Abstract
R. officinalis L. is an aromatic plant commonly used as condiment and for medicinal purposes. Biological activities of its extract were evaluated in this study, as antimicrobial effect on mono- and polymicrobial biofilms, cytotoxicity, anti-inflammatory capacity, and genotoxicity. Monomicrobial biofilms of Candida albicans, Staphylococcus aureus, Enterococcus faecalis, Streptococcus mutans and Pseudomonas aeruginosa and polymicrobial biofilms composed of C. albicans with each bacterium were formed in microplates during 48 h and exposed for 5 min to R. officinalis L. extract (200 mg/mL). Its cytotoxic effect was examined on murine macrophages (RAW 264.7), human gingival fibroblasts (FMM-1), human breast carcinoma cells (MCF-7), and cervical carcinoma cells (HeLa) after exposure to different concentrations of the extract, analyzed by MTT, neutral red (NR), and crystal violet (CV) assays. The anti-inflammatory activity was evaluated on RAW 264.7 non-stimulated or stimulated by lipopolysaccharide (LPS) from Escherichia coli and treated with different concentrations of the extract for 24 h. Interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α) were quantified by ELISA. Genotoxicity was verified by the frequency of micronuclei (MN) at 1000 cells after exposure to concentrations of the extract for 24 h. Data were analyzed by T-Test or ANOVA and Tukey Test (P ≤ 0.05). Thus, significant reductions in colony forming units per milliliter (CFU/mL) were observed in all biofilms. Regarding the cells, it was observed that concentrations ≤ 50 mg/mL provided cell viability of above 50%. Production of proinflammatory cytokines in the treated groups was similar or lower compared to the control group. The MN frequency in the groups exposed to extract was similar or less than the untreated group. It was shown that R. officinalis L. extract was effective on mono- and polymicrobial biofilms; it also provided cell viability of above 50% (at ≤ 50 mg/mL), showed anti-inflammatory effect, and was not genotoxic.
Impact statement
Rosmarinus officinalis L. extract effectively contributed to in vitro control of important species of microorganisms such as Candida albicans, Staphylococcus aureus, Enterococcus faecalis, Streptococcus mutans, and Pseudomonas aeruginosa in mono- and polymicrobial biofilms that are responsible for several infections in oral cavity as in other regions of the body. Furthermore, this extract promoted also cell viability above 50% at concentrations ≤ 50 mg/mL, excellent anti-inflammatory effect, showing inhibition or reduction of the synthesis of proinflammatory cytokines, being also non-genotoxic to cell lines studied. Thus, this extract may be a promising therapeutic agent that can be added in some medical and dental formulations such as toothpastes, mouthwashes, irrigating root canals, ointments, soaps, in order to control pathogenic microorganisms and biofilms, with anti-inflammatory effect and absence of cytotoxic and genotoxic.
Keywords: Rosmarinus officinalis L., antibiofilm activity, antiproliferative activity, antimutagenic activity, anti-inflammatory activity
Introduction
R. officinalis L. (Lamiaceae) is a perennial woody plant species, originated from the Mediterranean region, which currently can be found and cultivated in all continents as aromatic and ornamental plant; its leaves are commonly used as a condiment and also serve medicinal purposes.1 Its major constituents responsible for the pharmacological activities are 1,8-cineole (52.2%), camphor (15.2%), and α-pinene (12.4%).2
Biofilms can be described as a micro ecosystem formed by different species of microorganisms, surrounded by a protein extracellular matrix and polysaccharides produced by them. They can be adhered both to an abiotic surface, such as dental materials, prostheses, implants, endotracheal tube, pacemakers and catheters, or a biotic surface, such as host tissues.3–5 They are naturally found in interspecific associations in different niches. In these associations, a microorganism may favor or hinder the development of other, interfere with antimicrobial susceptibility, as well as interfere with the expression of genes that may lead to generation of pathogenic forms.6,7
C. albicans biofilm can be formed after contact with a suitable surface, and its development will depend on favorable conditions. In the initial stage, the adhered yeast forms initiate the formation of germ tube, and subsequently, in the intermediate phase, there will be an elongation of these hyphae and production of extracellular matrix, composed of carbohydrates and proteins. Following, it occurs the formation of a mature biofilm, composed by a yeasts base where the hyphal forms follow adhered, involved by the matrix.8,9 S. aureus is clinically relevant because it has high levels of systemic infections and mortality related to accumulation of its biofilm in medical devices.10 It has been estimated that about 27% of candidemias in nosocomial infections occurred in association with other microorganisms, being S. aureus the third most common.11 According to Harriott and Noverr,4 C. albicans can contribute to the formation of S. aureus biofilm, and thus increase the resistance of the bacteria to the action of antibiotics.
In a study conducted in the nematode Caenorhabditis elegans, with infection of C. albicans and E. faecalis, it was observed that there was no death of C. elegans due to this microbial association and an inhibition of the hyphae of C. albicans by E. faecalis action was also observed, which favored the survival of the nematode. Both microorganisms were commensal and non-pathogenic to C. elegans, but separately presented a highly pathogenic effect.12
In the oral cavity, C. albicans can form a complex microbial community with Streptococcus spp.13,14 and may influence the pathogenesis of dental caries, particularly in pediatric patients,15,16 which can form a virulent biofilm on the teeth surface of these patients.17,18
P. aeruginosa, in the oral cavity, may cause a more aggressive form of periodontitis just by their presence in supragingival biofilm.19 From the oral cavity, P. aeruginosa can be disseminated systemically and cause respiratory infection, particularly in hospitalized and immunosuppressed patients.20
The action of different types of R. officinalis L. extracts was reported on some tumor cell lines such as the ovarian cancer (SK-OV-3 and HO-8910), hepatocellular carcinoma (Bel-7402),21 colorectal cancer (SW620 and DLD-1), pancreas cancer (MIA-PaCa-2 and PANC-1),22 prostate cancer (LNCaP and 22Rv1),23 colorectal adenocarcinoma (LoVo), hepatocarcinoma (HepG2). On the target lineages in this study, MCF-724 and HeLa,25 the action of R. officinalis L has also been reported; however, in our study, we used three different cell viability tests and glycolic extract.
Studies have been undertaken in order to seek alternative ways to inhibit some inflammatory mechanisms that, for some reason, are biologically disarranged and tend to be harmful to the host. Thus, the anti-inflammatory effect of R. officinalis L. has been investigated regarding its ability to control the synthesis of proinflammatory cytokines, growth factors, nitric oxide (NO), and prostaglandin,26 and check its effect on immune cells migration.27
It was proposed that the ethanol extract of R. officinalis L. can promote protective effect against DNA damage.28 According to these authors, human lymphocytes exposed to R. officinalis L. extract showed no genotoxicity, in the group treated with the extract and exposed to hydrogen peroxide (H2O2), it was noticed DNA-protective effect.
Based on these findings, the objectives of this study were to evaluate some biological activities of R. officinalis L. glycolic extract, such as antimicrobial activity against C. albicans, S. aureus, E. faecalis, S. mutans, and P. aeruginosa in planktonic cultures and mono- and polymicrobial biofilms of C. albicans associated with S. aureus, E. faecalis, S. mutans, and P. aeruginosa, cytotoxicity to RAW 264.7, FMM-1, MCF-7 and HeLa, anti-inflammatory activity in LPS-stimulated RAW 264.7 and genotoxic activity on the cell lines studied.
Materials and methods
Plant extract and microbial strains
R. officinalis L. extract was commercially acquired (Mapric, SP, Brazil) at a concentration of 200 mg/mL of propylene glycol. This extract was obtained from leaves of the plant, being chemically composed of terpene derivatives such as pinene, camphene, free borneol and borneol acetate, cineol, camphor, besides sesquiterpenes, oleanolic acid, little tannin, bitter substances, acid saponin, and glucosidic compounds, according to the manufacturer.
Reference strains (ATCC – American Type Culture Collection) of C. albicans (ATCC 18804), S. aureus (ATCC 6538), E. faecalis (ATCC 4083), S. mutans (ATCC 35688), and P. aeruginosa (ATCC 15442) obtained from Institute of Science and Technology/UNESP were used in this study. Strains were kept frozen (−80℃) in Brain Heart Infusion broth (BHI – Himedia, Mumbai, India) with 20% glycerol, for bacteria, and Yeast Extract Peptone Dextrose broth (YPD – Himedia) with 16% glycerol, for C. albicans.
Antimicrobial activity against planktonic cultures – Broth microdilution method
For the determination of minimum inhibitory (MIC) and minimum microbicidal (MMC) concentrations of the extract, microdilution broth method was used, according to the Clinical and Laboratory Standards Institute (CLSI).29–31 Firstly, bacteria were grown in BHI agar (Himedia) and C. albicans in Sabouraud dextrose agar (SD – Himedia) for 24 h at 37℃ with 5% CO2 for S. mutans. Then, the microbial suspensions were prepared in sterile saline (0.9% NaCl). The turbidity of the suspensions was adjusted to 106 CFU/mL (colony forming units per milliliter) in spectrophotometer (Micronal, São Paulo, Brazil). The culture medium used for bacteria growth was Mueller Hinton broth (Himedia) and for C. albicans was used RPMI 1640 broth (Himedia) with glutamine, without bicarbonate and phenol red indicator, buffered to pH 7.0 ± 0.1 with MOPS [3-(N-morpholino) propanesulfonic acid] (Sigma-Aldrich, St. Louis, USA). The extract microdilutions were performed in 96-well plates (TPP, Trasadingen, Switzerland), where 100 µL of culture medium were added in 10 wells and 100 µL of R. officinalis L. extract (200 mg/mL) only in the first well, where serial dilutions (1:2) started till the 10th dilution. Then, 100 µL of the standardized microbial suspension were added in all the wells. Thus, the inoculum concentrations were approximately 5 × 105 CFU/mL for the bacteria, and 5 × 102 to 2.5 × 103 CFU/mL for C. albicans. The concentrations of the extract were diluted from 50 to 0.09 mg/mL. Wells for growth control (C-, medium plus inoculum) and medium (C+, medium alone) were added. After 24 h incubation, MIC was determined at the last well of the microplate which was not observed turbidity. For determining MMC, 100 µL of MIC and its previous wells were seeded on BHI or SB agar. After 48 h of incubation, the lowest concentration was determined which showed no microbial growth.
Antimicrobial activity against mono- and polymicrobial biofilms
Microorganisms were first cultured on solid medium (BHI or SD agar) and then in liquid medium (BHI broth or Yeast Nitrogen Base – YNB, Himedia) for 24 h at 37℃ (5% CO2 for S. mutans). The generated microbial suspension was centrifuged at 2000 rpm/10 min (MPW-350, Warsaw, Poland), the supernatant discarded and the pellet suspended in saline. This procedure was repeated twice. Thereafter, the turbidity of the suspension was adjusted to 107 CFU/mL in a spectrophotometer, and it was distributed in 96-well plates, 200 µL/well of this suspension. Plates were brought to incubation under agitation (37℃; 75 rpm – Quimis, Diadema, Brazil) for 90 min to initial adhesion of microorganisms. Then, the supernatant was discarded and added 200 µL of BHI or YNB broth. The plates were incubated for 48 h for the formation of biofilm; however, after 24 h, the culture medium was replaced by fresh medium.
The polymicrobial biofilms were formed in the same manner but equal parts of the standardized suspensions were added, i.e. 100 µL of each suspension and for growing in the wells, equal parts of BHI and YNB, 100 µL of each were also added.
After 48 h, biofilms were exposed to R. officinalis L. extract (200 mg/mL) (n = 10) for 5 min and saline was used as a negative control (n = 10). Cells affected by the extract were removed by washing with saline. Then, the biofilm has disaggregated by ultrasound homogenizer (Sonopuls HD 2200 – Bandelin Eletronic, Berlin, Germany) for 30 s and 25% power. The generated suspension was serially diluted and 100 µL were seeded into BHI or SD agar. In the case of polymicrobial biofilms, selective agar were used, as SD with chloramphenicol (1%) for C. albicans, BHI with 75 mg NaCl/mL medium for S. aureus, Mitis salivarius with 20% sucrose and 0.2 international units (IU) of bacitracin/mL medium for S. mutans, m-Enterococcus (Difco) for E. faecalis and MacConkey (Difco) for P. aeruginosa. After 48 h incubation, CFU were counted and CFU/mL were calculated.
Cell culture and preparation of test solutions
FMM-1 (Faculty of Dentistry, University of São Paulo, São Paulo, Brazil), RAW 264.7 (Rio de Janeiro Cell Bank, APABCAM, Rio de Janeiro, Brazil), MCF-7 and HeLa (Adolfo Lutz Institute, São Paulo, Brazil) were used in this study. The cells were maintained in Dulbecco's modified Eagle medium (DMEM – LGC, Cotia, Brazil) with 10% fetal bovine serum (Invitrogen, New York, USA) and 1% penicillin-streptomycin (Gibco, Grand Island, United States) at 37℃ and 5% CO2 with atmospheric humidity. Viable cells were quantified by Trypan blue (0.4%, Sigma-Aldrich) and in 96-well plates DMEM containing 4 × 104 cells was added (200 µL/well).
R. officinalis L. extract was diluted in DMEM at concentrations of 25, 50, and 100 mg/mL and DMEM was used as negative control (0 mg/mL), n = 10/group. After 24 h, each culture was exposed for 5 min. In order to discard cells that did not survive the treatment washes with phosphate-buffered saline (PBS) were performed. Then, cell viability tests were applied.
Cell viability tests – MTT, NR, and CV assays
In MTT assay, reductases present in viable cells break MTT generating formazan. Therefore, MTT solution (0.5 mg/mL PBS) was added (100 µL/well). After 1 h incubation, under protection from light, the supernatant was discarded and dimethyl sulfoxide (Sigma Aldrich) was added (100 µL/well). The plate was incubated (10 min) and agitated in a shaker (Solab, Piracicaba, Brazil) for more than 10 min. Through NR assay the incorporation of this dye into lysosomes of viable cells was checked. NR solution (20 µg/mL PBS) was added (100 µL/well) and after 2 h, the solution was removed and pure ethyl alcohol was added (100 µL/well). The plate was agitated in shaker for 15 min. CV assay was verified by DNA staining of viable cells. Firstly, cells were fixed for 10 min with 10% formaldehyde (Synth, São Paulo, Brazil) and then CV solution (0.2 mg/mL distilled water) was added (100 µL/well). After 15 min, the dye was discarded and the wells were washed with distilled water until no presence of the dye was found. Pure ethyl alcohol was added (100 µL/well) and the plate followed to shake for 10 min. In all tests, the absorbance of the wells was measured by spectrophotometer (Bio-Tek, Vermont, USA) at 570 nm and data generated were converted to cell viability percentage.
Anti-inflammatory activity
RAW 264.7 was cultured in 24-well plates (TPP) at a concentration of 5 × 105 cells/mL of DMEM for 24 h. In the group of non-LPS (Sigma-Aldrich) from E. coli, the supernatant was discarded and R. officinalis L. extract diluted in DMEM at concentrations of 25, 50, and 100 mg/mL was added, and DMEM was used as control (0 mg/mL) with n = 10/experimental group. In the group with LPS, in these concentrations 1 µg/mL LPS (n = 10/group) was added. After exposure for 24 h, the supernatant was collected in microtubes and stored at −20℃ for subsequent analysis of proinflammatory cytokines (IL-1β and TNF-α).
The levels of IL-1β and TNF-α, collected from RAW 264.7 supernatants, were analyzed by ELISA sandwich method. Commercial kits were used (R&D Systems, Minneapolis, USA) and DY401 catalog for IL-1β and DY410 catalog for TNF-α according to the manufacturer's guidance. The absorbance of the wells was assessed by microplate spectrophotometer (450 nm) and data were converted to picograms per milliliter (pg/mL), taking into account the standard curve values of IL-1β or TNF-α, with GraphPad Prism 5.0 software.
Genotoxicity – Micronucleus (MN) assay
The test was applied separately in all cell lines. Firstly, 2 × 104 cells/mL of DMEM were cultured in 24-well plates for 24 h. Then, the supernatant was discarded and R. officinalis L. extract diluted in DMEM (25, 50, 100 mg/mL) or only DMEM (0 mg/mL) were added with n = 2/experimental group. After incubation for 24 h, supernatant was discarded and washing was done with PBS to discard the non-viable cells. Subsequently, the cells were fixed for 10 min with 10% formaldehyde. After new washing, 200 µL of PBS and one drop of fluorshield with DAPI (Sigma-Aldrich) was added. The plate was taken to agitation for 5 min, and protected from light. Then, with the aid of a fluorescence microscope (Axiovert 200 – Zeiss, Jena, Germany), the MN frequency was observed in 1000 cells counted, both stained blue.
Statistical analysis
The results, analyzed by the GraphPad Prism 5.0 and Minitab 17, were presented as mean values (± standard deviation). It was considered statistically significant when P ≤ 0.05. The results of the antimicrobial activity were analyzed by T-Test or ANOVA and Tukey Test. Data of cell viability, anti-inflammatory activity, and genotoxicity were analyzed by ANOVA and Tukey Test.
Results
Action on planktonic cultures
Planktonic cultures of C. albicans, S. aureus, E. faecalis, S. mutans, and P. aeruginosa had growth inhibition at concentrations ≤ 50 mg/mL. However, C. albicans (3.13 mg/mL) and P. aeruginosa (6.25 mg/mL) showed elimination, other microorganisms showed MMC > 50 mg/mL (Table 1).
Table 1.
Antimicrobial effect of R. officinalis L. extract on planktonic forms
| Microorganism | mg/mL |
|
|---|---|---|
| MIC | MMC | |
| C. albicans | 0.78 | 3.13 |
| S. aureus | 25 | > 50 |
| E. faecalis | 50 | > 50 |
| S. mutans | 25 | > 50 |
| P. aeruginosa | 6.25 | 6.25 |
Note: Values (mg/mL) of minimal inhibitory concentration (MIC) and minimal microbicidal concentration (MMC) of R. officinalis L. extract (200 mg/mL), verified after 24 h of exposure.
Mono- and polymicrobial biofilms
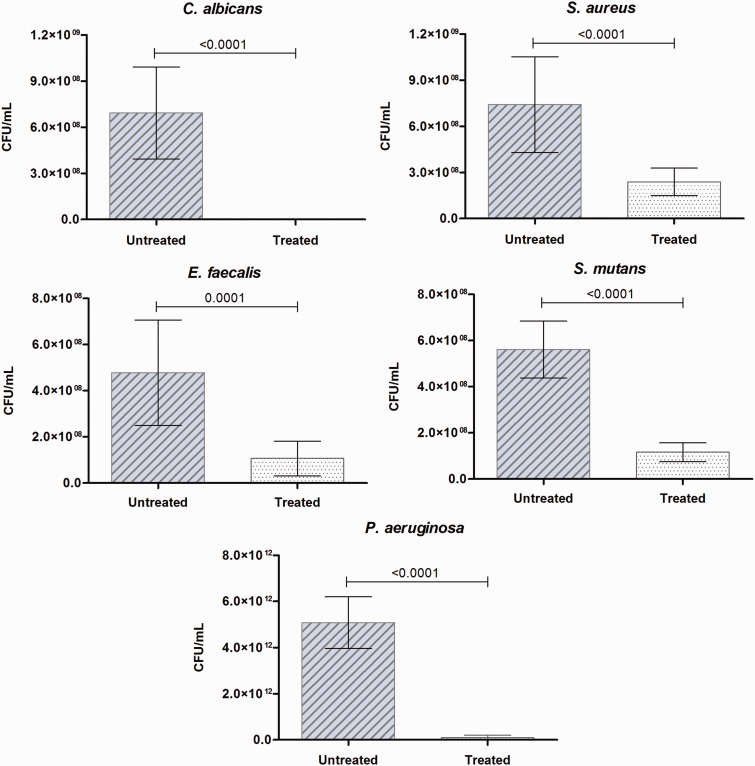
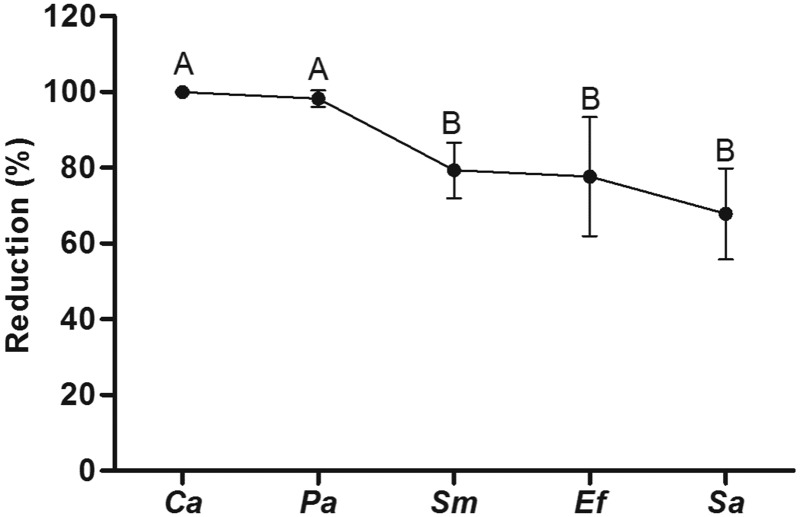
There was significant decrease of CFU/mL in monomicrobial biofilms of C. albicans, S. aureus, E. faecalis, S. mutans, and P. aeruginosa after treatment with R. officinalis L. extract (200 mg/mL) for 5 min (Figure 1). Thus, significant reductions were observed in these biofilms after exposure to the extract (Figure 2), most notably in C. albicans and P. aeruginosa, which showed complete elimination of biofilm.
Figure 1.
Action of R. officinalis L. extract on monomicrobial biofilms. Mean values (± standard deviation) of CFU/mL of C. albicans, S. aureus, E. faecalis, S. mutans and P. aeruginosa biofilms presented in untreated group (0.9% NaCl) and treated group with R. officinalis L. extract (200 mg/mL) for 5 min. P values follow on the columns (n = 10. T-Test, P ≤ 0.05). (A color version of this figure is available in the online journal.)
Figure 2.
Reduction percentage of monomicrobial biofilms. After exposure to R. officinalis L. extract (200 mg/mL) for 5 min, significant reductions were observed in the biofilms of C. albicans (Ca), S. aureus (Sa), E. faecalis (Ef), S. mutans (Sm) and P. aeruginosa (Pa). The groups were reunited according to their homogeneity. Statistically significant differences can be observed among groups with different superscript letters. (n = 10. ANOVA, Tukey test, P ≤ 0.05)
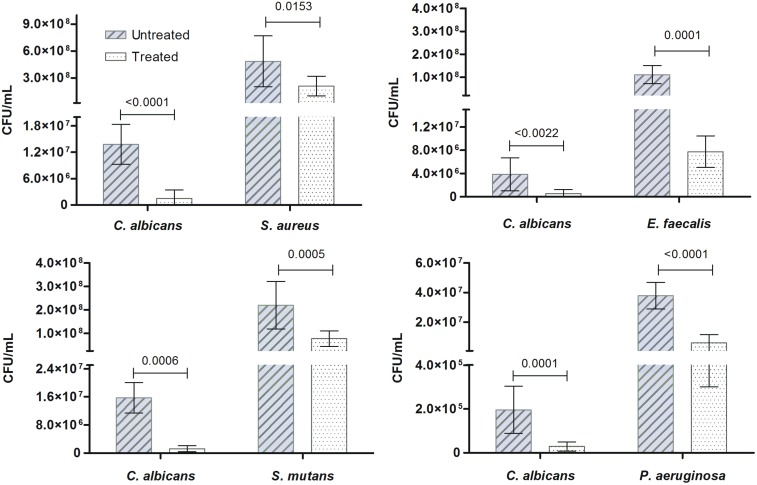
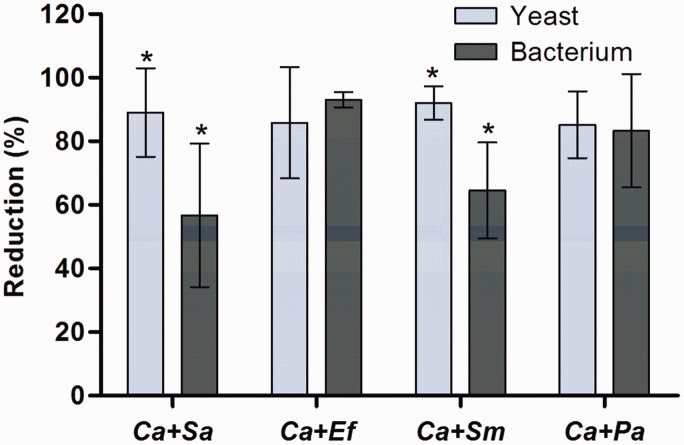
As in monomicrobial biofilms, the antibiofilm effect of R. officinalis L. extract was also observed in polymicrobial associations between C. albicans and each bacterium, i.e. S. aureus, E. faecalis, S. mutans, and P. aeruginosa (Figure 3). Although a similar amount of CFU/mL for each microorganism was used for biofilm formation; after 48 h of incubation, a significant difference was observed between CFU/mL amount of C. albicans and each bacterium, being greater the amount of bacteria in all the associations. However, the application of R. officinalis L. extract (200 mg/mL) for 5 min resulted in a significant decrease in CFU/mL number in all treated groups, taking into consideration the untreated group. Additionally, in Figure 4, it was observed that the rate of reduction of both, yeast and bacteria, in polymicrobial biofilm was similar in the associations of C. albicans and E. faecalis and C. albicans and P. aeruginosa. In biofilms composed by C. albicans and S. aureus and C. albicans and S. mutans, there was a greater reduction in yeast when compared to the bacterium (Figure 4).
Figure 3.
Action of R. officinalis L. extract on polymicrobial biofilms. Mean (± standard deviation) of CFU/mL of polymicrobial associations of C. albicans with S. aureus, E. faecalis, S. mutans and P. aeruginosa presented in the untreated group (0.9% NaCl) and treated groups with R. officinalis L. extract (200 mg/mL) for 5 min. P values follow on the columns (n = 10. T-Test, P ≤ 0.05). (A color version of this figure is available in the online journal.)
Figure 4.
Reduction percentage of polymicrobial biofilms. Data obtained in polymicrobial associations of C. albicans with S. aureus (Ca + Sa), E. faecalis (Ca + Ef), S. mutans (Ca + Sm) and P. aeruginosa (Ca + Pa) after exposure to R. officinalis L. extract (200 mg/mL) for 5 min. Statistically significant difference between the reductions in yeast and bacteria in each association can be observed among groups with superscript asterisks. (n = 10. T-Test, P ≤ 0.05). (A color version of this figure is available in the online journal.)
Cell viability of RAW 264.7, FMM-1, MCF-7, and HeLa
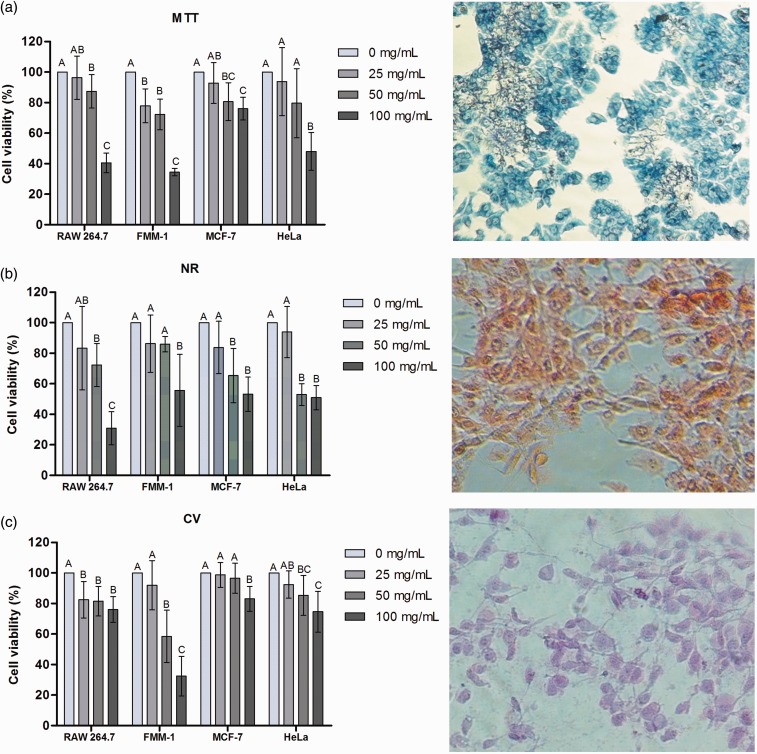
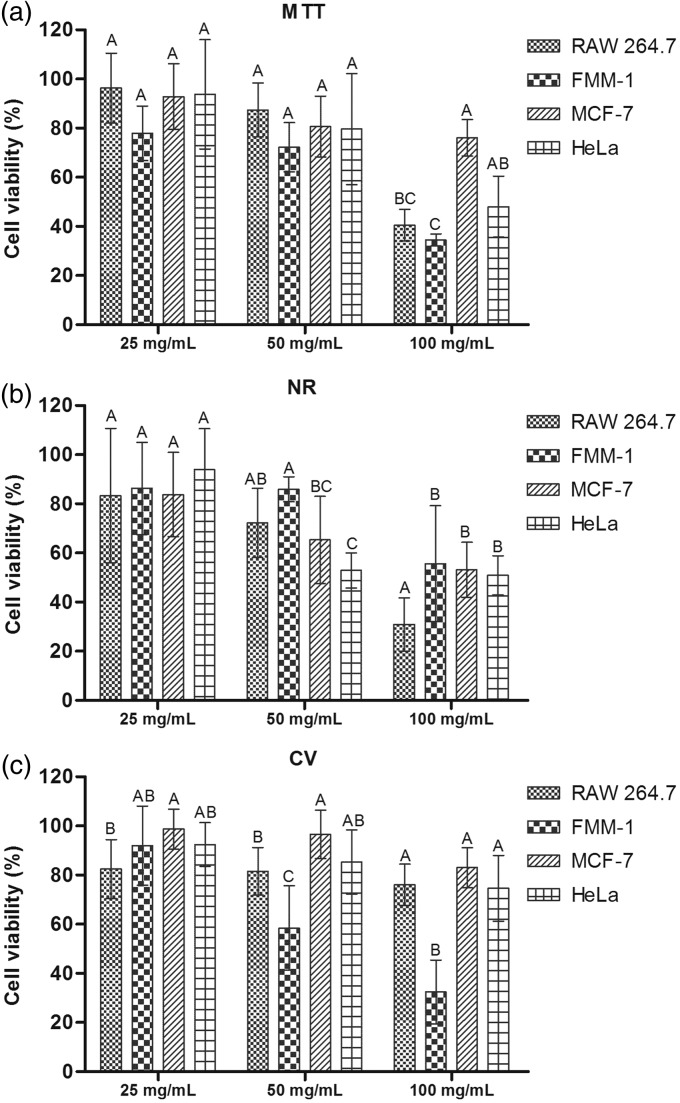
In Figure 5, the cell viability percentage can be seen in cultures exposed to concentrations of the extract analyzed by MTT test (Figure 5(a)), NR (Figure 5(b)) and CV (Figure 5(c)). The cell viability percentage of the lineages in each of the concentrations tested is demonstrated in Figure 6.
Figure 5.
Cell viability verified by MTT, NR and CV assays. After exposure of RAW 264.7, FMM-1, MCF-7 and HeLa at concentrations of 25, 50 and 100 mg/mL of R. officinalis L. extract, cell viability of the cultures, compared to the control group (0 mg/mL), were analyzed by: (a) reduction of MTT salt to formazan; (b) Incorporation of neutral red (NR) in the lysosomes; and (c) DNA staining with crystal violet (CV). Statistically significant differences among experimental groups can be observed with different superscript letters (n = 10. ANOVA, Tukey Test, P ≤ 0.05). Optical microscopy (200×). (A color version of this figure is available in the online journal.)
Figure 6.
Cell viability percentage obtained in each concentration of the R. officinalis L. Data were obtained in RAW 264.7, FMM-1, MCF-7 and HeLa cultures, after exposure for 5 min to concentrations of R. officinalis L. extract (25, 50 e 100 mg/mL), and analyzed by MTT (a), NR (b) and CV (c) assays. Different superscript letters indicate statistically significant differences among experimental groups. (n = 10. ANOVA, Tukey Test, P ≤ 0.05)
Anti-inflammatory activity
In the presence of LPS, it was found that the concentrations of R. officinalis L. extract afforded significant inhibition of cytokines production (Table 2). Likewise, in the quantification of TNF-α level was observed both in the absence or presence of LPS, there was significant inhibition of this cytokine production with the application of the plant extract concentrations.
Table 2.
Production of proinflammatory cytokines IL-1β and TNF-α by RAW 264.7, in the presence or absence of LPS
| Group (mg/mL) | Cytokine (pg/mL) |
|||
|---|---|---|---|---|
| IL-1β |
TNF-α |
|||
| no LPS | LPS | no LPS | LPS | |
| 0 | 2.7 ± 5.79A | 20.02 ± 11.17A | 19.74 ± 10.99A | 8125.46 ± 7305.34A |
| 25 | 0.91 ± 1.86A | 1.02 ± 2.24B | 3.65 ± 3.28B | 28.60 ± 34.66B |
| 50 | 1.44 ± 1.99A | 1.72 ± 3.63B | 3.87 ± 2.66B | 16.55 ± 8.86B |
| 100 | 0.27 ± 0.85A | 0B | 2.38 ± 2.51B | 4.77 ± 4.2B |
Note: Mean values (± standard deviation) of IL-1β and TNF-α (pg/mL) production by RAW 264.7 after contact with concentrations of 25, 50 or 100 mg/mL of R. officinalis L. extract for 24 h in the absence or presence of LPS (1 µg/mL). Statistically significant differences among experimental groups can be observed with different superscript letters (A and B in the table). (n = 10. ANOVA, Tukey Test, P ≤ 0.05)
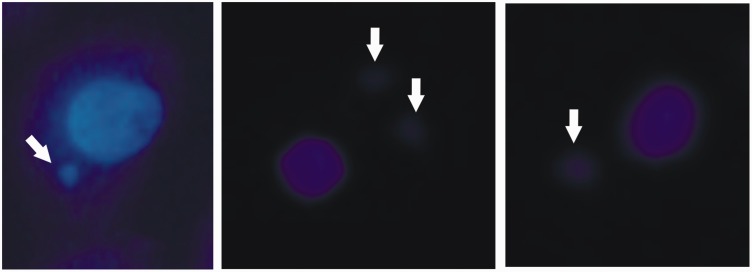
MN frequency
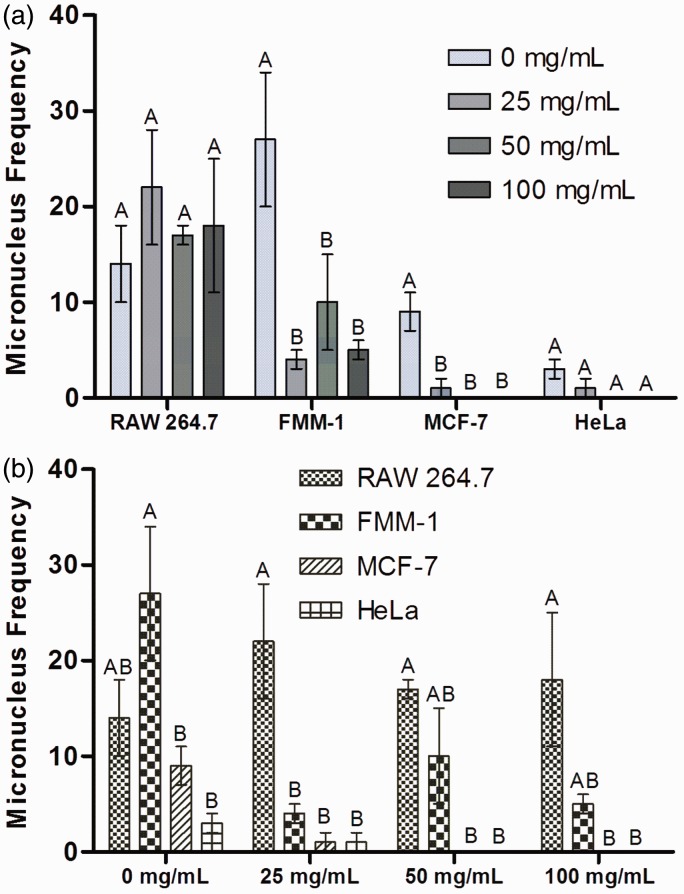
Several materials can cause damage to DNA, represented by the presence of small structures close to the cell nucleus called micronuclei (Figure 7). It was found that the frequency of MN was similar among treated and control (0 mg/mL) groups for RAW 264.7 and HeLa (Figure 8(a)). For FMM-1 and MCF-7, the concentrations of the extract afforded protection against DNA damage, since the presence of MN was lower than the control group. Additionally, the MN frequency formed by cell after exposure to each concentration (0, 25, 50, and 100 mg/mL) can be analyzed in Figure 8(b). At control group (0 mg/mL) it can be found that the formation of MN was higher in FMM-1 than in MCF-7 and HeLa. After exposure to 25 mg/mL, RAW 264.7 showed more MN than the other lineages. Concentrations of 50 and 100 mg/mL do not provide MN formation in MCF-7 and HeLa.
Figure 7.
Micronuclei (MN). MN are DNA fragments located around and close to the cell nucleus (indicated by white arrows), and may have variable size but always smaller than the cell nucleus and varied amount, as shown in the figure bottom right, which shows two MN. Its presence characterizes DNA damage, provided by intrinsic or extrinsic causes. After fixing the cells, previously treated or not with different concentrations of R. officinalis L. extract, DAPI dye was added and the nuclei and MN were observed through fluorescence microscopy (200×) and then the frequency of MN was determined after counting 1000 nuclei. (A color version of this figure is available in the online journal.)
Figure 8.
Micronuclei (MN) frequency presented by cells. RAW 264.7, FMM-1, MCF-7 and HeLa were exposed to concentrations of 25, 50 and 100 mg/mL of R. officinalis L. extract. After 24 h, MN frequency was counted. (a) MN frequency presented by the four cell lineages per 1000 cells counted. (b) MN frequency obtained in each experimental group (0, 25, 50 and 100 mg/mL). Statistically significant difference among treated groups and control groups can be observed with different superscript letters (n = 2. ANOVA, Tukey Test, P ≤ 0.05). (A color version of this figure is available in the online journal.)
Discussion
According to the results obtained on planktonic cultures, the concentration of 200 mg/mL on biofilms was applied, since controlling these communities require higher concentrations of antimicrobial agents in order to significantly affect their structures.32 Thus, after exposure to the extract, significant reductions were observed in monomicrobial biofilms of C. albicans (99.96 ± 0.07%), S. aureus (67.84 ±12.05%), E. faecalis (77.64 ± 15.67%), S. mutans (79.32 ±7.34%), and P. aeruginosa (98.23 ± 2.17%) (Figure 2). Although they were all affected by the extract, C. albicans and P. aeruginosa biofilms showed the highest reduction percentage.
Regarding polymicrobial biofilms, it was observed that there were significant reductions in both yeast and bacteria, after treatment with the extract (Figure 4). However, the reduction percentages showed by each microorganism varied after this exhibition. In association C. albicans with S. aureus, it was found that the yeast (89 ± 13.89%) showed the higher reduction percentage than the bacterium (56.75 ± 22.58%). It was reported that the development of C. albicans can be harmed by S. aureus, since this bacteria can easily adhere to C. albicans hyphae, forming a base composed by hyphae in which staphylococci adhere.4 Likewise, reduction of C. albicans (92.04 ± 5.24%) was greater than S. mutans (64.55 ± 15.12%) in this association. According to Pereira-Cenci et al.,33 this yeast favors the development of S. mutans biofilm. In polymicrobial biofilms, the different species may compete and this could harm or favor the development of each other.6,7
In association of C. albicans (85.87 ± 17.48%) with E. faecalis (93.03 ± 2.44%), there was no significant difference between the reductions presented by the yeast and the bacterium. Cruz et al.,12 noted that C. albicans associated with E. faecalis, during an in vivo infection (C. elegans), prevented bacterium cell death and the bacterium consequently inhibited the formation of C. albicans hyphae, which resulted in the survival of the host. Thus, the authors noted that these microorganisms were commensal for C. elegans and, out polymicrobial association, they were considered pathogenic to the host. The biofilm composed by C. albicans (85.19 ± 10.48%) and P. aeruginosa (83.33 ± 17.79%) also showed no significant difference between the reductions showed by the microorganisms. Morales et al.34 attributed to phenazine, an enzyme produced by P. aeruginosa, the regulation of the of fungal cells growth in polymicrobial biofilm, as well as the control of hyphal formation of C. albicans. Thus, some balance was noticed between the species in the biofilm formed by C. albicans and E. faecalis and C. albicans and P. aeruginosa.
Cell viability assays in RAW 264.7, FMM-1, MCF-7, and HeLa showed that the concentration of 100 mg/mL resulted in a significant decrease in cell viability in most groups, previously confirmed by the three tests. In some cases, cell viability reached levels lower than 50%, such as RAW 264.7, FMM-1, and HeLa cells, assessed by MTT assay (Figure 6(a)), in all lineages analyzed by NR assay (Figure 6(b)) and FMM-1, as verified by CV assay (Figure 4(c)). In many cases, the concentration of 25 mg/mL provided cell viability similar to the control group, verified on RAW 264.7, MCF-7, and HeLa cells, by MTT assay, on all lines, by NR assay, and on FMM-1, MCF 7 and HeLa, by CV assay. The concentration of 50 mg/mL showed some peculiarities, such as: (i) lower cell viability than the control group, but with percentages of viability higher the concentration of 100 mg/mL, as shown on RAW 264.7 and FMM-1 (MTT), RAW 264.7 (NR) and FMM-1 (CV); (ii) lower viability than the control, but similar to the concentration of 100 mg/mL, as seen in MCF-7 (MTT), MCF-7 and HeLa (NR) and HeLa cells (CV); (iii) show similarity compared to the control and 25 mg/mL, as observed in HeLa (MTT), FMM-1 (NR), and MCF-7 (CV); and presenting similarity between the concentrations of 25 and 100 mg/mL and the control, as noted in RAW 264.7 (CV). Thus, it can be suggested that some concentrations could interfere with cellular metabolism, harming its enzymatic action and also affecting the lysosomal activity. In this case, it may contribute to the interference of particles entrance and exit of the cell, damaging many of its functions.35 However, generally, they could promote inhibition of DNA damage; it was found that cell viability of RAW 264.7, MCF-7, and HeLa cells was above 50% after application of all concentrations, by CV assay. The extract may provide protective effects on DNA, especially on MCF-7 and HeLa. Thus, it can be noted that the cellular targets of therapeutic agents must be carefully studied, since certain cell structures may be more or less affected, interfering with its control and viability.
Regarding anti-inflammatory activity of R. officinalis L., it was noted that all concentrations analyzed demonstrated animmunomodulatory effect (Table 2). The production of IL-1β and TNF-α in the treated groups (100, 50, 25 mg/mL) was lower than in control group (0 mg/mL), demonstrating that the extract can inhibit the natural synthesis of these pro-inflammatory cytokines. In addition, in the groups stimulated with LPS, it was found that all concentrations also promoted immunomodulatory effect on the production of these cytokines, since there were significant reductions in their synthesis with the extract. Similarly, in the study of Yu et al.,26 the anti-inflammatory effect of R. officinalis L. on LPS-stimulated RAW 264.7 was also demonstrated.
In the genotoxicity test, where the MN frequency was verified, it was found that all tested concentrations did not stimulate DNA damage, i.e. they were DNA-protective, since the MN frequency was statistically similar to the control group for RAW 264.7 and significantly lower in the case of the other cells (Figure 8). The concentrations of 50 and 100 mg/mL completely inhibited the production of MN in HeLa and MCF-7. With this, it was evident that R. officinalis L. extract showed no mutagenic effect for the studied cells. This DNA-protective effect was also demonstrated on human lymphocytes exposed to H2O2 and treated with R. officinalis L. extract by Razavi-Azarkhiavi et al.28 Rosmarinic acid is one of the phytocompounds that has demonstrated antimutagenic effect.36
Increasingly, the number of medical and dental products based on medicinal plants has increased, because it has been proven them effectiveness in various application areas, as was evidenced in our study that showed control of mono- and polymicrobial biofilm, effect immunomodulatory and antimutagenic action of R. officinalis L. extract. Based on biological potential, this medicinal plant has great chances to be a promising therapeutic agent applied in some formulations as toothpastes, mouthwashes, irrigating root canals, ointments, soaps, among others.
In this study, R. officinalis L. extract acted on monomicrobial biofilms of C. albicans, S. aureus, E. faecalis, S. mutans, and P. aeruginosa as well as on polymicrobial biofilms formed by C. albicans with each bacterium. Regarding cell lines, the extract promoted cell viability above 50% (at ≤50 mg/mL). It showed significant anti-inflammatory effect, controlling the synthesis of IL-1β and TNF-α by LPS-stimulated RAW 264.7. In addition, it exhibited DNA-protective effect in all tested cells.
Authors’ contributions
JRO: conception, design of the experiments, interpretation of data, drafting of the manuscript. DJ: design of the experiments and drafting of the manuscript.
LWF: design of the experiments. FEO: review of the manuscript of the manuscript and prepared the linguistic correction of the manuscript. CPS: analysis and interpretation of data and review of the manuscript of the manuscript. SEAC: analysis and interpretation of data and review of the manuscript of the manuscript. AOCJ: conception, analysis and interpretation of data and review of the manuscript of the manuscript. LDO: conception, analysis and interpretation of data, critical analysis and review of the manuscript of the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Rašković A, Milanović I, Pavlović N, Ćebović T, Vukmirović S, Mikov M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Compl Altern Med 2014; 14: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.da Silva BN, Nakassugi LP, Faggion POJ, Kohiyama CY, Mossini SA, Grespan R, Nerilo SB, Mallmann CA, Alves Abreu Filho B, Machinski M., Jr Antifungal activity and inhibition of fumonisin production by Rosmarinus officinalis L. essential oil in Fusarium verticillioides (Sacc.) Nirenberg. Food Chem 2015; 166: 330–6. [DOI] [PubMed] [Google Scholar]
- 3.Kojic EM, Darouiche RO. Candida infections of medical devices. Clin Microbiol Rev 2004; 17: 255–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harriott MM, Noverr MC. Candida albicans and Staphylococcus aureus form polymicrobial biofilms: Effects on antimicrobial resistance. Antimicrob Agents Chem 2009; 53: 3914–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ammons MC, Tripet BP, Carlson RP, Kirker KR, Gross MA, Stanisich JJ, Copié V. Quantitative NMR metabolite profiling of methicillin-resistant and methicillin-susceptible Staphylococcus aureus discriminates between biofilm and planktonic phenotypes. J Proteome Res 2014; 13: 2973–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mastropaolo MD, Evans NP, Byrnes MK, Stevens AM, Robertson JL, Melville SB. Synergy in polymicrobial infections in a mouse model of type 2 diabetes. Infect Immun 2005; 73: 6055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Connell HA, Kottkamp GS, Eppelbaum JL, Stubblefield BA, Gilbert SE, Gilbert ES. Influences of biofilm structure and antibiotic resistance mechanisms on indirect pathogenicity in a model polymicrobial biofilm. Appl Environ Microbiol 2006; 72: 5013–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. Biofilm formation by the fungal pathogen Candida albicans: Development, architecture, and drug resistance. J Bacteriol 2001; 183: 5385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Fattani MA, Douglas LJ. Penetration of Candida biofilms by antifungal agents. Antimicrob Agents Chem 2004; 48: 3291–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katneni R, Hedayati SS. Central venous catheter-related bacteremia in chronic hemodialysis patients: Epidemiology and evidence-based management. Nat Clin Pract Nephrol 2007; 3: 256–66. [DOI] [PubMed] [Google Scholar]
- 11.Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN. Polymicrobial bloodstream infections involving Candida species: Analysis of patients and review of the literature. Diagn Microbiol Infect Dis 2007; 59: 401–6. [DOI] [PubMed] [Google Scholar]
- 12.Cruz MR, Graham CE, Gagliano BC, Lorenz MC, Garsin DA. Enterococcus faecalis inhibits hyphal morphogenesis and virulence of Candida albicans. Infect Immun 2013; 81: 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ten Cate JM, Klis FM, Pereira-Cenci T, Crielaard W, de Groot PW. Molecular and cellular mechanisms that lead to Candida biofilm formation. J Dent Res 2009; 88: 105–15. [DOI] [PubMed] [Google Scholar]
- 14.Gregoire S, Xiao J, Silva BB, Gonzalez I, Agidi PS, Klein MI, Ambatipudi KS, Rosalen PL, Bauserman R, Waugh RE, Koo H. Role of glucosyltransferase B in interactions of Candida albicans with Streptococcus mutans and with an experimental pellicle on hydroxyapatite surfaces. Appl Environ Microbiol 2011; 77: 6357–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Carvalho FG, Silva DS, Hebling J, Spolidorio LC, Spolidorio DM. Presence of mutans streptococci and Candida spp. in dental plaque/dentine of carious teeth and early childhood caries. Arch Oral Biol 2006; 51: 1024–8. [DOI] [PubMed] [Google Scholar]
- 16.Raja M, Hannan A, Ali K. Association of oral candidal carriage with dental caries in children. Caries Res 2010; 44: 272–6. [DOI] [PubMed] [Google Scholar]
- 17.Vadiakas G. Case definition, aetiology and risk assessment of early childhood caries (ECC): A revisited review. Eur Arch Paediatr Dent 2008; 9: 114–25. [DOI] [PubMed] [Google Scholar]
- 18.Parisotto TM, Steiner-Oliveira C, Silva CM, Rodrigues LK, Nobre-dos-Santos M. Early childhood caries and mutans streptococci: A systematic review. Oral Health Prev Dent 2010; 8: 59–70. [PubMed] [Google Scholar]
- 19.da Silva-Boghossian CM, do Souto RM, Luiz RR, Colombo AP. Association of red complex, A. actinomycetemcomitans and non-oral bacteria with periodontal diseases. Arch Oral Biol 2011; 56: 899–906. [DOI] [PubMed] [Google Scholar]
- 20.Raghavendran K, Mylotte JM, Scannapieco FA. Nursing home-associated pneumonia, hospital-acquired pneumonia and ventilator-associated pneumonia: the contribution of dental biofilms and periodontal inflammation. Periodontol 2000 2007; 44: 164–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang W, Li N, Luo M, Zu Y, Efferth T. Antibacterial activity and anticancer activity of Rosmarinus officinalis L. essential oil compared to that of its main components. Molecules 2012; 17: 2704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Vallinas M, Molina S, Vicente G, Zarza V, Martín-Hernández R, García-Risco MR, Fornari T, Reglero G, Ramírez de Molina A. Expression of microRNA-15 b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of Rosemary diterpenes in colon and pancreatic cancer. PLoS One 2014; 9: e98556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petiwala SM, Berhe S, Li G, Puthenveetil AG, Rahman O, Nonn L, Johnson JJ. Rosemary (Rosmarinus officinalis) extract modulates CHOP/GADD153 to promote androgen receptor degradation and decreases xenograft tumor growth. PLoS One 2014; 9: e89772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrelli M, Cristaldi B, Menichini F, Conforti F. Inhibitory effects of wild dietary plants on lipid peroxidation and on the proliferation of human cancer cells. Food Chem Toxicol 2015; 86: 16–24. [DOI] [PubMed] [Google Scholar]
- 25.Berrington D, Lall N. Anticancer activity of certain herbs and spices on the cervical epithelial carcinoma (HeLa) cell line. Evid Based Complement Alternat Med 2012; 2012: 564927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu MH, Choi JH, Chae IG, Im HG, Yang SA, More K, Lee IS, Lee J. Suppression of LPS-induced inflammatory activities by Rosmarinus officinalis L. Food Chem 2013; 136: 1047–54. [DOI] [PubMed] [Google Scholar]
- 27.Silva AM, Machado ID, Santin JR, de Melo IL, Pedrosa GV, Genovese MI, Farsky SH, Mancini-Filho J. Aqueous extract of Rosmarinus officinalis L. inhibits neutrophil influx and cytokine secretion. Phytother Res 2015; 29: 125–33. [DOI] [PubMed] [Google Scholar]
- 28.Razavi-Azarkhiavi K, Behravan J, Mosaffa F, Sehatbakhsh S, Shirani K, Karimi G. Protective effects of aqueous and ethanol extracts of rosemary on H2O2-induced oxidative DNA damage in human lymphocytes by comet assay. J Complement Integr Med 2014; 11: 27–33. [DOI] [PubMed] [Google Scholar]
- 29.CSLI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard, NCCLS document M7-A6, 6th ed USA: CLSI, 2003. [Google Scholar]
- 30.CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts. Fourth Informational Supplement M27-S4, USA: CLSI, 2012. [Google Scholar]
- 31.CLSI. Reference method for broth dilution in tests for determining the sensitivity to antifungal therapy of yeast. Approved standard, NCCLS document M27-A2. 2nd ed, USA: CLSI, 2002. [Google Scholar]
- 32.Lewis K. Riddle of biofilm resistance. Antimicrob Agents Chem 2001; 45: 999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pereira-Cenci T, Deng DM, Kraneveld EA, Manders EM, Del Bel Cury AA, ten Cate JM, Crielaard W. The effect of Streptococcus mutans and Candida glabrata on Candida albicans biofilms formed on different surfaces. Arch Oral Biol 2008; 53: 755–64.18395698 [Google Scholar]
- 34.Morales DK, Jacobs NJ, Rajamani S, Krishnamurthy M, Cubillos-Ruiz JR, Hogan DA. Antifungal mechanisms by which a novel Pseudomonas aeruginosa phenazine toxin kills Candida albicans in biofilms. Mol Microbiol 2010; 78: 1379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luzio JP, Pryor PR, Bright NA. Lysosomes: Fusion and function. Nat Rev Mol Cell Biol 2007; 8: 622–32. [DOI] [PubMed] [Google Scholar]
- 36.Furtado RA, de Araújo FR, Resende FA, Cunha WR, Tavares DC. Protective effect of rosmarinic acid on V79 cells evaluated by the micronucleus and comet assays. J Appl Toxicol 2010; 30: 254–9. [DOI] [PubMed] [Google Scholar]