Abstract
Therapeutic antibodies that block the programmed cell death protein-1 (PD-1) immune checkpoint pathway prevent T-cell downregulation and promote immune responses against cancer. Several PD-1 pathway inhibitors have shown robust activity in initial trials. This article reviews the preclinical evidence, rationale, and clinical pharmacology of blockade of PD-1 or its ligands as therapy for lung cancer and provides an overview of agents in development, clinical evidence to date, and implications for clinical application.
Background
First-line therapy for advanced non–small cell lung cancer (NSCLC), which accounts for ~85% of all lung cancers, is platinum-based chemotherapy.1,2 Patients with specific mutations may effectively be treated with targeted agents initially.2,3 However, most develop resistance to these therapies, with subsequent disease progression.2,3 As such, the average 5-year survival rate is 4% for patients diagnosed with advanced disease, highlighting a great need for improved treatment options.1 Immunotherapy is effective in patient subsets in some cancers (e.g., melanoma and renal cell carcinoma) and can increase survival.4,5,6 However, the limited activity of bacille Calmette-Guérin vaccination, interleukin (IL)-2, and interferons in clinical trials has promoted the perception that NSCLC is not an immunoresponsive tumor.7 Different immunologic approaches targeting immune checkpoint pathways are showing promise in development, and preclinical and clinical evidence provides rationale for investigating these newer immunotherapies in NSCLC and other tumors.
Rationale for Immune Checkpoint Inhibition
Upon emerging from the thymus, naive T cells circulate in blood through lymph nodes and seek foreign (“nonself”) antigens presented by specific antigen-presenting cells, typically dendritic cells.8 T cells can recognize not only pathogen-associated antigens but also abnormally expressed self-proteins—indicating mutated or transformed tumorigenic cells—as “nonself.” If T cells encounter their specific antigen in the context of appropriate costimulatory molecules, the cells become activated and upregulate activation and homing molecules. These T cells, termed effector T cells, are able to enter inflamed tissues in search of infected or cancerous cells. Among other functions, effector T cells can produce inflammatory cytokines and/or cytolytic granules, leading to apoptosis or necrosis of infected or tumor cells. Throughout the duration of an immune response, local and systemic downregulatory forces are in play to minimize damage to healthy cells and tissues. These can involve immunosuppressive cytokines, regulatory T cells (Tregs), and negative signaling from other cells.
Immune checkpoint pathways
Immune checkpoint pathways strongly downregulate T-cell activation with the intent of keeping nascent T-cell responses in check and reducing the likelihood of an immune attack against normal tissues. During tumorigenesis, however, cancer cells may exploit these co-inhibitory pathways to resist detection or avoid elimination by the adaptive immune system.8,9 The programmed cell death protein-1 (PD-1) is a critical checkpoint molecule that is expressed by T cells upon activation. The PD-1 checkpoint pathway is thought to act primarily in peripheral tissues to dampen ongoing immune responses and/or to prevent damage to self-tissues.9 PD-1 is expressed by B cells, natural killer (NK) cells, dendritic cells, and activated monocytes, in addition to T cells. PD-1 ligands—which include PD-L1 and PD-L2, among others—are expressed by macrophages and monocytes, and these can be induced in numerous cell types in an inflammatory environment.10
The ability of nonimmune cells to express ligands for PD-1, primarily PD-L1, is exploited by tumors as one way to avoid immune attack.11,12 Tumor cells can also downregulate antigen expression to avoid detection. In addition, production of immunosuppressive mediators and retention of Tregs and immune suppressor cells within the tumor microenvironment can dampen antitumor immune responses.11
This article focuses on the PD-1 pathway as a novel therapeutic target for oncology drug development.
Rationale for PD-1 Antagonism
PD-1 pathway and its role in cancer
Although most understanding of basic and tumor immunology comes from academic research, evidence from the clinic supports a role for the PD-1 pathway in human cancers. PD-L1 expression has been detected in lung, ovary, renal, and colon carcinomas and in malignant melanoma but not in normal tissues, including the lung, uterus, kidney, colon, or skin (nevi).13,14,15 PD-L1 expression by tumor cells is associated with a worse prognosis in breast cancer, gastric cancer, esophageal cancer, hepatocellular carcinoma, malignant melanoma, ovarian cancer, pancreatic cancer, renal cell carcinoma, and urothelial cancer.12
There is also evidence that human tumors can express PD-L2.16,17 NSCLC-associated fibroblasts constitutively express both PD-L1 and PD-L2. Decreased survival in patients with PD-L2–positive (vs. PD-L2–negative), esophageal, ovarian, or hepatocellular cancer has also been described. PD-1:PD-L2 binding has higher affinity and is slightly different than PD-1:PD-L1 binding, although whether this translates to different T-cell signaling and antitumor effects is unclear.16
If PD-1 ligands are involved in downregulating antitumor immune responses, then they would likely be acting on tumor-specific PD-1–expressing T cells. In support of this hypothesis, in both NSCLC and melanoma patients, higher levels of PD-1 were observed on tumor-infiltrating lymphocytes (TILs) than on circulating lymphocytes.14,18 Furthermore, in the peripheral blood of vaccinated melanoma patients, both melanoma antigen–specific cytotoxic lymphocytes and Tregs expressed PD-1.19 Finally, there was a negative correlation between tumor PD-L2 expression and the presence of CD8+ TILs in esophageal cancer.16
Preclinical support for PD-1/PD-L1 antagonism as a therapeutic intervention
Animal studies have suggested that the PD-1 pathway is involved in tumor immune evasion and that blockade of the PD-1 pathway can restore antitumor immune responses. Tumor cells expressing PD-L1 had increased resistance to T cell–mediated lysis and showed enhanced tumorigenesis and invasiveness, as compared with tumor cells lacking PD-L1 expression. These effects were reversed by administration of anti-PD-L1 antibody.20 Separately, growth of tumor cells was inhibited in PD-1-deficient mice, suggesting a strong antitumor immune response in the absence of PD-1:PD-L1 interactions.20,21 Furthermore, in mice with established tumors, administration of anti-PD-1 and/or anti-PD-L1 antibodies led to reduced tumor burden and increased survival.22,23
PD-1 pathway blockade may enhance other immunotherapeutic approaches, including those using nonoverlapping immune checkpoint pathways. As evidence, increased antitumor activity—relative to either single agent—was seen when anti–cytotoxic T lymphocyte–associated antigen 4 and anti-PD-1 antibodies were used concurrently, whereas sequential use showed efficacy similar to that of the single agents.24,25 Similarly, combination treatment with both anti–lymphocyte activation gene-3 (another T-cell-inhibitory receptor) and anti-PD-1 antibodies cured most mice of established tumors that were largely resistant to single-antibody treatment.26 In addition, there is preclinical evidence to suggest that PD-1 pathway blockade could be combined with other immunotherapeutic approaches, including administration of ILs, innate immune modulators, or vaccines. Combination of anti-PD-1 with recombinant IL-21 led to enhanced antitumor activity, with strong tumor growth inhibition and complete regression in the majority of mice.27 In a model of chronic viral infection leading to T-cell exhaustion, similar to tumor-mediated immunosuppression, combination treatment with anti-PD-1 and IL-2 was synergistic. It increased both the number of virus-specific CD8+ T cells and the ability of CD8+ T cells to kill infected cells.28 In other tumor models, use of anti-PD-L1 antibody with transfer of tumor-specific T cells led to eradication of PD-L1-expressing squamous cell carcinomas, in contrast to no tumor eradication with T-cell transfer alone.29 Combined treatment of anti-PD-1 antibody with CpG (a Toll-like receptor agonist) or with granulocyte macrophage colony-stimulating factor–secreting tumor cell immunotherapy prolonged the survival of tumor-bearing animals.30,31 Finally, multiple vaccination approaches had augmented responses when PD-1–pathway blockade was used.25,32,33,34
Studies of human antitumor responses provide some insight regarding why the PD-1 pathway seems to be a promising target for immunotherapy in solid tumors, including lung cancers. CD8+ TILs isolated from NSCLCs had increased expression of PD-1 and impaired functional responses (in vitro proliferation and inflammatory cytokine production) as compared with circulating CD8+ T cells or CD8+ T cells from healthy volunteers. Addition of anti-PD-L1 antibody significantly improved the ability of the CD8+ TILs to proliferate and produce interferon-γ in vitro.18 In a similar study using cultures of tumor-derived dendritic cells and TILs from ovarian cancer patients, addition of anti-PD-L1 antibody significantly increased interferon-γ production by TILs in response to tumor antigens. When these TILs were transferred to immunodeficient mice bearing the ovarian tumors, reduced tumor growth was seen as compared with that of mice in control groups.35
Some of the studies in which anti-PD-1 antibody enhanced antitumor vaccination responses also demonstrated immunologic changes that might help explain the apparent additive effects. In a short-term in vitro assay used to generate melanoma-specific T cells, addition of an anti-PD-1 antibody promoted the generation of melanoma antigen–specific cytotoxic lymphocytes and reduced their inhibition by Tregs.19 T cells isolated from melanoma antigen–vaccinated patients and exposed to melanoma antigens in vitro had augmented activation and expansion of functional effector cytotoxic lymphocytes when cultured in the presence of an anti-PD-1 antibody.36 In an in vitro immunization model using fused dendritic cells and tumor cells, anti-PD-1 antibody promoted T-cell polarization toward an activated phenotype and increased killing of tumor target cells.37
The PD-1 pathway may also be used by the innate immune system in some cancers. NK cells from multiple myeloma patients expressed PD-1, whereas NK cells from healthy patients did not.13 Treatment of PD-1+ NK cells with an anti-PD-1 antibody improved in vitro antitumor effects, including enhanced interferon-γ production and cytotoxic functions.13
Immunologic studies have identified potential mechanisms for these clinical responses. In the above-described preclinical studies, blockade of the PD-1 pathway led to the following:
Increased numbers of effector T cells through induction or expansion14,18,19,21,30,31,32,35
Augmented cytolytic activity of tumor-specific cells, leading to improved tumor lysis14,22,30,36,37,38
Enhanced production of proinflammatory cytokines14,18,19,21,23,30,34,35,38
Accumulation of effector T cells in the tumor sites via increased homing or persistence21,30,32,38
Reduced numbers of Tregs at the tumor site or reduced activity of Tregs19,31,32,37
Downregulation of potentially suppressive cytokines (IL-10)23
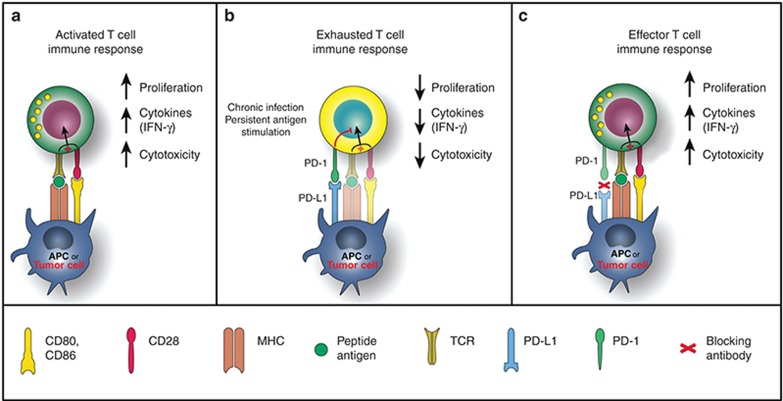
Figure 1 depicts the role of the PD-1 pathway in tumor immune evasion and the mechanism of action of PD-1 pathway blockade.39
Figure 1.
PD-1 in T-cell activation, exhaustion, and effector function. (a) T cells are activated via (i) binding of MHC plus peptide on an APC to the TCR and then (ii) binding of APC CD80/86 to T-cell CD28. In patients with cancer, tumor cells can also serve as APCs. Upon T-cell activation, PD-1 expression is induced. (b) In situations of chronic infection or persistent stimulation, PD-L1 signals through T-cell PD-1 to “turn off” T cells in order to minimize damage to healthy tissue (activation signaling is blocked). Tumor cells can upregulate PD-L1 in order to “turn off” T cells that might destroy them. (c) Blocking the PD-1/PD-L1 signaling pathway allows T cells to maintain their effector functions. In patients with cancer, activated tumor-specific T cells can kill tumor cells and secrete cytokines that activate/recruit other immune cells to participate in the antitumor response. APC, antigen-presenting cell; IFN-γ, interferon-γ; MHC, major histocompatibility complex; PD-1, programmed death-1; PD-L1, PD ligand 1; TCR, T-cell receptor. Reprinted from ref. 39.
PD-1 Pathway Inhibitors in Clinical Development
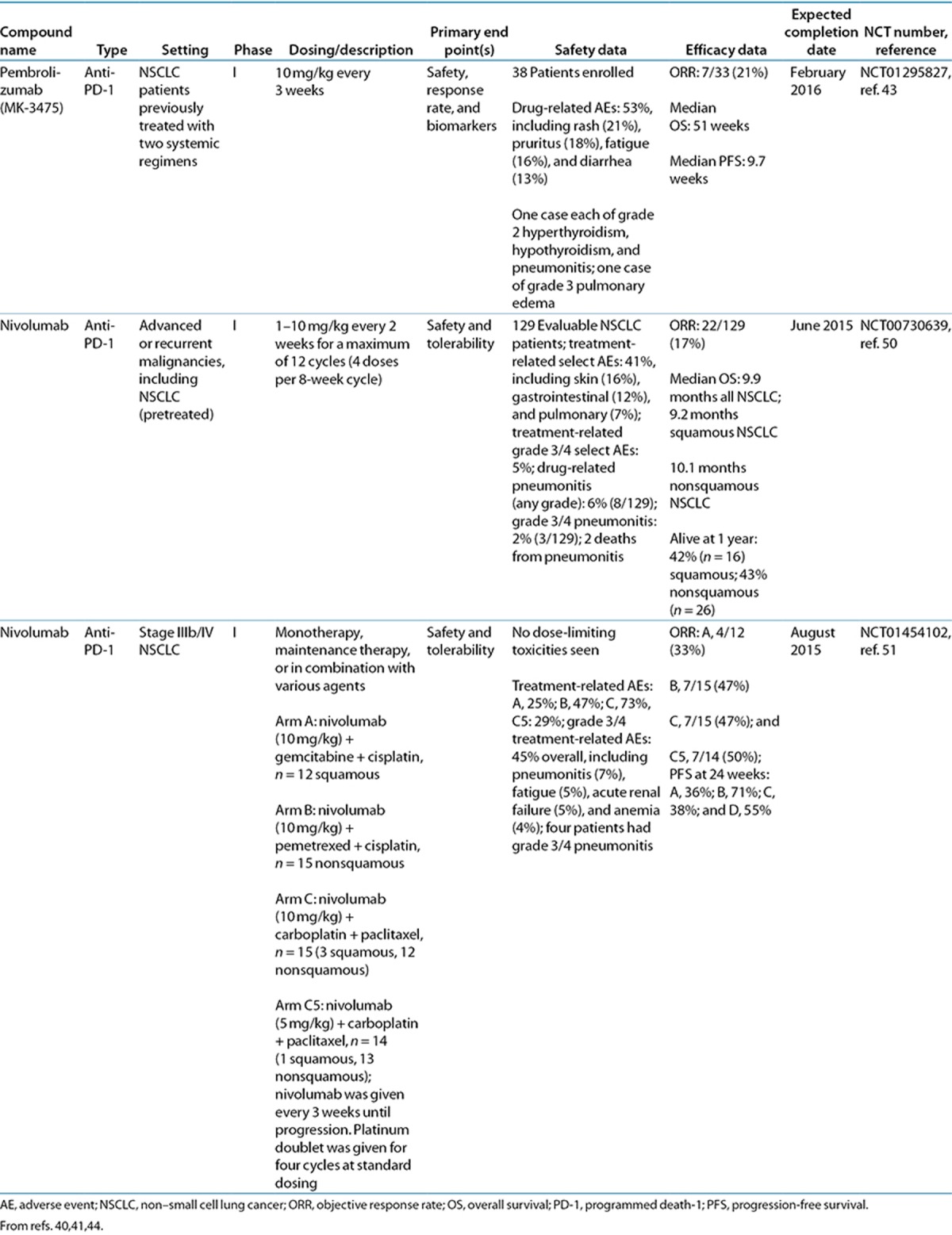
A number of promising agents targeting the PD-1 pathway are in clinical development: AMP-224, BMS-936559, MEDI4736, nivolumab, pembrolizumab (MK-3475), and pidilizumab (CT-011) (Table 1). Clinical trial data of these PD-1 pathway inhibitors in lung cancer, to date, are shown in Tables 2 and 3. AMP-224 and pidilizumab do not currently have any trials in lung cancer, and MEDI4736 currently has an ongoing phase I trial in multiple advanced cancers, including NSCLC.40 For all immune checkpoint inhibitors investigated to date, the safety profiles were largely characterized by immune-related adverse events (irAEs), in keeping with their mechanisms of action (Tables 2 and 3). Tolerability of the immune checkpoint inhibitors was generally good, with few dose-limiting toxicities reported.41,42,43,44,45,46,47,48,49 The irAEs are discussed in more detail below. Preliminary data suggest that PD-1 pathway inhibitors have activity in lung cancer.
Table 1. Characteristics of PD-1 checkpoint inhibitors.
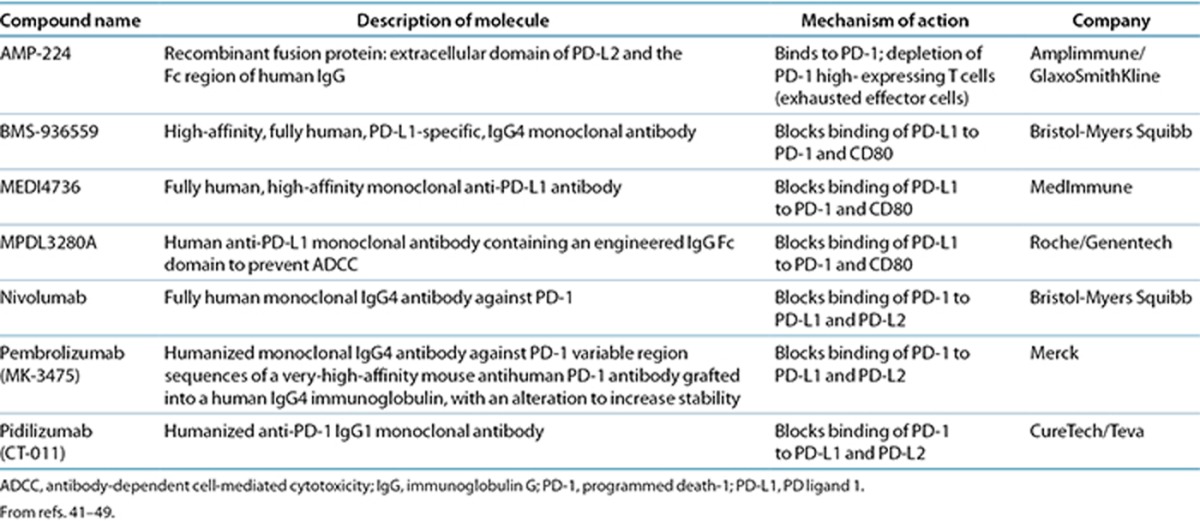
Table 2. Data to date of PD-L1 agents in lung cancer.
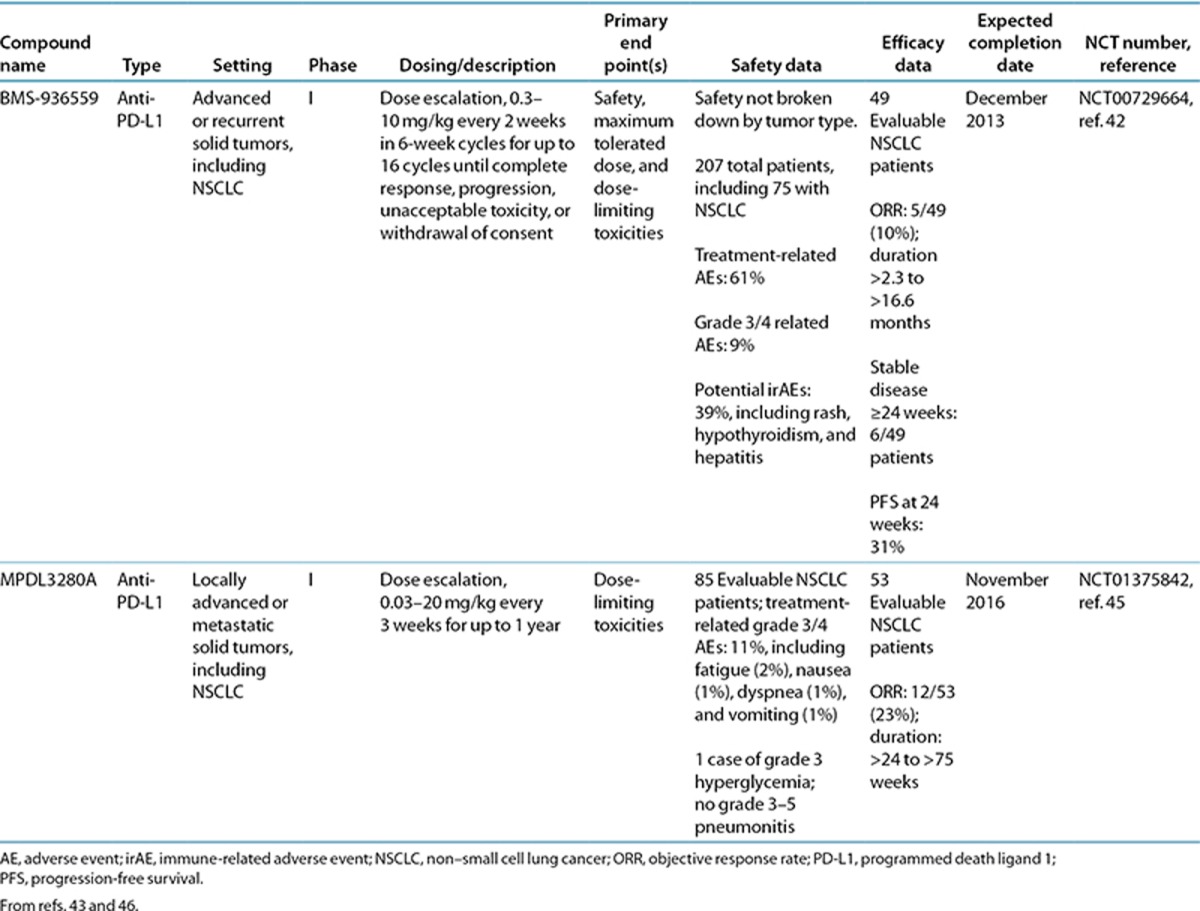
Table 3. Data to date of PD-1 agents in lung cancer.
PD-L1
Agents that target PD-L1 will inhibit PD-1:PD-L1 binding, as well as PD-L1 binding to CD80 on T cells.9 In the phase I trial of the PD-L1 inhibitor BMS-936559, objective responses were seen in 10% of patients (5 of 49) with advanced NSCLC (Table 2).42 When results were broken down by histological subtype, 1 of 13 evaluable patients with squamous NSCLC had an objective response, 3 patients had stable disease lasting ≥24 weeks, and 6 patients had progression-free survival at 24 weeks. Of 36 evaluable patients with nonsquamous subtype, 4 had an objective response, 3 had stable disease lasting ≥24 weeks, and 9 patients had progression-free survival at 24 weeks.
MPDL3280A, another antibody targeting PD-L1, showed an objective response rate of 23% (12/53) in patients with NSCLC who had been previously treated, with 55% having received at least 3 prior regimens (NCT01375842) (Table 2).45 In the interim analysis, clinical responses were seen in patients with both squamous and nonsquamous histologies, regardless of epidermal growth factor receptor mutation status. At the time of analysis, responses were ongoing in all but one responder with lung cancer; the duration of responses ranged from >24 to >75 weeks. On the basis of these promising results, two phase II trials are under way in NSCLC. The first involves monitoring objective responses of patients with PD-L1+ locally advanced or metastatic NSCLC receiving MPDL3280A monotherapy (NCT01846416). The second involves assessing overall survival vs. docetaxel in patients with advanced or metastatic NSCLC who failed platinum therapy (NCT01903993).40
PD-1
Antibodies targeting PD-1 will inhibit binding of PD-1 to both its ligands, PD-L1 and PD-L2. A humanized immunoglobulin G (IgG)-4 anti-PD-1 antibody, pembrolizumab (MK-3475), is being evaluated in an ongoing phase I trial in patients with advanced solid tumors, including patients with NSCLC (NCT01295827) (Table 3).43 In an interim analysis of previously treated NSCLC patients, the objective response rate was 21% overall using the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, and most responses were observed by 9 weeks. At the time of data cut, the preliminary median durations of overall survival and progression-free survival were 51 and 9.7 weeks, respectively.
A phase I trial enrolling patients with advanced NSCLC evaluating pembrolizumab in combination with cisplatin/pemetrexed or carboplatin/paclitaxel (NCT01840579) is ongoing. In addition, overall survival, progression-free survival, and safety of pembrolizumab (low dose or high dose) vs. docetaxel are being evaluated in a phase II/III trial in previously treated NSCLC patients (NCT01905657). Two more studies are planned that will start enrolling patients with advanced NSCLC later this year. A phase I/II study will evaluate the safety, tolerability, and efficacy of pembrolizumab in different combinations with chemotherapy, targeted agents (bevacizumab, erlotinib, and gefitinib), and ipilimumab (NCT02039674); and a phase I study will study responses and safety of pembrolizumab monotherapy in patients with PD-L1+ tumors (NCT02007070).40
Nivolumab, a fully human IgG4 PD-1 immune checkpoint inhibitor antibody, has undergone the most extensive clinical evaluation in lung cancer among the PD-1 pathway inhibitors. Evidence of activity both as a monotherapy in squamous and nonsquamous NSCLC and in combination with conventional chemotherapy has been demonstrated in patients with NSCLC (Table 3).50,51 In pretreated advanced NSCLC patients, nivolumab monotherapy had an overall response rate of 17% (22/129), not including 6 patients with immune-related responses.50 Half of responding patients showed a response by the first assessment at 8 weeks. The estimated median response duration was 74.0 weeks (range: >6.1 to >133.9 weeks), and responses were ongoing in 45% of patients at the time of analysis. Overall survival was 42% at 1 year and 24% at 2 years.
Nivolumab has nine active clinical trials in NSCLC at the time of writing, including trials in patients with advanced or metastatic solid tumors that include NSCLC.40 Phase I and I/II trials combine nivolumab with various other therapies including chemotherapies, targeted agents (bevacizumab or erlotinib), or other immunotherapies: IL-21, ipilimumab, anti–lymphocyte activation gene 3, or lirilumab, which targets a key inhibitory receptor on NK cells (killer cell immunoglobulin-like receptor). Furthermore, there are two phase II and two phase III trials of nivolumab in patients with NSCLC only. The phase II studies are investigating (i) nivolumab monotherapy as a third-line treatment in patients with squamous NSCLC (NCT01721759) or (ii) nivolumab therapy following azacitidine and entinostat vs. oral azacitidine in patients with advanced NSCLC (NCT01928576). The phase III trials, both fully enrolled, are comparing nivolumab to docetaxel in previously treated NSCLC; one study involves patients with squamous cell NSCLC (NCT01642004), whereas the other is focusing on patients with nonsquamous histologies (NCT01673867). In addition, a planned phase III trial evaluating nivolumab vs. chemotherapy (investigator’s choice) as first-line treatment of PD-L1+ advanced NSCLC will start enrolling patients later this year (NCT02041533), as will a planned phase III nivolumab safety study in patients with advanced NSCLC (NCT02066636).40
There is initial evidence that combination strategies that involve immune checkpoint blockade may also have additive effects in the clinic. In patients with advanced melanoma, combination therapy with nivolumab and ipilimumab showed preliminary activity much greater than that seen in previous experience with either agent alone: 40% of patients on a concurrent regimen had an objective response, and 65% had evidence of clinical activity.52 Results of the ongoing trials of checkpoint inhibitors in lung cancer will provide further insight into new therapeutic targets and inform approaches for checkpoint inhibitor use in patients who currently have limited treatment options.
Practical Implications of an Immunotherapeutic Mechanism of Action
Drug disposition and considerations for different patient populations
The approved or in-development immune checkpoint inhibitors are all therapeutic monoclonal antibodies, with the exception of AMP-224, and all are administered as an i.v. infusion (Table 1). Because immune checkpoint inhibitors act to stimulate the immune system, patients with a history of autoimmune disease or other diseases requiring immunosuppressive therapy, including corticosteroids at supraphysiological doses, are probably not appropriate candidates for immunotherapy. Such patients were and are excluded from clinical trials.40,42,44,49,52
Because the PD-1 pathway inhibitors have, to date, reported phase I data only, limited evidence is available to inform their potential in individual subpopulations of patients. All IgG antibody subtypes can cross the placenta; therefore, the use of the checkpoint inhibitors will probably be contraindicated in pregnancy.
Potential effects on cytochrome P450 enzymes
Drug–drug interactions or toxicity profiles of PD-1 pathway inhibitors in combination with other drugs are not well established. Therapeutic monoclonal antibodies are not metabolized by cytochrome P450 (CYP) enzymes but are instead cleared by renal filtration or via receptor-mediated mechanisms.53 As such, they are not expected to have direct drug–drug interactions involving CYP enzymes.53,54 However, there is evidence that cytokines involved in effector T-cell responses can alter regulation of many drug transporters and levels of CYP enzymes. Administration of high-dose IL-2 to patients with hepatocellular carcinoma has been shown to decrease expression of multiple CYP enzymes, and administration of IL-2 to cancer patients has been proposed to cause clinically important drug interactions.53,55,56 Immune-modulating antibodies used in cancer may have cytokine-mediated effects on CYP enzymes, although direct evidence is currently unavailable.
Clinical responses with immunotherapy
The anticancer mechanism of action is different with checkpoint inhibitors as compared with chemotherapy. Standard lung cancer chemotherapies and targeted agents act directly on cancer cells to inhibit tumor growth or cause tumor cell death via apoptosis, necrosis, or both.57 Chemotherapies work by interrupting DNA synthesis, replication, and/or repair or by inhibiting normal cell division by other means. Targeted agents work to inactivate mutated or overexpressed proteins that confer growth/survival advantages, thereby reducing the aggressiveness of the tumor.3,58,59 The effectiveness of these standard therapies is measured radiologically by tumor shrinkage.60
In contrast to conventional cytotoxic agents, immunotherapies are designed to stimulate antitumor immune responses so that tumors are destroyed via normal immune processes. This antitumor activity occurs by contact-dependent and contact-independent interactions between T cells and tumor cells that are facilitated by a host of other immune cells, including dendritic cells, NK cells, and B cells.61 As a clinical consequence, antitumor responses that are delayed, as compared with those for chemotherapy or targeted agents, may occur because it may take time for an antitumor immune response to be mobilized and prove effective at killing tumor cells.
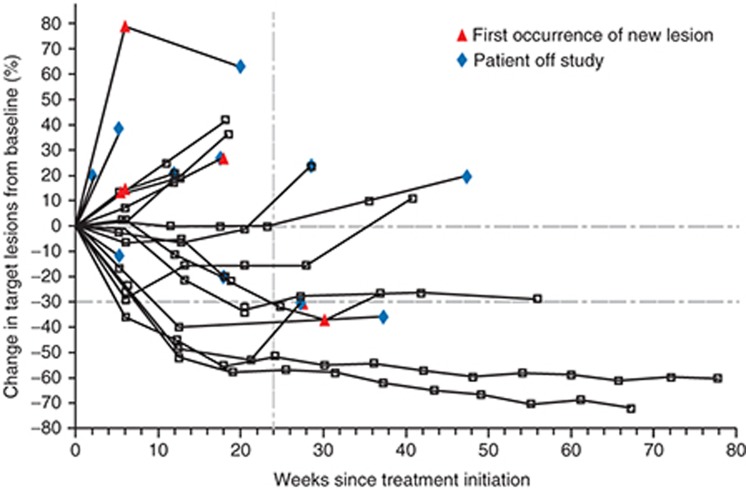
Immune checkpoint inhibitor trials have also reported pseudoprogression, whereby the tumor volume increases after initiation of checkpoint inhibitor therapy but is then followed by tumor regression or a prolonged reduction in tumor burden in the presence of new lesions (Figure 2).42,43,45,49,50,62,63 This may be explained by an influx of effector T cells, dendritic cells, and other immune cells into the tumor after activation by immune-modulating therapy, and not by an increase in tumor cells. These unconventional responses may be associated with favorable survival.62,63
Figure 2.
Activity of anti-PD-L1 antibody in patients with advanced NSCLC. Shown is the tumor burden (assessed as the longest linear dimension) over time in patients with NSCLC who received 10 mg of anti–PD-L1 antibody per kilogram body weight. In most patients who had an objective response, responses were durable and were evident by the end of cycle 2 (12 weeks) of treatment, regardless of the drug dose or tumor type. The vertical dashed line marks the 24-week time point at which the rate of progression-free survival was calculated. Tumor regression followed both conventional and immune-related patterns of response, such as a prolonged reduction in the tumor burden in the presence of new lesions. NSCLC, non–small cell lung cancer; PD-L1, programmed death ligand 1. Reprinted from ref. 42.
To better characterize responses with immunotherapy, immune-related response criteria have been defined. With these criteria, in contrast with RECIST criteria, new lesions do not necessarily represent progressive disease. Moreover, instead of using the sum of the product of perpendicular diameters of index lesions to determine tumor burden, the immune-related response criteria calculate an overall tumor burden based on the sum of the product of perpendicular diameters of index and new measurable lesions.63 Because these immune-related response criteria are still undergoing prospective validation in clinical trials, their usefulness across the spectrum of solid tumors, or even across the spectrum of immune checkpoint inhibitors under development, is an open question. Nevertheless, clinicians and researchers should be aware of the possibility for differences in responses and know how to accurately confirm responses when using immunotherapies so as to make well-informed treatment and investigation decisions.
Response rates with PD-1 pathway inhibitors in NSCLC are evolving; however, it is clear that a substantial proportion of patients achieve clinically meaningful and prolonged responses. Nivolumab monotherapy showed a median response duration of 74.0 weeks (range: >6.1 to >133.9 weeks), and responses were ongoing in 45% of responding patients (10/22) at the time of analysis.50 In an interim analysis, 7 of the 38 NSCLC patients receiving pembrolizumab (MK-3475) had ongoing responses at >60 weeks.43 Preliminary data for MPDL3280A showed that responses in 9 of 53 NSCLC patients were ongoing at the time of analysis, with the duration of responses ranging from >24 to >75 weeks.45 These highly encouraging response data in a population with a historically dismal prognosis support efforts to fully understand and characterize mechanisms of clinical and biological response. Ongoing efforts to refine patient selection are encouraging, and collaborations between pharmacologists and immunologists are critical to elucidating T-cell activation drivers to optimize responses in a greater number of patients.
Monitoring, management, implications, and possible predictors of irAEs
Health-care providers and patients must also be on the alert for irAEs with immune checkpoint inhibitors, which can occur at any time after the start of treatment and which require prompt diagnosis and interventions for effective management.64,65 Types of irAEs reported with immune checkpoint inhibitors include dermatologic (rash, pruritus, and vitiligo), gastrointestinal (diarrhea and colitis), endocrine (hypothyroidism and hyperthyroidism), and hepatic (hepatitis and increased liver function enzymes) events, as well as pneumonitis, uveitis, infusion-related events, and fatigue.5,40,42,44,49,52,66
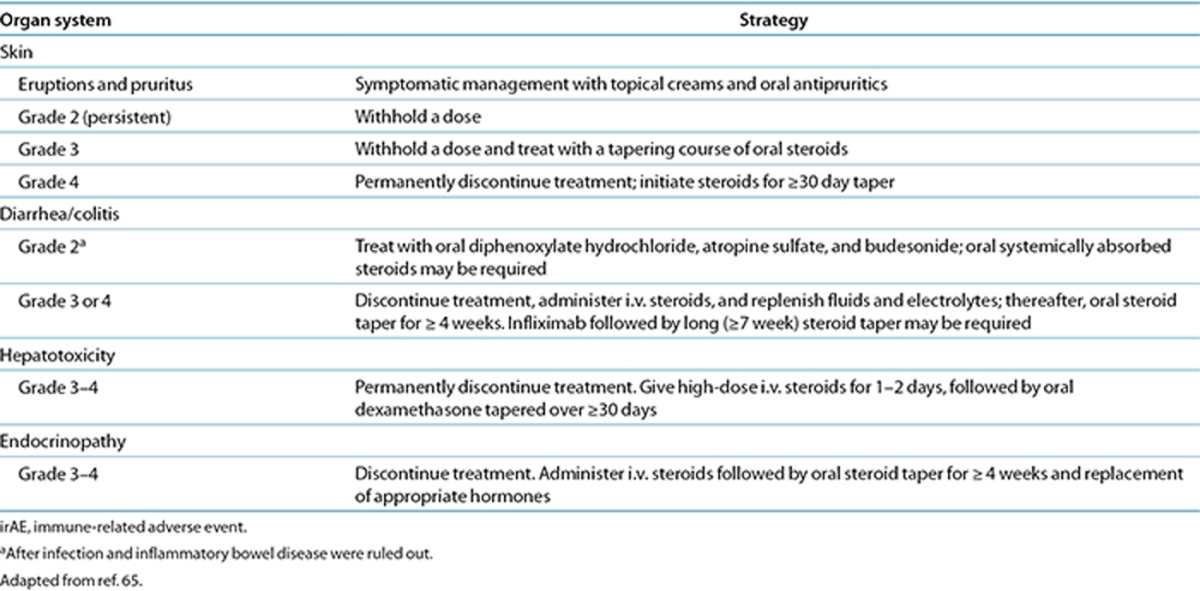
Across the different immune checkpoint inhibitors, some differences in incidence and type of grade 3/4 irAEs have been observed.5,42,44,49 Further studies will be needed to determine differences between these therapies. However, trials of the newer checkpoint inhibitors have successfully used approaches to managing irAEs similar to those used for managing ipilimumab-related irAEs (Table 4),65 which suggests that development of class-wide management principles is reasonable.42,44,49 General strategies included the administration of corticosteroids for irAEs, a delay in a scheduled dose for moderate irAEs, and discontinuation of therapy for severe reactions. Dose reductions are not recommended for the management of irAEs. Appropriate clinical management is critical for successful outcomes. This includes ruling out perforation in colitis, seeking appropriate consultation (e.g., endocrinology for hypophysitis or adrenal insufficiency or pulmonology for pneumonitis), and increasing the monitoring frequency of patients experiencing irAEs, including monitoring of liver function and timely follow-up of patients with diarrhea or worsening pulmonary symptoms. To date, no clear relationship between the incidence or severity of irAEs and response has been noted, making additional biomarker development necessary for prediction of initial and subsequent response to immune checkpoint therapy.
Table 4. Management strategies for irAEs with ipilimumab.
The effect of dose on the incidence and severity of irAEs is also unclear. In the nivolumab phase I study, no maximum tolerated dose was defined across the 0.1, 1, 3, or 10 mg/kg doses tested.49 However, patients receiving doses associated with a greater likelihood of response appear to be more likely to discontinue therapy due to an AE throughout treatment.44,49 Similarly, in the BMS-936559 anti-PD-L1 antibody study, no maximum tolerated dose was defined across the 0.3, 1, 3, or 10 mg/kg doses tested, and the percentages of patients with any grade AE or grade 3/4 AEs were similar across the 1–10 mg/kg doses, with numerically higher rates of all AEs in the 1 mg/kg group.42
More challenging is the current lack of predictors of irAEs in a given patient receiving a specific dose. The pharmacokinetic profiles of these IgG monoclonal antibodies show dose linearity. For example, pharmacokinetic results for BMS-936559 reported geometric mean area under the curve (0–14 days) values of 2,210, 7,750, and 36,620 μg/ml/h for doses of 1, 3, and 10 mg/kg, respectively.42 The coefficient of variation ranged from 34 to 59%. After the first dose, geometric mean peak levels at these dose levels were 27, 83, and 272 μg/ml, respectively (coefficient of variation: 30–34%).
Peripheral pharmacodynamic markers are unlikely to be of benefit because peripheral blood mononuclear cells from 65 patients receiving nivolumab (0.1–10 mg/kg every 2 weeks) showed a median PD-1 receptor occupancy of 64–70% across dose levels.49 However, relationships between receptor occupancy in peripheral blood and that in other tissues remain poorly understood, and therefore heterogeneity of occupancy in tumor and target irAE tissues (e.g., colon and liver) may contribute to AEs.
Future Directions and Conclusions
The use of immunotherapy to unlock the immune system’s ability to eradicate cancer cells is an exciting new avenue for treatment of solid tumors, even in an extremely aggressive disease with poor prognosis such as advanced lung cancer, but the exact clinical applications are still not clear. Ongoing trials investigating combination immunotherapy with additional therapeutic agents, the impact of treatment sequence of immunotherapy with chemotherapy, and the possible role of maintenance therapy with checkpoint inhibitors should advance our knowledge and, hopefully, treatment options.
In lung cancer therapy, the emergence of targeted agents with mechanisms of action based on driver mutations have introduced a set of standard predictive biomarkers to aid in clinical decision making. Immune checkpoint blockade, however, is designed to act on a complex and intact immunological pathway rather than individual mutations or antigens; as such, identification of a predictive biomarker has proven challenging. Answers as to whether biomarkers such as PD-L1 can predict tumor responsiveness to agents targeting this pathway have been equivocal so far.
In summary, the goal of immunotherapy is to prolong survival and quality of life for patients with lung cancer by stimulating the patient’s own immune system to combat cancer. This new treatment approach, however, is still early in lung cancer, and more data from mechanistic and clinical studies are needed on strategies to optimize the clinical impact of these therapies.
Acknowledgments
The author takes full responsibility for the contents of this publication and confirms that the article reflects his viewpoint and expertise. The author acknowledges StemScientific, funded by Bristol-Myers Squibb, for writing and editorial support. Neither Bristol-Myers Squibb nor StemScientific influenced the content of the article, nor did the author receive financial compensation for authoring it.
Footnotes
The author declared no conflict of interest.
References
- Molina, J.R., Yang, P., Cassivi, S.D., Schild, S.E. & Adjei, A.A. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 83, 584–594 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-small cell lung cancer. Version 2, 2013. <http://www.nccn.com>. Accessed 8 August 2013.
- Jänne, P.A., Engelman, J.A. & Johnson, B.E. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J. Clin. Oncol. 23, 3227–3234 (2005). [DOI] [PubMed] [Google Scholar]
- Yervoy® (ipilimumab) [prescribing information]. Princeton, NJ: Bristol-Myers Squibb Company, 2013. <http://www.yervoy.com/pdf/pi_yervoy.pdf>. Accessed 2 December 2013. [Google Scholar]
- Hodi, F.S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasch, E. et al. State of the science: an update on renal cell carcinoma. Mol. Cancer Res. 10, 859–880 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Moundhri, M., O’Brien, M. & Souberbielle, B.E. Immunotherapy in lung cancer. Br. J. Cancer 78, 282–288 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy, K. Janeway’s Immunobiology 8th edn. (Garland Science, New York, 2011). [Google Scholar]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keir, M.E., Butte, M.J., Freeman, G.J. & Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26, 677–704 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadus, M.R. et al. Lung cancer: a classic example of tumor escape and progression while providing opportunities for immunological intervention. Clin. Dev. Immunol. 2012, 160724 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, W. & Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 8, 467–477 (2008). [DOI] [PubMed] [Google Scholar]
- Benson, D.M. Jr et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank, C. et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int. J. Cancer 119, 317–327 (2006). [DOI] [PubMed] [Google Scholar]
- Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002). [DOI] [PubMed] [Google Scholar]
- Rozali, E.N., Hato, S.V., Robinson, B.W., Lake, R.A. & Lesterhuis, W.J. Programmed death ligand 2 in cancer-induced immune suppression. Clin. Dev. Immunol. 2012, 656340 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim, R. et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin. Cancer Res. 15, 6341–6347 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Huang, S., Gong, D., Qin, Y. & Shen, Q. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell. Mol. Immunol. 7, 389–395 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W., Lau, R., Yu, D., Zhu, W., Korman, A. & Weber, J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int. Immunol. 21, 1065–1077 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai, Y., Ishida, M., Tanaka, Y., Okazaki, T., Honjo, T. & Minato, N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc. Natl. Acad. Sci. USA 99, 12293–12297 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai, Y., Terawaki, S. & Honjo, T. PD-1 blockade inhibits hematogenous spread of poorly immunogenic tumor cells by enhanced recruitment of effector T cells. Int. Immunol. 17, 133–144 (2005). [DOI] [PubMed] [Google Scholar]
- Hirano, F. et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 65, 1089–1096 (2005). [PubMed] [Google Scholar]
- Okudaira, K. et al. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int. J. Oncol. 35, 741–749 (2009). [DOI] [PubMed] [Google Scholar]
- Selby, M. et al. Antitumor activity of concurrent blockade of immune checkpoint molecules CTLA-4 and PD-1 in preclinical models. J. Clin. Oncol. 31, abstr. 3061 (2013). [Google Scholar]
- Curran, M.A., Montalvo, W., Yagita, H. & Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 107, 4275–4280 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo, S.R. et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res. 72, 917–927 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jure-Kunkel, M. et al. Nonclinical evaluation of the combination of mouse IL-21 and anti- mouse CTLA-4 or PD-1 blocking antibodies in mouse tumor models. J. Clin. Oncol. 31, abstr. 3019 (2013). [Google Scholar]
- West, E.E. et al. PD-L1 blockade synergizes with IL-2 therapy in reinvigorating exhausted T cells. J. Clin. Invest. 123, 2604–2615 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strome, S.E. et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 63, 6501–6505 (2003). [PubMed] [Google Scholar]
- Li, B., VanRoey, M., Wang, C., Chen, T.H., Korman, A. & Jooss, K. Anti-programmed death-1 synergizes with granulocyte macrophage colony-stimulating factor–secreting tumor cell immunotherapy providing therapeutic benefit to mice with established tumors. Clin. Cancer Res. 15, 1623–1634 (2009). [DOI] [PubMed] [Google Scholar]
- Mangsbo, S.M., Sandin, L.C., Anger, K., Korman, A.J., Loskog, A. & Tötterman, T.H. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J. Immunother. 33, 225–235 (2010). [DOI] [PubMed] [Google Scholar]
- Mkrtichyan, M. et al. Anti-PD-1 synergizes with cyclophosphamide to induce potent anti-tumor vaccine effects through novel mechanisms. Eur. J. Immunol. 41, 2977–2986 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster, W.S. et al. Targeting molecular and cellular inhibitory mechanisms for improvement of antitumor memory responses reactivated by tumor cell vaccine. J. Immunol. 179, 2860–2869 (2007). [DOI] [PubMed] [Google Scholar]
- Zhou, Q. et al. Blockade of programmed death-1 pathway rescues the effector function of tumor-infiltrating T cells and enhances the antitumor efficacy of lentivector immunization. J. Immunol. 185, 5082–5092 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel, T.J. et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9, 562–567 (2003). [DOI] [PubMed] [Google Scholar]
- Wong, R.M. et al. Programmed death-1 blockade enhances expansion and functional capacity of human melanoma antigen-specific CTLs. Int. Immunol. 19, 1223–1234 (2007). [DOI] [PubMed] [Google Scholar]
- Rosenblatt, J. et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J. Immunother. 34, 409–418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomi, T. et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin. Cancer Res. 13, 2151–2157 (2007). [DOI] [PubMed] [Google Scholar]
- McDermott, D.F. & Atkins, M.B. PD-1 as a potential target in cancer therapy. Cancer Med. 2, 662–673 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinicaltrials.gov. <http://clinicaltrials.gov/ct2/show/NCT01903993>. Accessed 14 February 2014.
- Berger, R. et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 14, 3044–3051 (2008). [DOI] [PubMed] [Google Scholar]
- Brahmer, J.R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon, E.B. et al. Preliminary clinical safety and activity of MK-3475 monotherapy for the treatment of previously treated patients with non-small cell lung cancer (NSCLC). Abstr. MO18.02. <https://www.webges.com/cview/library/wclc/home>. Accessed 2 December 2013.
- Hamid, O. et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N. Engl. J. Med. 369, 134–144 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, L. et al. An analysis of the relationship of clinical activity to baseline EGFR status, PD-L1 expression and prior treatment history in patients with non-small cell lung cancer (NSCLC) following PD-L1 blockade with MPDL3280A (anti-PDL1). Abstr. MO18.01. <https://www.webges.com/cview/library/wclc/home>. Accessed 2 December 2013.
- Infante, J.R. et al. Clinical and pharmacodynamic (PD) results of a phase I trial with AMP-224 (B7-DC Fc) that binds to the PD-1 receptor. J. Clin. Oncol. 31, abstr. 2013 (2013). [Google Scholar]
- Smothers, J.F., Hoos, A., Langermann, S., Marshall, S., May, R. & Fleming, M.E. AMP-224, a fusion protein that targets PD-1. Ann. Oncol. 24, abstr. L02.04 (2013). [Google Scholar]
- Stewart, R.A. et al. MEDI4736: delivering effective blockade of immunosupression to enhance tumour rejection: monoclonal antibody discovery and preclinical development. Cancer Res. 71, abstr. LB-158 (2011). [Google Scholar]
- Topalian, S.L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer, J.R. et al. Nivolumab (anti-PD-1; BMS-936558; ONO-4538) in patients with non-small cell lung cancer (NSCLC): overall survival and long-term safety in a phase 1 trial. Abstr. MO18.03. <https://www.webges.com/cview/library/wclc/home>. Accessed 2 December 2013.
- Rizvi, N.A. et al. A phase I study of nivolumab (anti-PD-1; BMS-936558, ONO-4538) plus platinum-based doublet chemotherapy (PT-doublet) in chemotherapy-naive non-small cell lung cancer (NSCLC) patients (pts). Abstr. 8072. <http://meetinglibrary.asco.org/content/84225>. Accessed 2 December 2013.
- Wolchok, J.D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen, H. & Hermann, M. Immunological response as a source to variability in drug metabolism and transport. Front. Pharmacol. 3, 8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yervoy (ipilizumab) Summary of Product Characteristics. <http://www.medicines.org.uk/emc/medicine/24779>. Accessed 5 September 2013.
- Evers, R. et al. Critical review of preclinical approaches to investigate cytochrome p450-mediated therapeutic protein drug-drug interactions and recommendations for best practices: a white paper. Drug Metab. Dispos. 41, 1598–1609 (2013). [DOI] [PubMed] [Google Scholar]
- Lee, J.I., Zhang, L., Men, A.Y., Kenna, L.A. & Huang, S.M. CYP-mediated therapeutic protein-drug interactions: clinical findings, proposed mechanisms and regulatory implications. Clin. Pharmacokinet. 49, 295–310 (2010). [DOI] [PubMed] [Google Scholar]
- Cepeda, V., Fuertes, M.A., Castilla, J., Alonso, C., Quevedo, C. & Pérez, J.M. Biochemical mechanisms of cisplatin cytotoxicity. Anticancer. Agents Med. Chem. 7, 3–18 (2007). [DOI] [PubMed] [Google Scholar]
- Bang, Y.J. The potential for crizotinib in non-small cell lung cancer: a perspective review. Ther. Adv. Med. Oncol. 3, 279–291 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd, F.A. et al.; National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med. 353, 123–132 (2005). [DOI] [PubMed] [Google Scholar]
- Eisenhauer, E.A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- Zitvogel, L., Tesniere, A. & Kroemer, G. Cancer despite immunosurveillance: immunoselection and immunosubversion. Nat. Rev. Immunol. 6, 715–727 (2006). [DOI] [PubMed] [Google Scholar]
- Saenger, Y.M. & Wolchok, J.D. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 8, 1 (2008). [PMC free article] [PubMed] [Google Scholar]
- Wolchok, J.D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009). [DOI] [PubMed] [Google Scholar]
- Weber, J. Review: anti-CTLA-4 antibody ipilimumab: case studies of clinical response and immune-related adverse events. Oncologist 12, 864–872 (2007). [DOI] [PubMed] [Google Scholar]
- Weber, J.S., Kähler, K.C. & Hauschild, A. Management of immune-related adverse events and kinetics of response with ipilimumab. J. Clin. Oncol. 30, 2691–2697 (2012). [DOI] [PubMed] [Google Scholar]
- Herbst, R.S. et al. A study of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic tumors. J. Clin. Oncol. 31, abstr. 3000 (2013). [Google Scholar]