of HA population. (e) Localizing area of HABP2 population. *P < 0.05, **P < 0.01, ***P < 0.001. HYAL-LE: hyaluronidase-injected lymphedema group; PBS-LE: PBS-injected lymphedema group;. (A color version of this figure is available in the online journal.)
Amelioration of lymphedema by hyaluronidase treatment in the right hind limb mouse model
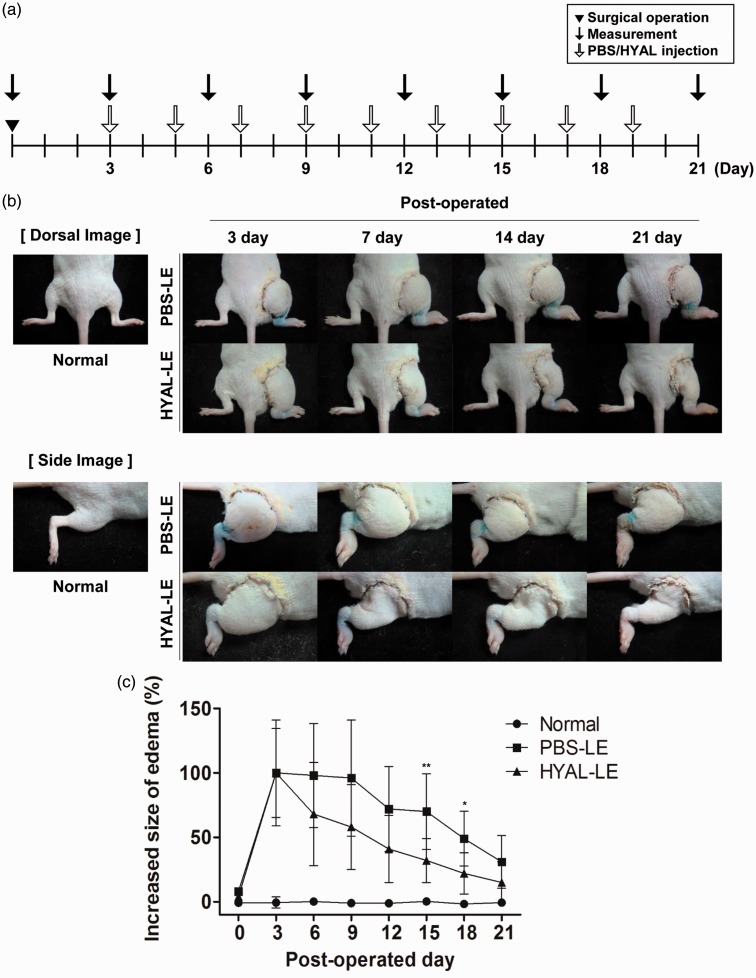
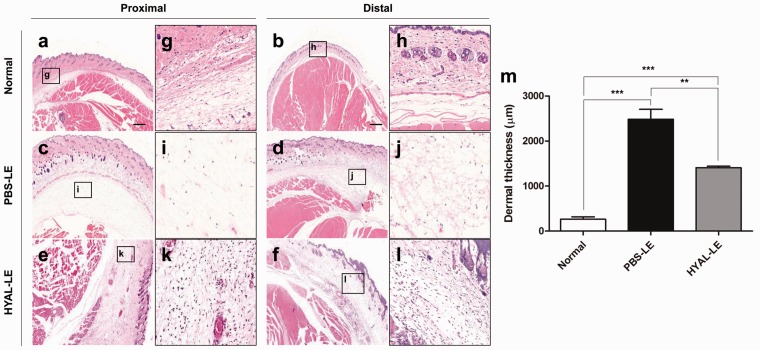
Swelling was measured every three days and agents (PBS or hyaluronidase) were used for treatment every two days (Figure 3(a)). On day 3 postsurgery, the right hind limb was swollen to a maximum edema size. After hyaluronidase injection, both the dorsal and side images of the lymphedema model showed outstanding levels of edema alleviation in the right hind limb (Figure 3(b)). The HYAL-LE group showed a more significant reduction in edema in the right hind limb than did the PBS-LE and normal groups (Figure 3(c)). Hyaluronidase had local effects on the right hind limb, whereas no other physiologic changes were observed in other regions. To investigate the histological changes following hyaluronidase treatment, H&E staining was performed on two regions of the thigh: proximal and distal regions (Figure 4). As compared to the normal mouse group (Figure 4(a), (b), (g), and (h)), the PBS-LE group showed an irregular epidermal/dermal junction and a three- to fourfold greater expansion of tissues between the epidermis and bone (Figure 4(c), (d), (i), and (j)). Both the proximal and distal regions of the HYAL-LE group (Figure 4(e), (f), (k), and (l)) had swollen tissues that were significantly reduced compared to the PBS-LE group. Dermal layer of the PBS-LE group was 9.46 times thicker compared with that of the normal group. In contrast, the HYAL-LE group showed a significant reduced dermal layers (normal 262.66 ± 96.90, PBS-LE 2485.46 ± 376.75, HYAL-LE 1408.01 ± 59.45 µm) (Figure 4(m)).
Figure 3.
Changes in the thickness of the operated thigh after hyaluronidase injection. (a) Experimental schedule. (b) Dorsal and side view images of changes in the lymphedema-induced limb thickness caused by hyaluronidase treatment at each time point. (c) Quantitative analysis demonstrating a reduction in thigh thickness in the hyaluronidase-treated group (n = 8) compared with the PBS-treated group (n = 9) (the normal group, n = 5). PBS: phosphate buffered saline. (A color version of this figure is available in the online journal.)
Figure 4.
Histological changes in the lymphedema-induced thigh upon treatment with hyaluronidase (hematoxylin and eosin staining, low magnification; 50x and high magnification; 200x). Proximal images were taken from the tissues of 10 mm distal to operated sites. In contrast, distal images were taken from the tissues of 20 mm distal to operated point. ((e) and (f): 50x, (k) and (l): 200x) the images in HYAL-LE group were the tissue from seven days postoperation with hyaluronidase injection in a lymphedema group. (m) Dermal thickness of the right leg in each group was quantified. HYAL-LE: hyaluronidase-injected lymphedema group. (A color version of this figure is available in the online journal.)
Changes in HA caused by hyaluronidase promote the growth of lymphatic vessels in a mouse limb model
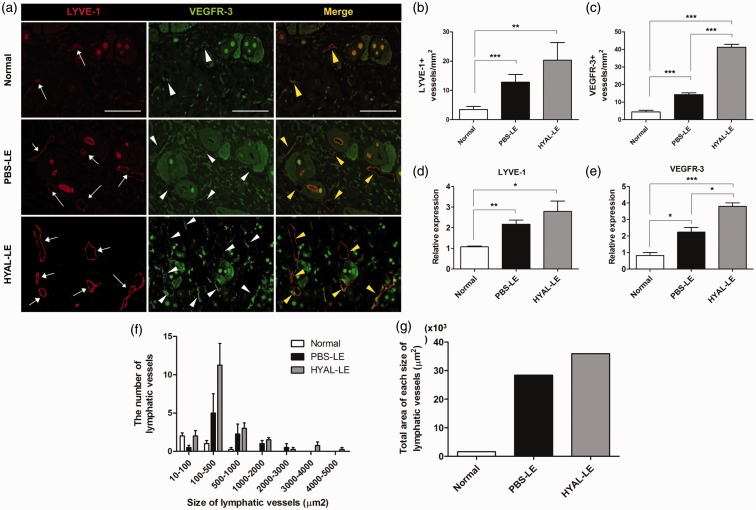
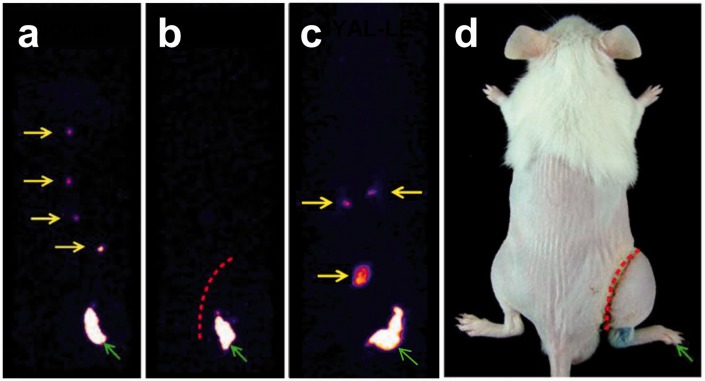
Morphological changes and the number of lymphatic vessels on the dermal layer were identified by immunohistochemical analysis with anti-LYVE-1 and anti-VEGFR-3 antibodies which are markers for lymphatic endothelial cells (Figure 5(a)). The PBS-LE group had large and dilated lymphatic vessels consistent with lymphatic dysfunction. However, the HYAL-LE group had denser and newly elongated LYVE-1-positive and VEGFR-3-positive lymphatic vessels. The HYAL-LE group showed a 1.5-fold increase in the number of LYVE-1-positive lymphatic vessels compared to the PBS-LE group (normal 3.47 ± 0.51, PBS-LE 12.84 ± 1.31, HYAL-LE 20.34 ± 2.99 per mm2) (Figure 5(b)). In addition, the number of VEGFR-3-positive lymphatic vessels was increased 2.89-fold more in the HYAL-LE group than in the PBS-LE group (normal 4.40 ± 0.55, PBS-LE 14.31 ± 0.55, HYAL-LE 41.29 ± 0.95 per mm2) (Figure 5(c)). Expression of VEGFR-3 was 6.83 times and 14.49 times higher than in the PBS-LE and normal groups, respectively. Significant increases in the mRNA levels of both LYVE-1 and VEGFR-3 were also observed in mouse tissue treated with hyaluronidase compared to the other groups (LYVE-1: normal 1.07 ± 0.03, PBS-LE 2.17 ± 0.19, HYAL-LE 2.79 ± 0.49/VEGFR-3: normal 0.82 ± 0.17, PBS-LE 2.24 ± 0.27, HYAL-LE 3.79 ± 0.21) (Figure 5(d) and (e)). When we measured the number of each size of lymphatic vessels in HYAL-LE group, HYAL-LE group showed higher in numbers than PBS-LE group. Especially, the number of lymphatic vessels was more dramatically increased at the size of 10–100 and 100–500 µm2 of HYAL-LE group (Figure 5(f)). Lymphangioscintigraphy detected enhanced lymphatic drainage after hyaluronidase injection (Figure 6). In the normal group, lymphatic flow circulated, and the draining lymph nodes were clearly visible from the right hind limb to body. Imaging performed at postsurgery 7 day showed successful surgical blockade of lymphatic fluid in the PBS-LE group. Treatment with hyaluronidase induced the proliferation of lymphatic endothelial cells.
Figure 5.
Effects of hyaluronidase on the promotion of lymphangiogenesis. (a) Immunofluorescence staining. LYVE-1-positive and/or VEGFR-3-positive staining in a sectioned right hind limb. Samples were prepared from the tissues of a normal group, a PBS-treated lymphedema group (seven days postoperation), and a hyaluronidase-treated lymphedema group (seven days postoperation). (b) Evaluation of the number of LYVE-1-positive lymphatic vessels. (c) Evaluation of the number of VEGFR-3-positive lymphatic vessels. (d) Changes in the expression of LYVE-1 mRNA. (e) Changes in the expression of VEGFR-3 mRNA. (f) Measurement of the number of each size of lymphatic vessels. (g) Comparison of total area of each size of lymphatic vessels between each group. *P < 0.05, **P < 0.01, ***P < 0.001. LYVE: lymphatic vessel endothelial hyaluronan receptor; VEGFR: vascular endothelial growth factor receptor. (A color version of this figure is available in the online journal.)
Figure 6.
Lymphangioscintigraphy. Tc-99 m-HSA was injected intradermally into the right hind footpad (green arrow). (a) In normal mice, draining lymph nodes (LNs) (yellow arrows) were clearly detected. (b) In PBS-treated mice, no visualization of lymph nodes was seen. (c) After injecting hyaluronidase, increased uptake by LNs was detected in the HYAL-LE group. (d) Schematic picture of the lymphangioscintigraphic images. HSA: human serum albumin; HYAL-LE: hyaluronidase-injected lymphedema group; PBS: phosphate buffered saline. (A color version of this figure is available in the online journal.)
Discussion
Acquired lymphedema is a physiological disease caused by the accumulation of interstitial fluids due to structural defects and malfunction of the lymphatic system.14 This study represented a possibility to remedy acquired lymphedema using hyaluronidase therapy in lymphedematous regions. Hyaluronidase modulates HA accumulated in lymphedematous tissues and, therefore, we expected that fragmentation of HA might alleviate acquired lymphedema.
The development of reproducible and constant animal model to study acquired lymphedema is important. The absence of the appropriate animal model and the short continuity of lymphedema in models have hindered further substantive studies on lymphedema therapy.15 Most lymphedema studies have used surgically induced lymphatic disease in the tails of rodents, such as rats and mice,16,17 or ears of rabbits.18,19 Although such lymphedema models are easily prepared by simple surgical operation, they do not mimic the symptoms shown in lymphedema patients well. Furthermore, their disease regions do not correspond to those of patients. Irradiation therapy has been known to affect the risk of lymphedema after cancer treatment.20 There were several trials to induce lymphedema by irradiation with gamma ray targeting for limbs in vivo.21,22 Some models lasted lymphedema for several months. However, technical limits against local irradiation to specific lymph nodes and side effects from damage to a wide range of normal tissue by proton scattering of radiation were also observed. Therefore, the combined experimental model system with the newly developed surgical protocol and tailor-made local irradiation system is required for further investigation of lymphedema.
In this study, using a new strategy to mimic the symptoms observed in lymphedema using the right whole extremity of a mouse, we evaluated the therapeutic effect of hyaluronidase on lymphedema. Our model required blocking the proper lymph nodes and lymphatic vessels in the regions between an extremity and the body to induce lymphedema in the limbs. It was difficult to identify which lymphatic components were essential in order to cause lymphedema with the minimum amount of damage to the tissue in a small mouse when the lymphatic system was surgically blocked. In reference to reported studies that used different protocols,23,24 our lymphedema mouse model was developed by surgical elimination and aggregation of specific lymphatic components, including the superficial inguinal lymph node, the popliteal lymph node, the deep inguinal lymph node, and a femoral lymphatic vessel (Figure 1(a)). Recently, co-administration of human adipose-derived stem cells and VEGF-C hydrogel as a means of improving lymphangiogenesis in a lymphedema mouse model was investigated (Figure 1(b)).23 They induced lymphedema on the footpad. However, their lymphedematous tissue was insufficient to distinguish between the swelling caused by surgery and inflammation toxicity caused by the methylene blue used for staining or blockage of lymphatic components. We performed careful trials to minimize inflammation during the surgical procedures since we intended to identify specific changes caused by lymphatic damages without unintended tissue damage, such as toxicity caused by staining dyes and bleeding by sharp surgical instruments. Furthermore, all targeted lymphatic components were stained using the minimum concentration of methylene blue and they were incised by opening muscles along the texture of the muscles without tissue damage. As a result, our animal model has outstanding swelling in the lymphedematous limb, as compared to the contralateral limb (Figure 1(c) to (e)). This model is an animal model that simulates the representative symptoms of acquired lymphedema through lymphatic dysfunction.
Lymphedema is known to be filled with protein-rich interstitial fluids.25 Specifically, it has been reported that a lymphedematous leg contains approximately eight times more HA than the contralateral leg.10 Consistent with this finding, our carbazole assay performed with HA prepared from tissues from each experimental group indicated that the increased total concentration of HA in lymphedematous tissues was reduced after administration of hyaluronidase (Figure 2(a) and (b)). We saw induced expression of HA and HABP2 in hyaluronidase-injected lymphedema mice. IHC, performed with HA and HABP2 antibodies (Figure 2(c)), was used to localize the distribution of HAs. The localization of HA was increased, along with the total HA content and symptoms of lymphedema, and showed a decrease after treatment with hyaluronidase (Figure 2(d)). HABP2 is a known marker for LMWHA and, in this study, the expression of HABP2 was consistently correlated with the decrease in the volume of lymphedematous tissue and with the enhanced LMWHA accumulation (Figure 2(e)). Although it does not inform a precise quantitative value for LMWHA accumulation, it has been known that HABP2 expression level and LMWHA accumulation are strongly correlated. HMWHA can be cleaved into LMWHA via hyaluronidase.26 HMWHA accumulation decreases HABP2 expression whereas LMWHA accumulation increases HABP2 expression.27 In addition, LMWHA has been a well-known factor for stimulation of HABP2 expression by directly binding to HABP2.28 There have been several reports that LMWHA promotes LYVE-1 expression29,30 and elevates the expression of HABP2 in endothelial cells.31 As a marked decrease in swelling was seen after injecting hyaluronidase into the lymphedematous limb (Figure 3(a) and (b)), it is likely that the mechanism of lymphedema might be modulated by HA regulation.
In the recent study, however, Jeong et al.32 demonstrated that hyaluronidase treatment led to a decrease in lymphedema volume and reduction in neutrophils near normal histological appearance (thickness of dermis) in mouse tail. Moreover, Nekoroski et al.33 also confirmed that the recombinant human hyaluronidase, which kept longer hyaluronidase enzymatic activity, alleviated symptoms of lymphedema in mouse tail lymphedema. We also investigated the change in the amount and size of HA contents in lymphedematous tissues by hyaluronidase (Figure 2(a) and (b)) and visualized it on cross-section tissue (Figure 2(c)). Taken together, we believe that HA modulation by hyaluronidase treatment can provide the potent therapeutic strategy on lymphedema remedy.
One of the most typical characteristics of lymphedema is swelling and expansion between the dermis and epidermis layers.34 Also, it is clearly different from the histological conditions and the size of swelling caused by simple inflammation. H&E staining demonstrated that the expanded layer of lymphedematous tissue that was filled with excess of fluid shrank (Figure 4).
Previous studies for lymphedema therapy have mainly focused on stimulating lymphangiogenesis via exogenous treatment or endogenous expression of growth factors, such as VEGF-C or D.35,36 Nevertheless, this approach cannot be applied to actual clinical use because the main obstacle would be concern over possible tumor metastasis caused by growth factors.37,38 Therefore, unlike conventional trends in lymphedema studies, we focused on targeting one major ECM component for lymphedema therapy. HA has a high water binding capacity.39,40 When the accumulated HA in our lymphedema animal is degraded by injecting hyaluronidase, fluids trapped by HA can be released and are free to move. In addition, a recent study reported that LMWHA was required for lymphangiogenesis through interactions with its receptor LYVE-1.29 We expected that the increased LMWHA caused by hyaluronidase would enhance lymphangiogenesis in the injected region. LYVE-1 has an essential role in biogenesis of lymphatic endothelial cell and in controlling lymphangiogenesis.41 LYVE-1 and VEGFR-3 staining have been used for the quantitative study about the formation of new lymphatic vessels such as in the avascular cornea in their mouse corneal model.42 Thus, LYVE-1 and VEGFR-3 were used for selective markers of the lymphatic endothelium.42 They showed the dilation of lymphatic vessels (Figure 5(a)) and change in the number of lymphatic vessels (Figure 5(b) and (c)) in the dermis layer of hyaluronidase-treated group, which resulted that hyaluronidase treatment led to a reduction in the volume of the lymphedematous region. Also their improved expression (Figure 5(d) and (e)) might demonstrate that lymphatic systems were regenerated. When western blotting for the VEGFR-3 proteins was performed to investigate proliferation of lymphatic endothelial cells by hyaluronidase for 21 days, we determined the highly gradual increase in protein expression of VEGFR-3 in HYAL-LE group during the entire observation period (Figure S2). Based on the high increase in the small size of lymphatic vessels in HYAL-LE group, this result indicated that hyaluronidase treatment could induce small sprouting lymphatic vessels, followed by the promotion of proliferation of lymphatic endothelial cells (Figure 5(f)). Furthermore, lymphangioscintigraphy images from the HYAL-LE group showed ameliorated circulation of lymphatic fluid by regeneration of the lymphatic system blocked from the lower extremity of the body (Figure 6). In this respect, injection of hyaluronidase induced lymphangiogenesis by stimulating regeneration of lymphatic components.
In summary, we developed a model for acquired lymphedema in the mouse hind limb which presents with various symptomatic features detected in lymphedema patients. This newly developed lymphedema model showed more distinct symptoms with respect to volume and morphology in the right lower limb than any other lymphedema animal model. Consequently, control of accumulated HA by hyaluronidase in lymphedematous tissues could repair the histopathological abnormalities in lymphedema by reducing swelling and regenerating the impaired lymphatic circulation. We believe that signaling of the degraded HAs known as LMWHA might have an important role in therapeutic effects on lymphedema. Several sizes of HA fragments have had important roles in specific mechanisms.29,43,44 Further studies about signaling mechanism of various size of HAs and biochemical profiling of various HA sizes produced by hyaluronidase treatment should be performed in vitro cell lines or in vivo model system to understand the mode of action of LMWHA in the lymphangiogenesis among lymphedema symptoms.
Materials and methods
Creation of a new mouse lower limb model of lymphedema
In order to develop a lymphedema model, we designed a new experimental mouse model system (ICR, eight-week-old, male, 33–35 g, Dae Han Bio Link Co., Ltd, Korea) for the induction of lymphedema in the right lower limb. The lymphedema model mouse was prepared by eliminating three lymph nodes (Superficial inguinal lymph node, Popliteal lymph node, Deep inguinal lymph node) and the Femoral lymphatic vessel (SPDF removal group). During surgical procedures, highly controlled incision and electrocauterization were used without bleeding (Figure S1).
A detailed Supplementary Materials and Methods is available online.
Supplementary Material
Acknowledgements
This research was supported by the Global PH.D Fellowship Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2015H1A2A1034469). This work was partly supported by the Korean Association for Vitamin Research and Professor Chang-Hwan Yeom M.D.
Authors’ contributions
KR and SL conceived the experiments. KR, SC, JP, WK conducted the experiments. KR, BCY, SK, CY, KL and SL analyzed the results. All authors reviewed the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental Material
Supplementary material for this paper can be found at http://journals.sagepub.com/doi/suppl/10.1177/1535370216688570
References
- 1.Szuba A, Rockson SG. Lymphedema: classification, diagnosis and therapy. Vasc Med 1998; 3: 145–56. [DOI] [PubMed] [Google Scholar]
- 2.Rockson SG. Lymphedema. Am J Med 2001; 110: 288–95. [DOI] [PubMed] [Google Scholar]
- 3.Szuba A, Rockson S. Lymphedema: anatomy, physiology and pathogenesis. Vasc Med 1997; 2: 321–321. [DOI] [PubMed] [Google Scholar]
- 4.Ryan TJ, De Berker D. The interstitium, the connective tissue environment of the lymphatic, and angiogenesis in human skin. Clin Dermatol 1995; 13: 451–8. [DOI] [PubMed] [Google Scholar]
- 5.Aschen S, Zampell JC, Elhadad S, Weitman E, Andrade MDB, Mehrara BJ. Regulation of adipogenesis by lymphatic fluid stasis part II: expression of adipose differentiation genes. Plast Reconstr Surg 2012; 129: 838–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Can Med Assoc J 2006; 175: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrek JA, Senie RT, Peters M, Rosen PP. Lymphedema in a cohort of breast carcinoma survivors 20 years after diagnosis. Cancer 2001; 92: 1368–77. [DOI] [PubMed] [Google Scholar]
- 8.Kissin M, Della Rovere GQ, Easton D, Westbury G. Risk of lymphoedema following the treatment of breast cancer. Br J Surg 2005; 73: 580–4. [DOI] [PubMed] [Google Scholar]
- 9.Ko DSC, Robert L, Guenter K, Cosimi AB. Effective treatment of lymphedema of the extremities. JAMA Surg 1998; 133: 452–58. [DOI] [PubMed] [Google Scholar]
- 10.Liu N-F, Zhang L-R. Change of tissue fluid hyaluronic acid in peripheral lymphedema. Lymphology 1998; 31: 173–9. [PubMed] [Google Scholar]
- 11.Weissmann B, Meyer K. The structure of hyalobiuronic acid and of hyaluronic acid from umbilical Cord1, 2. J Am Chem Soc 1954; 76: 1753–7. [Google Scholar]
- 12.Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev 2006; 106: 818–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu N, Zhang L. Changes of tissue fluid hyaluronan (hyaluronic acid) in peripheral lymphedema. Lymphology 1998; 31: 173–9. [PubMed] [Google Scholar]
- 14.Granzow JW, Soderberg JM, Kaji AH, Dauphine C. Review of current surgical treatments for lymphedema. Ann Surg Oncol 2014; 21: 1195–201. [DOI] [PubMed] [Google Scholar]
- 15.Rockson SG. Diagnosis and management of lymphatic vascular disease. J Am Coll Cardiol 2008; 52: 799–806. [DOI] [PubMed] [Google Scholar]
- 16.Saito Y, Nakagami H, Morishita R, Takami Y, Kikuchi Y, Hayashi H, Nishikawa T, Tamai K, Azuma N, Sasajima T. Transfection of human hepatocyte growth factor gene ameliorates secondary lymphedema via promotion of lymphangiogenesis. Circulation 2006; 114: 1177–84. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwagi S, Hosono K, Suzuki T, Takeda A, Uchinuma E, Majima M. Role of COX-2 in lymphangiogenesis and restoration of lymphatic flow in secondary lymphedema. Lab Invest 2011; 91: 1314–25. [DOI] [PubMed] [Google Scholar]
- 18.Gong-Kang H, Yuan-Pai H. An experimental model for lymphedema in rabbit ear. Microsurgery 1983; 4: 236–42. [DOI] [PubMed] [Google Scholar]
- 19.Yoon Y-S, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest 2003; 111: 717–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warren LE, Miller CL, Horick N, Skolny MN, Jammallo LS, Sadek BT, Shenouda MN, O’Toole JA, MacDonald SM, Specht MC. The impact of radiation therapy on the risk of lymphedema after treatment for breast cancer: a prospective cohort study. Int J Radiat Oncol Biol Phys 2014; 88: 565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang C-Y, Nguyen DH, Wu C-W, Fang Y-HD, Chao K-T, Patel KM, Cheng M-H. Developing a lower limb lymphedema animal model with combined lymphadenectomy and low-dose radiation. Plast Reconstr Surg Glob Open 2014; 2: e121–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanter MA, Slavin SA, Kaplan W. An experimental model for chronic lymphedema. Plast Reconstr Surg 1990; 85: 573–80. [DOI] [PubMed] [Google Scholar]
- 23.Hwang JH, Kim IG, Lee JY, Piao S, Lee DS, Lee TS, Ra JC, Lee JY. Therapeutic lymphangiogenesis using stem cell and VEGF-C hydrogel. Biomaterials 2011; 32: 4415–23. [DOI] [PubMed] [Google Scholar]
- 24.Chung JK, Kwon YJ, Lee TS, Park HS, Yoo YS, Choi GH, Hahn S, Hwang JH, Lee JY. An experimental study for mouse lymphedema model. Korean J Vasc Endovasc Surg 2011; 27: 114–9. [Google Scholar]
- 25.Warren AG BH, Borud LJ. Lymphedema: a comprehensive review. Ann Plast Surg 2007; 59: 464–72. [DOI] [PubMed] [Google Scholar]
- 26.Lennon FE, Mirzapoiazova T, Mambetsariev N, Mambetsariev B, Salgia R, Singleton PA. Transactivation of the receptor-tyrosine kinase ephrin receptor A2 is required for the low molecular weight hyaluronan-mediated angiogenesis that is implicated in tumor progression. J Biol Chem 2014; 289: 24043–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirzapoiazova T, Mambetsariev N, Lennon FE, Mambetsariev B, Berlind JE, Salgia R, Singleton PA. HABP2 is a novel regulator of hyaluronan-mediated human lung cancer progression. Front Oncol 2015; 5: 164–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lennon FE, Singleton PA. Hyaluronan regulation of vascular integrity. Am J Cardiovasc Dis 2011; 1: 200–200. [PMC free article] [PubMed] [Google Scholar]
- 29.Wu M, Du Y, Liu Y, He Y, Yang C, Wang W, Gao F. Low molecular weight hyaluronan induces lymphangiogenesis through LYVE-1-mediated signaling pathways. PLoS One 2014; 9: e92857–e92857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu M, He P, Liu Y, He Y, Du Y, Wu M, Zhang G, Yang C, Gao F. Hyaluroan-regulated lymphatic permeability through S1P receptors is crucial for cancer metastasis. Med Oncol 2015; 32: 1–8. [DOI] [PubMed] [Google Scholar]
- 31.Wygrecka M, Markart P, Fink L, Guenther A, Preissner KT. Elevated protein levels and altered cellular expression of factor VII-activating protease (FSAP) in the lungs of patients with acute respiratory distress syndrome (ARDS). Thorax 2007; 62: 880–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jeong H, Roh K, Kim G, Kim Y, Lee J, Lee M, Sim Y. Hyaluronidase treatment of acute lymphedema in a mouse tail model. Lymphology 2013; 46: 160–72. [PubMed] [Google Scholar]
- 33.Nekoroski T, Paladini RD, Sauder DN, Frost GI, Keller GA. A recombinant human hyaluronidase sustained release gel for the treatment of post-surgical edema. Int J Dermatol 2014; 53: 777–85. [DOI] [PubMed] [Google Scholar]
- 34.Tabibiazar R, Cheung L, Han J, Swanson J, Beilhack A, An A, Dadras SS, Rockson N, Joshi S, Wagner R. Inflammatory manifestations of experimental lymphatic insufficiency. PLoS Med 2006; 3: e254–e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vlahakis NE, Young BA, Atakilit A, Sheppard D. The lymphangiogenic vascular endothelial growth factors VEGF-C and-D are ligands for the integrin α9β1. J Biol Chem 2005; 280: 4544–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karkkainen MJ, Saaristo A, Jussila L, Karila KA, Lawrence EC, Pajusola K, Bueler H, Eichmann A, Kauppinen R, Kettunen MI. A model for gene therapy of human hereditary lymphedema. Proc Natl Acad Sci USA 2001; 98: 12677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 2001; 7: 192–8. [DOI] [PubMed] [Google Scholar]
- 38.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S-I, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med 2001; 7: 186–91. [DOI] [PubMed] [Google Scholar]
- 39.Duranti F, Salti G, Bovani B, Calandra M, Rosati ML. Injectable hyaluronic acid gel for soft tissue augmentation. Dermatol Clin 1998; 24: 1317–25. [DOI] [PubMed] [Google Scholar]
- 40.Lupton JR, Alster TS. Cutaneous hypersensitivity reaction to injectable hyaluronic acid gel. Dermatol Surg 2000; 26: 135–7. [DOI] [PubMed] [Google Scholar]
- 41.Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 2002; 21: 1505–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cao R, Lim S, Ji H, Zhang Y, Yang Y, Honek J, Hedlund E-M, Cao Y. Mouse corneal lymphangiogenesis model. Nat Protoc 2011; 6: 817–26. [DOI] [PubMed] [Google Scholar]
- 43.Park B-G, Lee CW, Park JW, Cui Y, Park Y-S, Shin W-S. Enzymatic fragments of hyaluronan inhibit adipocyte differentiation in 3T3-L1 pre-adipocytes. Biochem Biophys Res Commun 2015; 467: 623–8. [DOI] [PubMed] [Google Scholar]
- 44.Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS One 2011; 6: e26078–e26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.