Abstract
Sinomenium acutum has been long used in the preparations of traditional medicine in Japan, China and Korea for the treatment of various disorders including rheumatism, fever, pulmonary diseases and mood disorders. Recently, it was reported that Sinomenium acutum, has sedative and anxiolytic effects mediated by GABA-ergic systems. These experiments were performed to investigate whether sinomenine (SIN), an alkaloid derived from Sinomenium acutum enhances pentobarbital-induced sleep via γ-aminobutyric acid (GABA)-ergic systems, and modulates sleep architecture in mice. Oral administration of SIN (40 mg/kg) markedly reduced spontaneous locomotor activity, similar to diazepam (a benzodiazepine agonist) in mice. SIN shortened sleep latency, and increased total sleep time in a dose-dependent manner when co-administrated with pentobarbital (42 mg/kg, i.p.). SIN also increased the number of sleeping mice and total sleep time by concomitant administration with the sub-hypnotic dosage of pentobarbital (28 mg/kg, i.p.). SIN reduced the number of sleep-wake cycles, and increased total sleep time and non-rapid eye movement (NREM) sleep. In addition, SIN also increased chloride influx in the primary cultured hypothalamic neuronal cells. Furthermore, protein overexpression of glutamic acid decarboxylase (GAD65/67) and GABAA receptor subunits by western blot were found, being activated by SIN. In conclusion, SIN augments pentobarbital-induced sleeping behaviors through GABAA-ergic systems, and increased NREM sleep. It could be a candidate for the treatment of insomnia.
Keywords: Sinomenium acutum, Sinomenine, Pentobarbital, GABAA-ergic systems, Electroencephalogram (EEG), Insomnia
INTRODUCTION
Insomnia can be defined as the inability to initiate or maintain sleep. It is also one of the most common health problems in modern society. There are individual differences in the degree of insomnia. So, it is not easy to indicate the severity of insomnia by the absolute amount of sleep time. The research has been performed to recognize sleep status or insomnia of the elder and young peoples. Over the last decade, neuroscientists have more interests in herbal medicines, which contain phytochemicals for the treatment of insomnia. The alternative herbs for the treatment of insomnia has progressed in the past decades. Many herbs such as St, John’s wort, kava kava, valerian, and passion flower have been introduced in European countries (Kim et al., 2011). Herbs as sleep aids have been becoming more popular as alternative medicines.
γ-Aminobutyric acid (GABA), the main inhibitory neurotransmitter of the central nervous system (CNS), is the most prevalent target for treating insomnia. It is well established that activation of GABAA-ergic neurons plays an important role in sleep. Glutamic acid decarboxylase (GAD65/67), an enzyme responsible for the synthesis of GABA also plays a crucial role in sleep. On the other hand, The GABAA receptors complex consists of a Cl− ionophore principally coupled to GABA, barbiturate, benzodiazepine (BZ), steroid, and picrotoxin binding sites (Macdonald and Olsen, 1994; Sieghart, 1995). Basic subunits of GABAA receptors are composed to α (1∼6), β (1∼3) and γ (1∼3) (Seifi et al., 2014). These binding sites trigger the chloride channel’s opening with resulting membrane hyperpolarization (Wang and Xu, 2006). GABAA-ergic drugs have induced sedative–hypnotic effects in animals and humans (Abourashed et al., 2004). Depending on the configuration of the structural subunits, it determines the pharmacological properties of the GABAA receptors. α1–Subunits in GABAA receptors mediate sedation, amnesia, and ataxic effects of benzodiazepine (BZ), whereas α2–and α3-subunits are involved in their anxiolytic-like and muscle-relaxing actions. α5–-Subunits are involved in at least some of the memory impairment caused by BZ (Macdonald and Olsen, 1994).
Sinomenine (SIN; Fig. 1), an alkaloid derived from Sinomenium acutum, is a chief ingredient which has been reported to have a variety of pharmacological effects including anti-rheumatism, immunomodulation and anxiolytic-like effects (Chen et al., 1997; Li et al., 2003; Rao et al., 2017). However, it has not been studied whether SIN is sedative or affects sleep behaviors. Form these experiments, we focused on whether SIN enhances pentobarbital-induced sleeping behaviors and modulate sleep architecture via GABAA-ergic systems in rodents.
Fig. 1.
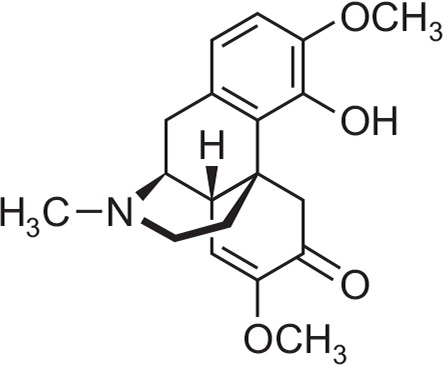
Chemical structure of sinomenine (SIN).
MATERIALS AND METHODS
Animals
The animals used for experiments were 4-week-old ICR male mice and 8-week-old male Sprague Dawley (SD) rats weighing 20–25 g and 300–320 g, respectively. They were purchased from Samtako (Osan, Korea). All rodents were housed in acrylic cages (45×60×23 cm), and were kept at least 1 week for acclimation time. The room condition was maintained at 22 ± 2°C, relative humidity (50–52%), and a 12-h light/dark cycle with ad libitum feeding. The behavioral experiments were performed between 10:00 and 17:00 and were carried out in accordance with the Principle of Laboratory Animal Care (NIH publication No. 85-23, revised 1985). This experiment was performed in accordance with the Animal Care and Use Guidelines of Chungbuk National University, Korea.
Drugs and reagents
SIN was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan). Diazepam (ample), pentobarbital (ample), and muscimol were acquired, respectively, from the following companies: Samjin Pharm. (Seoul, Korea), Hanlim Pharm. Co., Ltd. (Seoul, Korea), and Tocris (Cookson, Avonmouth, UK or Ellisville, MO, USA). Fetal bovine serum and DMEM were obtained from GIBCO (Grand Island, NY, USA). The Cl–sensitive fluorescence probe N-(ethoxycarbonyl-methyl)-6-methoxyquinolinium bromide (MQAE) was purchased from Sigma-Aldrich (St Louis, MO, USA). The specific rabbit polyclonal antibodies against GABAA receptors subunits, GAD65/67 and the corresponding conjugated anti-rabbit immunoglobulin G-horseradish peroxidase, were obtained from Abcam Inc (Cambridge, UK).
Locomotor activity measurement
Spontaneous locomotor activity was measured by a tilting-type ambulometer (AMB-10, O’Hara, Tokyo, Japan) for 1 h (Morton et al., 2011). The mice in each group had 10 min of adaptation time in the activity cages (20 cm in diameter and 18 cm in height). Diazepam (2 mg/kg, p.o.) and SIN (20 and 40 mg/kg, p.o.) dissolved in distilled water and 0.01% DMSO, respectively, were administered 30 min and 60 min prior to the experiment, respectively.
Pentobarbital-induced sleeping behaviors measurement
All mice were fasted for a day, and all experiments were carried out between 1:00 and 5:00 p.m. Pentobarbital was diluted in 0.9% physiological saline. Muscimol (0.2 mg/kg, i.p.) and SIN (20 and 40 mg/kg, p.o.) were orally administered before 15 min and 60 min, respectively, and then pentobarbital (42 mg/kg) was injected intraperitoneally (i.p.). After the pentobarbital, the mice were moved to another cage. Sleep latency was recorded as time elapsed after the pentobarbital injection. Sleep was recorded as the time between the elapse and the righting of animals. The mice that failed sleep within 15 min were excluded from the experiments (Wolfman et al., 1996; Hu, 2012).
EEG telemetry transmitter implantation and data collection
After the pentobarbital was administered (50 mg/kg, i.p.), the SD rats were placed on a pad in the stereotaxic apparatus under aseptic conditions. Transmitters (TA11CTA-F40, Data Sciences International, St. Paul, MN, USA) were implanted under the skin after the scalp incision. In detail, the skull periosteum was removed, and then two holes for were drilled to insert electric lines (A: 2.0 [Bregma], L: 1.5; P: 7.0 [Bregma], L: 1.5 contra-lateral) (Paxinos et al., 1985). The transmitter lines were subcutaneously connected to the skull, and dental cement was used to fix the electric lines to the skull. The incisions were sewn up with a silk suture. An antibiotic was given to all rats after surgery (5 million unit potassium penicillin-G Injection, Keunwha, Seoul, Korea). After the transmitters were implanted, the rats were given a week of recovery time. SIN (40 mg/kg, p.o.) were orally administered before 15 min of experiment. All signals were transmitted by AD converter (Eagle PC300, Los Gatos, CA, USA) and stored in the computer, and the computer could also graphically display the results. Fast Fourier transform (FFT) analysis generated power density values from 0 to 20.0 Hz with a resolution of 0.5 Hz. Mean FFT was also in the range of 0 and 20.0 Hz for every 10 sec. EEG data in all rats were recorded for 4 h from 11:00 a.m. to 5:00 p.m. (Sanford et al., 2006).
Data analysis
Sleep cycles were graphically recorded and saved in Sleep-Sign 2.1 software (KISSEI Comtec Co Ltd, Matsumoto, Japan). Data were classified into wakefulness, non-rapid eye movement (NREM), and rapid eye movement (REM) for every 10 sec (Tokunaga et al., 2007). Wakefulness and NREM states were found in high-frequency and slow waves, respectively. δ-wave (0.75–4.0 Hz) and θ-wave (5.0–9.0 Hz, peak at 7.5 Hz) increased in the low EEG waves during REM sleep. Wakefulness, NREM, REM, and total sleep time (NREM+REM) were recorded for each rat for 6 h. The EEG power was set up at 0.5–20.0 Hz in 0.5 Hz bins. Sleep architecture was evaluated in three waves in the range of 8.0–13.0 Hz (Ma, 2009). Data were calculated as relative values in Microsoft Excel.
Cell culture
Primary cultures of the SD rats’ hypothalamus cells were tested for 7–8 days (Ma et al., 2007). The cells were seeded at 1.0×105 cells in 96-well microplates coated with poly-L-lysine (50 μg/mL; Sigma, St. Louis, MO, USA). The DMEM used for cell cultures contained 10% fetal bovine serum, glutamine (2.0 mM), gentamicin (100 μg/mL), antibiotic-antimycotic solution (10 μg/mL; Sigma), and potassium chloride (25 mM). Cells were incubated at 37°C in a humidified atmosphere of 5% CO2/95% O2 air. After 16 h, the 96-well plates were added into cytosine arabinofuranoside (final concentration 10 μM; Sigma) to inhibit non-neuronal cell growth.
Measurement of intracellular Cl− influx
MQAE (a sensitive fluorescent substance for Cl−) was used to measure Cl− influx in the rats’ cerebellum cells following the method of West and Molloy (1996). After overnight MQAE treatment, the cells were washed three times in a buffer (pH 7.4) that contained 2.4 mM HPO42−, 0.6 mM H2PO4−, 10 mM HEPES, 10 mM D-glucose, and 1.0 mM MgSO4. The fluorescence data were measured according to excitation wavelength 320 nm and emission wavelength 460 nm by Elisa Reader (SpectraMax M2e Multi-mode, PA, USA) (Wagner et al., 2010). The data were calculated as F/F0 based on the Cl− data ratios. F is the fluorescence of each sample, and F0 is the fluorescence without Cl− ions.
Western blotting
Protein samples were extracted from the rat’s hypothalamus cell cultures. SIN (final concentration 40 mg/ml) was dissolved in 0.01% DMSO. The control sample was treated in the same solvent as that used in the SIN treatment. After diazepam or SIN administration, the cells were extracted and treated with a cold lysis buffer [25 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1.0 mM CaCl2, 1% Triton X-100, 1.0 mM PMSF, 10 μl/mL aprotinin, 1.0 mM NaF, and 2.0 mM sodium ortho-vanadate]. Supernatant extracts were recovered after centrifugation at 13,000×g at 4°C for 20 minutes. Protein concentration was measured using Bradford protein analysis and stored at −20°C. The same amounts of protein were placed in 10% SDS-polyacrylamide gel, and then the electrophoresis was loaded. The protein was transferred to PVDF membranes (Hybond-P, GE Healthcare, Amersham, UK) using semidry transfer. The blots were blocked for 1 h at room temperature with 5.0% (w/v) BSA [applied to all primary antibodies except for glyceraldehyde 3-phosphate dehydrogenase (GAPDH)], and 5.0% (w/v) skim milk (only applied to GAPDH) in tris-buffered saline solution (TBS) containing 0.1% Tween-20. Both specific rabbit polyclonal antibodies against GABAA receptor subunits and rabbit anti-GAD65/67 polyclonal antibody at the appropriate dilution in TBST and 5.0% BSA (1:2,500 for all the primary antibodies used) were incubated overnight at 4°C. After washing with TBST, the blots were treated 1:3,000 dilution of a secondary antibody at room temperature for 4 h (goat anti-rabbit, IgG). A the secondary antibody was detected using ECL light-emitting substrate (Roche Diagnostics, Mannheim, Germany)(Han et al., 2010).
Statistical analysis
All statistical analysis was performed with SigmaStat software (SPSS Inc., Chicago, USA). Experimental results are shown as mean ± SEM, and significance was measured with analysis of variance (ANOVA). When there were significant differences, values were compared with Student’s t-test. However, in sub-hypnotic pentobarbital-induced sleep, the falling asleep/total was compared using Chi-square test. p was considered significant at less than 0.05.
RESULTS
Effect of SIN on locomotor activity in mice
SIN (20 and 40 mg/kg, p.o.) was administered to the mice. Locomotor activity was significantly inhibited by SIN at 40 mg/kg. The locomotor activity by diazepam (2.0 mg/kg, i.p.) as a positive control was also decreased compared with that of the control (Fig. 2). The value of locomotor activity to oral administration with SIN at 40 mg/kg were decreased by approximately 41.2%. Positive control DZ also decreased by approximately 56.3%, compared with that of the control. From these preliminary experiments, we suggest that SIN might be sedative.
Fig. 2.
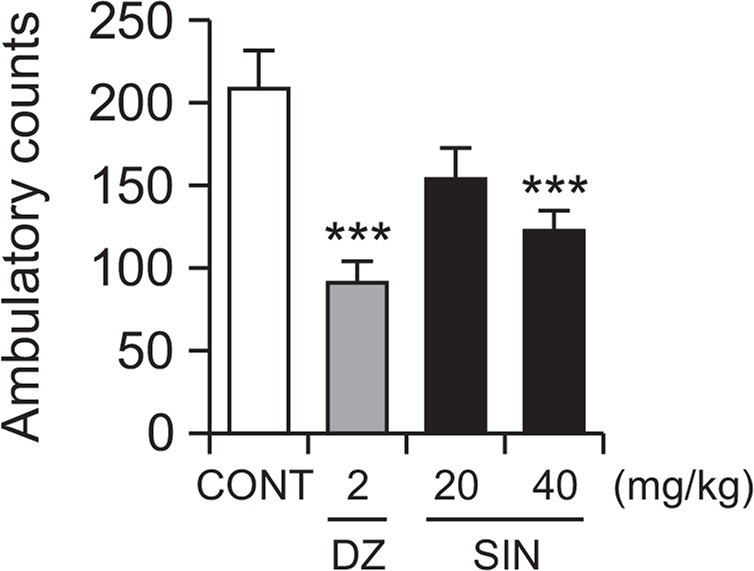
Effect of SIN on locomotor activity test. Ambulation activity was measured for 1 h, 30 min after oral administration of diazepam and 1 h after administration of SIN. Each column shows the mean ± SEM. The significance of the compound’s effects was assessed using ANOVA. Where there was significant variability, the individual values were compared using Student’s t-test. ***p<0.005, compared with the control.
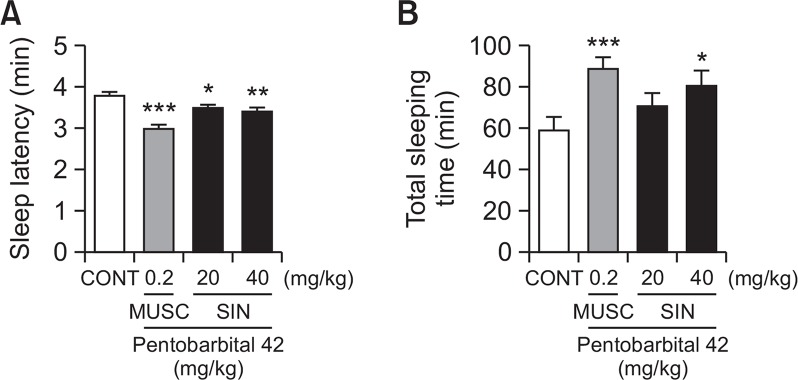
Effects of SIN on pentobarbital-induced sleeping behaviors in mice
SIN (20 and 40 mg/kg, p.o.) reduced the sleep latency of sleep and induced dose-dependently total sleep time in pentobarbital induced sleeping mice. Pretreatment with mucsimol (0.2 mg/kg, i.p.) as a positive control 30 min before the pentobarbital (42 mg/kg, i.p.) also increased sleeping time and decreased sleep latency (Fig. 3). The time of sleep latency to oral administration with SIN 40 mg/kg were decreased by approximately 10.1%. Positive control (muscimol) also decreased by approximately 24.8%, compared with that of the control. Total sleep time SIN 40 mg/kg group were increased by approximately 36.7%. Positive control MUSC also increased by approximately 50.6%, compared with that of the control. We suggest that SIN could reduce sleep latency and increase total sleep.
Fig. 3.
Effects of SIN on sleep onset and duration in pentobarbital-treated mice. Mice were deprived of sleep for 24 h prior to the experiment. Pentobarbital (42 mg/kg, i.p.) was administered to mice following administration of muscimol or SIN, and sleep latency (A) and total sleep time (B) were measured. Each column shows the mean ± SEM. The significance of the compounds’ effects was assessed using ANOVA. Where there was significant variability, the individual values were compared using Student’s t-test. *p<0.05, **p<0.01, ***p<0.005, compared with that of the control.
Effects of SIN on sleep onset by sub-hypnotic dosage of pentobarbital in mice
SIN (40 mg/kg, p.o.) reduced the sleep latency and prolonged total sleep time induced by a sub-hypnotic pentobarbital dose (28 mg/kg, i.p.). Similarly, muscimol (a GABA receptor agonist) significantly is affected pentobarbital-induced sleep (Table 1). Total sleep time to sub-hypnotic dosage with at SIN 40 mg/kg were increased by approximately 31.5%. Positive control MUSC also increased by approximately 53.9%, compared with that of the control. We suggest that SIN would interact with GABAA receptors.
Table 1.
Effects of SIN on number of sleep mice and total sleep time treated by sub-hypnotic dose of pentobarbital (28 mg/kg, i.p.)
| Group | Dose (mg/kg) | No. of animals | Total Sleep time (min) |
|---|---|---|---|
| Control | 0 | 6/12 | 30.8 ± 2.6 |
| Muscimol | 0.2 | 12/12* | 47.4 ± 1.8*** |
| SIN | 20 | 8/13 | 34.7 ± 2.0 |
| 40 | 10/13 | 40.5 ± 2.3** |
Each value reflects the mean ± SEM. Where there was significant variability, the individual values were compared using Chi-square and Student’s t-test.
p<0.05,
p<0.01
p<0.005, compared with the control.
Effects of SIN on sleep-wake cycles
SIN (40 mg/kg, p.o.) significantly reduced sleep-wake cycles. The count of sleep-wake cycle were decreased by approximately 19.8%, compared with that of the control (Fig. 4). The sleep structure shows that the sleep time is shorter than the total sleep time (Fig. 5); In other words, SIN reduced wakefulness.
Fig. 4.
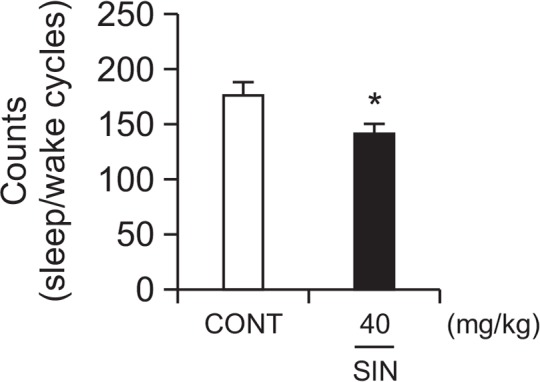
Effect of SIN on numbers of sleep-wake cycles. Where there was significant variability, the individual values were compared using Student’s t-test. *p<0.05, compared with that of the control.
Fig. 5.
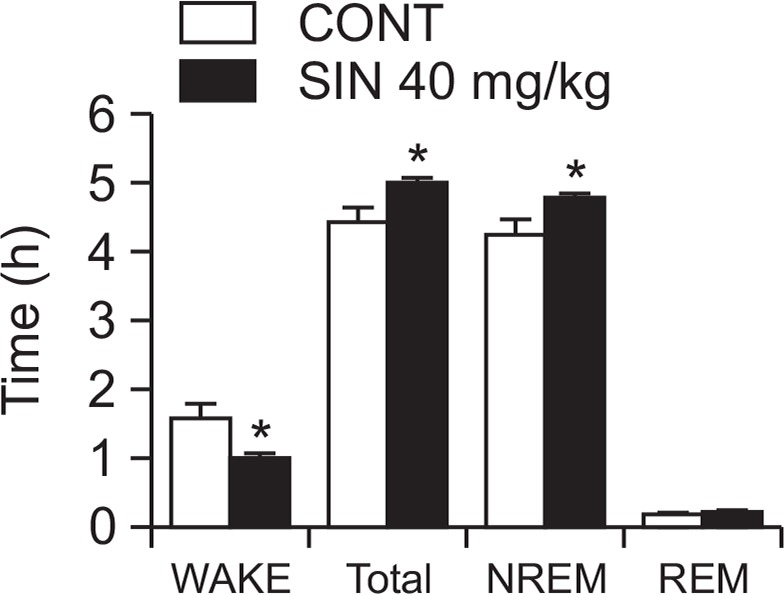
Effect of SIN on rat sleep architecture. The data show the mean ± SEM of time spent, which separated the wakefulness and sleep (NREM and REM) states. The significance of the compounds’ effects was assessed using ANOVA. Where there was significant variability, the individual values were compared using Student’s t-test. *p<0.05, compared with that of the naïve control.
Effects of SIN on sleep architectures
After EEG analysis, we found that SIN (40 mg/kg, p.o.) significantly prolonged total sleep time, especially NREM sleep. SIN also decreased wakefulness (Fig. 5). The wake time of sleep architectures at SIN 40 mg/kg were decreased by approximately 36.5%, then increased total sleep and NREM sleep by approximately 13.0% and 12.7% respectively, compared with that of the control.
Effects of SIN on intracellular Cl− influx in primary cultured hypothalamus cells
SIN (10 μM) significantly increased intracellular Cl− influx, resulting in the hyperpolarization of the neuronal cell membrane. In addition, Pentobarbital (10 μM) also significantly increased intracellular Cl− influx in primary cultured hypothalamus cells. The intensities of Cl− influx in hypothalmus cells treared with SIN 0.1, 1 and 10 μM were approximately 38.7%, 54.9% and 65.6% respectively. Positive control PENT also increased by approximately 55.9%, compared with that of the control (Fig. 6).
Fig. 6.
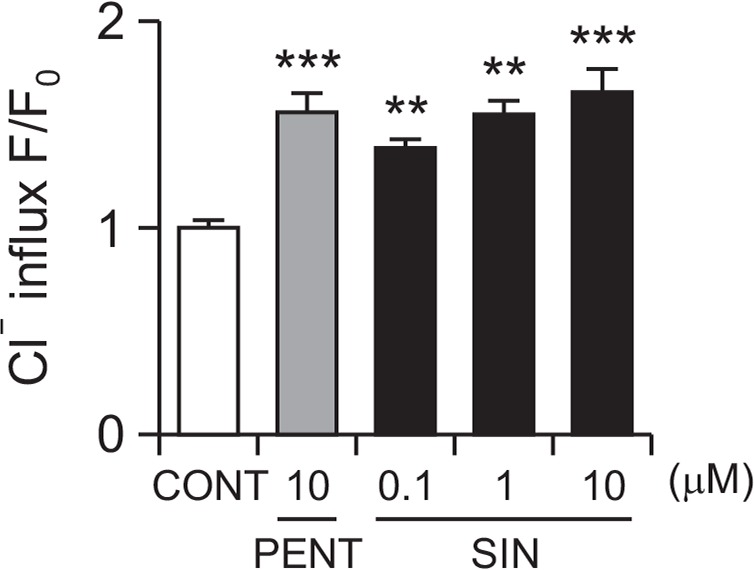
Effect of SIN on Cl− influx in primary cultured cerebellar granule cells. After the hypothalamic neuronal cells were cultured for 8 days, the cells were incubated with MQAE overnight, and then SIN (0.1, 1, and 10 μM) and pentobarbital (10 μM) were added 1 h prior to measurement. Each column shows the mean ± SEM. The significance of the compounds’ effects was assessed using ANOVA. Where there was significant variability, the individual values were compared using Student’s t-test. **p<0.01, ***p<0.005, compared with that of the control.
Effects of SIN on expression of GAD65/67
GAD65/67 expression was induced by SIN (40 mg/kg, p.o.) in the rats’ primary hypothalamic neuron cells. The intensities of GAD65/67 expression translocated to the plasma membrane in hypothalamic tissue treated with SIN was increased by approximately 85.8% compared with that of the control tissue (Fig. 7). We suggest that SIN activates GAD65/67.
Fig. 7.
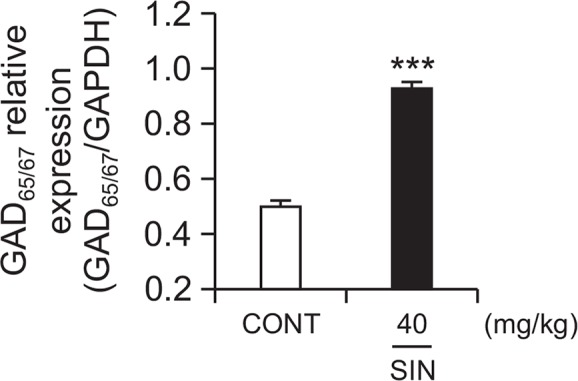
Effect of SIN on the expression of GAD; the GAD65/67 expression was induced by SIN (40 mg/kg) in the hypothalamic neuronal cells of the mice. GAPDH levels were needed in order to normalize the protein expression. Each column shows the mean ± SEM. The significance of the compounds’ effects was assessed using ANOVA. Where there was significant variability, the individual values were compared using Student’s t-test. ***p<0.005 compared with that of the control.
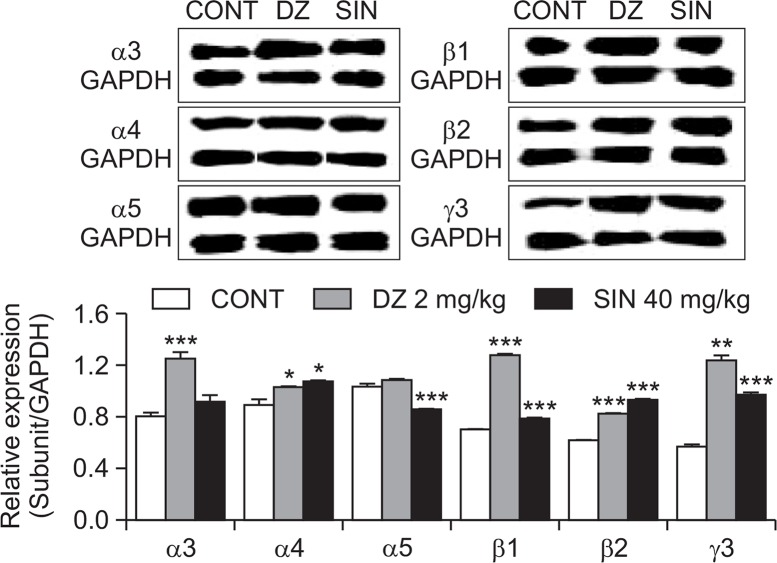
Effects of SIN on expression of GABAA receptors subunits
From these experiments, the GABAA receptor subtype activation was measured by western blotting. All subtypes of GABAA receptors except α5 subtype were overexpressed with the SIN (40 mg/kg, p.o.). DZ as a positive control also showed similar patterns. The expression of GABA subunit (α4, β1, β2, γ3) increased in hypothalamic sample treated with SIN were by approximately 20.6%, 11.9%, 50.7% and 70.4% respectively. Positive control DZ (α3, α4, β1, β2, γ3) also increased by approximately 55.4%, 15.6%, 81.9%, 33.3% and 116.9% respectively, compared with that of the control (Fig. 8).
Fig. 8.
Effects of SIN on expression of GABAA receptor subunits. Immunoblots are shown of lysed hypothalamic neuronal cells that were treated for 1 h following SIN. GAPDH levels were needed in order to normalize the protein expression. Each column shows the mean ± SEM. The significance of the effects of the compounds was assessed using ANOVA. Where there was significant variability, the individual values were compared using Student’s t-test. *p<0.05, **p<0.01, ***p<0.005, compared with that of the control.
DISCUSSION
Many alkaloids such as SIN, disinoimenine, sinactine, sinoactine, acutumine, and magnoflorine, as well as the lignan syringaresinol have been isolated from Sinomenium acutum (Bao et al., 2005; Wang et al., 2007; Jin et al., 2008). Among them, SIN has been reported to have a variety of pharmacological effects including anti-rheumatism, immunomodulation and sedative effects (Chen et al., 1997; Li et al., 2003; Rao et al., 2017). Recently, it was reported that Sinomenium acutum, has sedative and anxiolytic effects mediated by GABA-ergic systems. In addition, they reported that SIN exerts considerable antinociceptive property for neuropathic pain via GABAA-mediated mechanism, and it could be useful for the management of chronic painful conditions such as neuropathic pain (Zhu et al., 2014). Based on previous studies, we focused on the hypnotic effect of SIN as the ultimate goal of the experiment. The preliminary experiment results demonstrate that SIN inhibited locomotor activity, showing sedative effects in mice. We investigated the effects of different dosages of SIN and muscimol in rodents with pentobarbital treatment and found that SIN enhanced pentobarbital-induced sleep, similar to muscimol, It is suggested that potentiation of SIN’s hypnotic effect can interact with GABAA-ergic systems.
The sleep architectures of rat after oral SIN administration were also analyzed. The spontaneous electrical activity from the rat brain can be recorded by SSG over a short period of time, and sleep/wake cycles can be measured using EEG frequency analysis. Spectral EEG analysis technique of sleep/wake cycles can be used as a tool for insomnia treatment. We found that SIN itself modulated sleep architectures; SIN reduced the counts of sleep/wake cycles in rats, which is important in treating insomnia. Sleep can be divided three stages, wake, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. REM Sleep is also composed of rapid eye movement (REM) sleep and non-REM (NREM) sleep. REM sleep is the major source of dreams, whereas synchronous cortical oscillations, called slow waves, are observed during NREM sleep. Both stages are unique to certain mammalian species, and therefore, REM and NREM sleep are thought to be involved in higher-order brain functionssleep is most often associated with vivid dreaming and a high level of brain activity. The other phase of sleep, NREM sleep or slow wave sleep (SWS), is usually associated with reduced neuronal activity. As one goes to sleep, the low voltage fast EEG of waking gradually gives way to a slowing of frequency and, as sleep moves toward the deepest stages, there is an abundance of delta waves, EEG waves with a frequency of 0.5 to <4 Hz and of high amplitude (McCarley, 2007). According to the sleep research, REM sleep was characterized by fast-wave sleep along with muscle atonia, brain activation, and eye movement. NREM sleep was discovered to play a role in restoring physiological functions (Siegel, 2005). REM sleep is a distinctive sleep stage that alternates with episodes of NREM sleep (Trachsel et al., 1991; Gottesmann, 1996; Datta and Hobson, 2000). About 25 percent of sleep is spent in an REM sleep and the remaining 75 percent is for the NREM sleep. We especially focused on determining whether SIN increased REM, NREM, and total sleep time and alter sleep architectures. From these experiments, it is suggested that SIN could enhance total sleep and NREM sleep in rats, reducing sleep/wake cycles.
Activating GABAA–ergic transmission is important for treating insomnia. From the first in vitro experiments, we found that SIN increased intracellular Cl− influx in primary cultured hypothalamic neuronal cells of rats, resulting in hyperpolarization of neuronal cells in the CNS. These effects can be caused by the increased levels of GABA in the neuronal cells. GABA is synthesized from glutamate exclusively in GABAA-ergic neurons by GAD, which consists of two isoforms with molecular weights of 65-kDa and 67-kDa (Bu et al., 1992). GAD65/67 is the rate-limiting enzyme in GABA biosynthesis which also plays an important role in maintaining GABA level in the brain (Tillakaratne et al., 1995). GABA activates the ionotropic GABA receptors on the presynaptic, postsynaptic, and extrasynaptic neurons. GABA is released to the synapse which is the extracellular space existing between the neurons. The GABAA receptor Cl− channel opens after binding with GABA to give a net inward flux of negative Cl− (outward current), hyperpolarizing the membrane and reducing neuronal firing (Macdonald and Olsen, 1994). Protein expression levels of GAD65/67 were measured in primary cultured hypothalamic neuronal cells; SIN increased protein expression levels in these cells. It is suggested that SIN activates GAD65/67. SIN also increased Cl− influx, similar to pentobarbital. This result shows that SIN can lead GABAA receptors to open the Cl− channel.
The expression levels of GABAA receptor α-, β- and γ-subunits were also investigated. The most abundant GABAA receptor subunit composition, α1, β2 and γ2, including the cerebellum, is related to the hypnotic/sedative effect of GABAA receptors (Rudolph and Mohler, 2006). Previous studies have shown α1 subunit associated with sedation (Rudolph et al., 1999; McKernan et al., 2000), the α2/3 subunits associated with anxiety (Low et al., 2000; Crestani et al., 2001), and the α5 subunit associated with temporal and spatial memory (Collinson et al., 2002; Crestani et al., 2002). Structural and physiological heterogeneities of GABAA receptors as well as the differential distribution of its receptor subtypes in specific brain areas provide an important basis for the development of therapeutic drugs. We examined expression patterns of α-subunits (α3, α4, α5), β-subunits (β1, β2) and γ-subunit (γ3) in GABAA receptors in hypothalamic cells. SIN, non-selectively activated the subunits of GABAA receptors in these experiments. All subunits which we have tested except for α3, α5- subunits were over-expressed by SIN. On the other hand, diazepam increased high protein levels in the α3, α4, β1, β2, γ3 subunits. The activation of GABAA receptors by SIN seem unlikely with those of diazepam.
The present study provides evidence that SIN possesses not only sleep-prolonging but also sleep quality-enhancing effects when administered orally to rats. SIN itself decreases sleep/wake cycle and increases total sleep time. GABAA-ergic transmissions including GAD65/67, intracellular chloride influx, and GABAA receptor subtypes were reduced by SIN. In conclusion, SIN can be a candidate for the treatment of insomnia.
Acknowledgments
This work was financially supported by the National Research Foundation of Korea (NRF and MSIP) (no. MRC 2008-0062275).
Footnotes
CONFLICT OF INTEREST
All authors declare no conflict of interest.
REFERENCES
- Abourashed EA, Koetter U, Brattström A. In vitro binding experiments with a Valerian, hops and their fixed combination extract (Ze91019) to selected central nervous system receptors. Phytomedicine. 2004;11:633–638. doi: 10.1016/j.phymed.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Bao GH, Qin GW, Wang R, Tang XC. Morphinane alkaloids with cell protective effects from Sinomenium acutum. J Nat Prod. 2005;68:1128–1130. doi: 10.1021/np050112+. [DOI] [PubMed] [Google Scholar]
- Bu DF, Erlander MG, Hitz BC, Tillakaratne NJ, Kaufman DL, Wagner-McPherson CB, Evans GA, Tobin AJ. Two human glutamate decarboxylases, 65-kDa GAD and 67-kDa GAD, are each encoded by a single gene. Proc Natl Acad Sci USA. 1992;89:2115–2119. doi: 10.1073/pnas.89.6.2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-J, Shiao MS, Lee SS, Wang SY. Effect of Cordyceps sinensis on the proliferation and differentiation of human leukemic U937 cells. Life Sci. 1997;60:2349–2359. doi: 10.1016/S0024-3205(97)00291-9. [DOI] [PubMed] [Google Scholar]
- Collinson N, Kuenzi FM, Jarolimek W, Maubach KA, Cothliff R, Sur C, Smith A, Otu FM, Howell O, Atack JR, McKernan RM, Seabrook GR, Dawson GR, Whiting PJ, Rosahl TW. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Blüthmann H, Möhler H, Rudolph U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crestani F, Löw K, Keist R, Mandelli M, Möhler H, Rudolph U. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- Datta S, Hobson JA. The rat as an experimental model for sleep neurophysiology. Behav Neurosci. 2000;114:1239–1244. doi: 10.1037/0735-7044.114.6.1239. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. The transition from slow-wave sleep to paradoxical sleep: evolving facts and concepts of the neurophysiological processes underlying the intermediate stage of sleep. Neurosci Biobehav Rev. 1996;20:367–387. doi: 10.1016/0149-7634(95)00055-0. [DOI] [PubMed] [Google Scholar]
- Han S, Niu W, Li H, Hu L, Yuan Y, Xu G. Effect of hydroxyl and amino groups on electrochemiluminescence activity of tertiary amines at low tris(2,2′-bipyridyl)ruthenium(II) concentrations. Talanta. 2010;81:44–47. doi: 10.1016/j.talanta.2009.11.037. [DOI] [PubMed] [Google Scholar]
- Hu Z, Kim C-S, Oh EH, Lee MK, Eun JS, Hong JT, Oh KW. Methanol extract of zizyphi spinosi semen augments pentobarbital-induced sleep through the modification of GABAergic systems. Nat Prod Sci. 2012;18:67–75. [Google Scholar]
- Jin HZ, Wang XL, Wang HB, Wang YB, Lin LP, Ding J, Qin GW. Morphinane alkaloid dimers from Sinomenium acutum. J Nat Prod. 2008;71:127–129. doi: 10.1021/np0704654. [DOI] [PubMed] [Google Scholar]
- Kim CS, Han JY, Kim S, Hong JT, Oh KW. Herbs for the treatment of insomnia. Biomol. Ther. (Seoul) 2011;19:274–281. doi: 10.4062/biomolther.2011.19.3.274. [DOI] [Google Scholar]
- Li SP, Zhao KJ, Ji ZN, Song ZH, Dong TT, Lo CK, Cheung JK, Zhu SQ, Tsim KW. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003;73:2503–2513. doi: 10.1016/S0024-3205(03)00652-0. [DOI] [PubMed] [Google Scholar]
- Low K, Crestani F, Keist R, Benke D, Brünig I, Benson JA, Fritschy JM, Rülicke T, Bluethmann H, Möhler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- Ma Y, Eun JS, Lee KS, Lee ES, Kim CS, Hwang BY, Oh KW. Methanol extract of longanae arillus regulates sleep architecture and EEG power spectra in restraint-stressed rats. Nat Prod Sci. 2009;15:213–221. [Google Scholar]
- Ma Y, Han H, Eun JS, Kim HC, Hong JT, Oh KW. Sanjoinine A isolated from Zizyphi Spinosi Semen augments pentobarbital-induced sleeping behaviors through the modification of GABA-ergic systems. Biol Pharm Bull. 2007;30:1748–1753. doi: 10.1248/bpb.30.1748. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–330. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- McKernan RM, Rosahl TW, Reynolds DS, Sur C, Wafford KA, Atack JR, Farrar S, Myers J, Cook G, Ferris P, Garrett L, Bristow L, Marshall G, Macaulay A, Brown N, Howell O, Moore KW, Carling RW, Street LJ, Castro JL, Ragan CI, Dawson GR, Whiting PJ. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. Identification of a physiological role for leptin in the regulation of ambulatory activity and wheel running in mice. Am J Physiol Endocrinol Metab. 2011;300:E392–E401. doi: 10.1152/ajpendo.00546.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J. Neurosci. Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Rao S, Liu S, Zou L, Jia T, Zhao S, Wu B, Yi Z, Wang S, Xue Y, Gao Y, Xu C, Li G, Xu H, Zhang C, Liang S. The effect of sinomenine in diabetic neuropathic pain mediated by the P2X3 receptor in dorsal root ganglia. Purinergic Signal. 2017;13:227–235. doi: 10.1007/s11302-016-9554-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Mohler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Sanford LD, Yang L, Liu X, Tang X. Effects of tetrodotoxin (TTX) inactivation of the central nucleus of the amygdala (CNA) on dark period sleep and activity. Brain Res. 2006;1084:80–88. doi: 10.1016/j.brainres.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Seifi M, Brown JF, Mills J, Bhandari P, Belelli D, Lambert JJ, Rudolph U, Swinny JD. Molecular and functional diversity of GABA-A receptors in the enteric nervous system of the mouse colon. J Neurosci. 2014;34:10361–10378. doi: 10.1523/JNEUROSCI.0441-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of γ-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Tillakaratne NJ, Medina-Kauwe L, Gibson KM. γ-Aminobutyric acid (GABA) metabolism in mammalian neural and nonneural tissues. Comp Biochem Physiol A Physiol. 1995;112:247–263. doi: 10.1016/0300-9629(95)00099-2. [DOI] [PubMed] [Google Scholar]
- Tokunaga S, Takeda Y, Niimoto T, Nishida N, Kubo T, Ohno T, Matsuura Y, Kawahara Y, Shinomiya K, Kamei C. Effect of valerian extract preparation (BIM) on the sleep-wake cycle in rats. Biol Pharm Bull. 2007;30:363–366. doi: 10.1248/bpb.30.363. [DOI] [PubMed] [Google Scholar]
- Trachsel L, Tobler I, Achermann P, Borbély AA. Sleep continuity and the REM-nonREM cycle in the rat under baseline conditions and after sleep deprivation. Physiol Behav. 1991;49:575–580. doi: 10.1016/0031-9384(91)90283-T. [DOI] [PubMed] [Google Scholar]
- Wagner C, Vargas AP, Roos DH, Morel AF, Farina M, Nogueira CW, Aschner M, Rocha JB. Comparative study of quercetin and its two glycoside derivatives quercitrin and rutin against methylmercury (MeHg)-induced ROS production in rat brain slices. Arch Toxicol. 2010;84:89–97. doi: 10.1007/s00204-009-0482-3. [DOI] [PubMed] [Google Scholar]
- Wang W, Xu TL. Chloride homeostasis differentially affects GABAA receptor- and glycine receptor-mediated effects on spontaneous circuit activity in hippocampal cell culture. Neurosci Lett. 2006;406:11–16. doi: 10.1016/j.neulet.2006.06.064. [DOI] [PubMed] [Google Scholar]
- Wang X, Jin H, Li Z, Qin G. 8-demethoxyrunanine from Sinomenium acutum. Fitoterapia. 2007;78:593–595. doi: 10.1016/j.fitote.2007.03.018. [DOI] [PubMed] [Google Scholar]
- West MR, Molloy CR. A microplate assay measuring chloride ion channel activity. Anal Biochem. 1996;241:51–58. doi: 10.1006/abio.1996.0377. [DOI] [PubMed] [Google Scholar]
- Wolfman C, Viola H, Marder M, Wasowski C, Ardenghi P, Izquierdo I, Paladini AC, Medina JH. Anxioselective properties of 6,3′-dinitroflavone, a high-affinity benzodiazepine receptor ligand. Eur J Pharmacol. 1996;318:23–30. doi: 10.1016/S0014-2999(96)00784-4. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Sun Y, Zhu J, Fang T, Zhang W, Li JX. Antinociceptive effects of sinomenine in a rat model of neuropathic pain. Sci Rep. 2014;4:7270. doi: 10.1038/srep07270. [DOI] [PMC free article] [PubMed] [Google Scholar]