Abstract
Atopic dermatitis (AD) is a common inflammatory skin disorder mediated by inflammatory cells, such as macrophages and mast cells. Rifampicin is mainly used for the treatment of tuberculosis. Recently, it was reported that rifampicin has anti-inflammatory and immune-suppressive activities. In this study, we investigated the effect of rifampicin on atopic dermatitis in vivo and in vitro. AD was induced by treatment with 2, 4-dinitrochlorobenzene (DNCB) in NC/Nga mice. A subset of mice was then treated with rifampicin by oral administration. The severity score and scratching behavior were alleviated in the rifampicin-treated group. Serum immunoglobulin E (IgE) and interleukin-4 (IL-4) levels were also ameliorated in mice treated with rifampicin. We next examined whether rifampicin has anti-atopic activity via suppression of mast cell activation. Rifampicin suppressed the release of β-hexosaminidase and histamine from human mast cell (HMC)-1 cultures stimulated with compound 48/80. Treatment with rifampicin also inhibited secretion of inflammatory mediators, such tumor necrosis factor-α (TNF-α) and prostaglandin D2 (PGD2), in mast cells activated by compound 48/80. The mRNA expression of cyclooxygenase 2 (COX-2) was reduced in the cells treated with rifampicin in a concentration-dependent manner. These results suggest that rifampicin can be used to treat atopic dermatitis.
Keywords: Rifampicin, NC/Nga mice, Mast cell, Atopic dermatitis
INTRODUCTION
Atopic dermatitis (AD) is an inflammatory skin disorder associated with epidermal hyper-reactivity to allergens such as dust mites, pollen, and mold spores (Leung et al., 2004).
AD is related to immunoglobulin E (IgE) overproduction and is well characterized by increased inflammatory infiltration that leads to elevated serum levels of IgE (Bergmann et al., 1998; Chang and Shiung, 2006). Mast cells are effector cells in the inflammatory activity associated with allergic disorders, including asthma and AD. Histamine released from mast cells is a major mediator leading to hypersensitivity, and remains the most characterized and potent vasoactive mediator near allergic lesions (Petersen et al., 1996). With the release of proinflammatory cytokines, such as tumor necrosis factor (TNF)-α, interleukin-4 (IL-4), IL-8, and IL-13, mast cells induce allergic inflammation (Kalesnikoff and Galli, 2008). In general, using medications of adequate potency, mainly corticosteroids, is conventional in the treatment of the vast majority of AD patients (Sidbury and Hanifin, 2000). However, the long-term use of steroids is not desirable because of the systemic adverse effects, including skin atrophy, secondary infection, and acne (Furue et al., 2003). Therefore, alternative therapeutic agents are required for the treatment of AD.
Rifampicin is mainly used to treat mycobacterial infections, such as Hansen’s disease (leprosy) and tuberculosis (Eule et al., 1974; Loeffler, 1999). Rifampicin inhibits bacterial RNA synthesis by inhibiting bacterial DNA-dependent RNA polymerase (Wehrli et al., 1968; di Mauro et al., 1969; Tsai and Saunders, 1973). Recently, it has been reported that rifampicin exhibits anti-inflammatory effects as well as plays a role in relieving neuropathic pain and in immune modulation (Bellahsene and Forsgren, 1980; Kim et al., 2009; Wang et al., 2013). Rifampicin was also reported to possess therapeutic effects against psoriasis in clinical practice (Tsankov and Angelova, 2003). However, the anti-allergic effects of rifampicin are not clear. In this study, an atopic in vivo and in vitro model was used to assess the effects of rifampicin therapy for AD. To this end, we investigated the effect of rifampicin on AD using NC/Nga mice, which are genetically predisposed to develop an AD-like skin disease (Matsuda et al., 1997; Tsudzuki et al., 1997). Furthermore, we examined the effect of rifampicin on degranulation, secretion of TNF-α and prostaglandin D2 (PGD2), and mRNA expression of cyclooxygenase 2 (COX-2) in human mast cell (HMC)-1 cultures.
MATERIALS AND METHODS
Reagents
Rifampicin, compound 48/80, disodium cromoglycate (cromolyn), p-nitrophenyl-N-acetyl-β-D-glucosaminide [PN-(GlcNAc)2], thiazolyl blue tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), toluidine blue dye, CaCl2, MgCl2, NaHCO3, and glucose were purchased from Sigma-Aldrich Korea (Yongin, Korea). The histamine enzyme-linked immunosorbent assay (ELISA) kit was obtained from IBL International GmbH (Hamburg, Germany). We purchased the human PGD2 ELISA kit from Cusabio (Wuhan, China) and the TNF-α ELISA kit from eBioscience (San Diego, USA). The total RNA isolation kit was purchased from GeneAll (Seoul, Korea). Fluo-3 AM was obtained from Molecular Probes (Eugene, OR, USA).
Animals
Four-week-old male NC/Nga mice were purchased from Central Laboratory Animal, Inc (Woomyundong, Seoul, Korea). They were housed under standard conditions of ambient temperature (23 ± 2°C) and humidity (55 ± 10%), with free access to chow pellets and water for one week before the start of the experiments. The experimental groups included 5–7 animals per drug and dose. Animals were treated and maintained according to the Animal Care and Use Guidelines of Sahmyook University, Korea.
AD induction and treatment with rifampicin
The dorsal surface of each mouse was shaved with animal clippers before the experiments. The dorsal region was treated with 2, 4-dinitrochlorobenzene (DNCB) to induce AD (Jung et al., 2014). After induction of AD, mice were treated with rifampicin by oral administration (Fig. 1A). Dexamethasone was used as a control. Mice were randomly assigned to one of five groups at the start of the experiment (n=5–7): Group A, normal control; Group B, AD control; Group C, rifampicin treatment (25 mg/kg); Group D, rifampicin treatment (50 mg/kg); Group E, dexamethasone treatment (1 mg/kg).
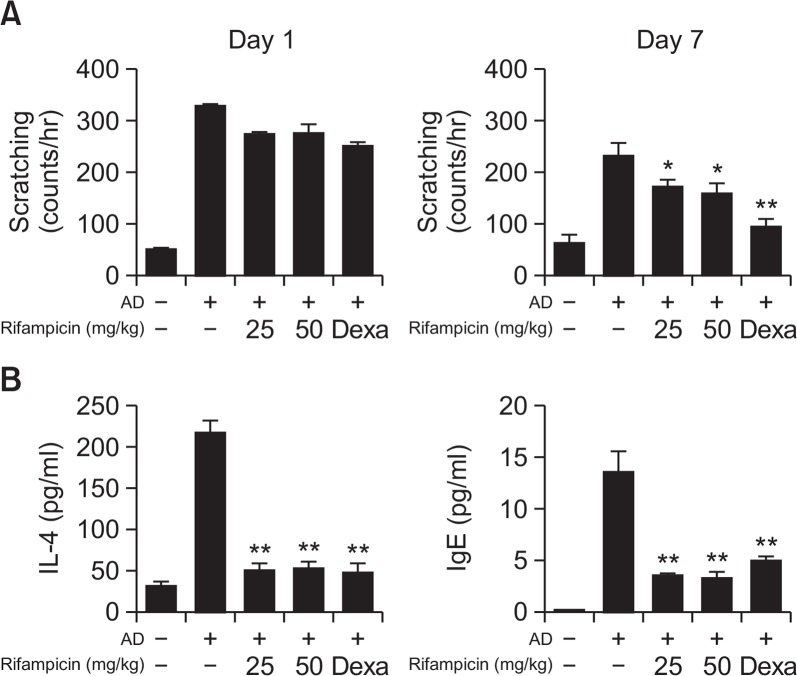
Fig. 1.
The effect of rifampicin on AD-induced NC/Nga mice (n=5–7). The mice were observed 7 days after oral administration of rifampicin. Dexamethasone (1 mg/kg) was used as a positive control. (A) The scheme of the animal experiment. (B) Image of the lesions from AD-induced mice at day 1 and day 7 after rifampicin treatment. (C) Rifampicin significantly mitigated five symptoms: erythema/darkening, edema/population, excoriations, lichenification/prurigo, and dryness 7 days after treatment. The improvement in skin lesions was evaluated based on the skin severity score. *p<0.05, **p<0.01, when compared with the AD-induced and untreated mice.
Skin severity score and scratching behavior
Mice were photographed before and after rifampicin treatment. The skin severity test was evaluated once a day after completing treatment as previously described (Suto et al., 1999). Briefly, the development of (1) erythema/hemorrhage, (2) dryness/scaling, (3) edema, (4) erosion/excoriation, and (5) lichenification was scored as 0 (none), 1 (mild), 2 (moderate), or 3 (severe). The sum of the individual scores was taken as the dermatitis score, ranging from 0 to 15. Scratching behavior was recorded on video as previously described (Umeda et al., 2006; Chung et al., 2012). The scratching number was counted as the number of times a mouse scratched the dorsal skin lesion within a period of 30 min. The number of scratches in the non-treatment group was regarded as the baseline scratching behavior before the start of treatment.
Blood collection for serologic tests
Mice were killed 1, 3, or 7 days after treatment with rifampicin, and cardiac blood was collected for the measurement of serum IgE and IL-4 levels. Blood was separated by centrifugation at 3,000 g for 10 min at 4°C, and the serum was stored at −80°C until use.
Cell culture and treatment
The HMC-1 cell line was kindly provided by Prof. Jang (College of Korean Medicine, Kyung Hee University, Korea). HMC-1 cells were cultured in Dulbecco’s modified Eagle medium (DMEM, Welgene, Seoul, Korea) with 10% heat-inactivated fetal bovine serum (FBS) and 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C and 5% CO2 with 95% humidity. The cells were treated with the indicated concentrations of rifampicin for the indicated times and were then stimulated with compound 48/80 (10 μg/ml) for 30 min. The cell culture supernatants and cell lysates were used for ELISA and reverse-transcription polymerase chain reaction (RT-PCR), respectively.
Measurement of cell viability
The trypan blue exclusion method and MTT assay were used to determine viable and dead cells to test the cytotoxicity of rifampicin. Cell viability was evaluated by the colorimetric MTT assay. Cells were seeded in 96-well plates (2×105 cells/well) and incubated for 24 h. After treatment with rifampicin, 20 μl of MTT solution (5 mg/ml) was added for 4 h at 37°C. Subsequently, the medium was removed, and 100 μl DMSO was added to extract formazan. The plate was shaken for 15 min. A microplate reader was used to read the absorbance at 540 nm.
β-Hexosaminidase (β-HEX) secretion assay
Degranulation of HMC-1 cells was measured by β-HEX assay. The amount of β-HEX released into the medium was determined as described previously (Schwartz et al., 1979). HMC-1 cells were grown in 24-well plates for one day. After washing twice with Tyrode’s buffer (137 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1.1 mM MgCl2, 11.9 mM NaHCO3, 0.6 mM NaH2PO4, 5.6 mM glucose, pH 7.2), the cells were pretreated with different concentrations of rifampicin (6.25, 12.5, 25, or 50 μM) for 30 min at 37°C. Cromolyn, which is a mast cell stabilizer, was used as a positive control. Next, the cells were treated with compound 48/80 (10 μg/ml) for 30 min at 37°C. The supernatant (50 μL) was transferred to a 96-well plate and incubated with 50 μL of 5 mM PN-(GlcNAc)2 for 1 h at 37°C. The assay was terminated by the addition of stop solution (200 μL/well of 0.1 M Na2CO3/NaHCO3 pH 10.2). The absorbance was measured with a microplate reader at 405 nm (Schwartz and Austen, 1980).
Histamine release assay
HMC-1 cells (5×105 cells/well) were incubated for 12 h at 37°C. The cells were treated with various concentrations of rifampicin for 2 h and then treated with compound 48/80 (10 μg/ml) for 30 min. Cromolyn was used as a positive control. The cell culture supernatants were collected, centrifuged at 400 g for 5 min at 4°C, and then used for the measurement of histamine release using an ELISA kit. The assay was performed according to the manufacturer’s instructions.
Intracellular calcium
To measure intracellular calcium levels, HMC-1 cells were pre-incubated with 5 μM Fluo-3 AM for 30 min at 37°C and then washed with Tyrode’s buffer. The cells were treated with rifampicin for 30 min at 37°C and then stimulated with compound 48/80. After 10 min, intracellular calcium levels were analyzed using a fluorescence microscope (Olympus CKX41, Olympus Corp., Tokyo, Japan). Cromolyn was used as a positive control.
Measurement of PGD2 and TNF-α production
HMC-1 cells (1×106 cells/well) were incubated for 12 h at 37°C. The cells were treated with various concentrations of rifampicin for 2 h and then with compound 48/80 (10 μg/ml) for 30 min. The cells were washed with PBS and incubated for 18 h. The supernatants were collected, centrifuged at 400 g for 5 min at 4°C, and used for PGD2 and TNF-α ELISA tests, according to the manufacture’s protocol.
RT-PCR
RT-PCR was performed as previously described (Kwon et al., 2016). HMC-1 cells (1×106/well) were seeded in plates. The cells were pretreated with various concentrations of rifampicin at 37°C for 2 h. After washing, the cells were stimulated with compound 48/80 (10 μg/ml) for 4 h. Extraction of total RNA was carried out with an RNA isolation kit (GeneAll), and cDNA was synthesized using an RT premix (Bioneer, Seoul, Korea). RT-PCR was used to determine COX-2 mRNA expression levels. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a standard control. The PCR reaction was performed with a Bio-Rad PCR system (Thermal cycler, Bio-Rad, USA). After PCR, the products were electrophoresed on 1% agarose gels containing ethidium bromide. The COX-2 band signal was normalized to that of GAPDH. Table 1 shows the primer sequences used in this study.
Table 1.
RT-PCR primer sequences for the genes used in this study
| Target gene | Primer sequence |
|---|---|
| COX-2 | Forward: 5′-TTCAAATGAGATTGTGGGAAAATTGCT-3′ Reverse: 5′-AGATCATCTCTGCCTGAGTATCTT-3′ |
| GAPDH | Forward: 5′-CAAAAGGGTCATCATCTC-3′ Reverse: 5′-CCTGCTTCACCACCTTCTTG-3′ |
Measurement of IgE and cytokines
Mouse serum and cell culture supernatants were assayed for IgE, IL-4, and TNF-α using ELISA kits (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions, and as described previously (Lim et al., 2015; Park et al., 2016).
Statistical analysis
All data were analyzed with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical comparisons of data were performed by using Student’s t-tests. A value of p<0.05 or p<0.01 indicated statistical significance.
RESULTS
Rifampicin improves AD in the NC/Nga mouse model
To investigate the effect of rifampicin on AD, the NC/Nga mice were orally administered the drug. The skin lesions of the mice were observed until 7 days after the treatment. Rifampicin improved AD-like symptoms, which was comparable to the effect of dexamethasone (Fig. 1B). The lesions were also evaluated with severity scores and scratching behavior assessments. As shown in Fig. 1C, the severity score significantly decreased in the rifampicin-treated group after 7 days compared to that of the non-treated group. To determine whether the effect of rifampicin was dose dependent, two different doses (25 and 50 mg/kg) were administered to the AD-induced mice. As we expected, rifampicin reduced the skin severity score in a dose-dependent manner. We next investigated the effects of rifampicin on scratching behavior in NC/Nga mice. The treatment with rifampicin significantly reduced scratching behavior in the AD mice on day 7 after treatment compared to that of the untreated AD mice (Fig. 2A).
Fig. 2.
The effect of rifampicin on scratching behavior, serum IgE, and IL-4 levels in AD-induced NC/Nga mice. The AD-induced mice were observed 7 days after oral rifampicin administration. Dexamethasone (1 mg/kg) was used as a positive control. (A) Scratching behavior and (B) serum IL-4 and IgE levels were measured at the indicated times. Experimental values are given as means ± SEM (n=5). *p<0.05, **p<0.01, when compared with the AD-induced and untreated mice.
As it has been reported that AD is an IgE-mediated type I hypersensitivity reaction and its major characteristic is the hyperproduction of IgE (Chang and Shiung, 2006), total serum IgE levels were measured in the presence or absence of rifampicin and the values were compared with those of the controls. As shown in Fig. 2B, when AD was induced, the IgE levels were higher than those of the non-treated control group, and the levels in the AD mice treated with rifampicin were significantly attenuated on day 7 after treatment. IL-4 levels were also increased in the AD-induced mice, and treatment with rifampicin alleviated this increase (Fig. 2B). Consistent with the results of the skin severity score and scratching behavior assessments, rifampicin exhibited a strong inhibitory effect on IgE and IL-4 secretion in the AD mouse model.
Effect of rifampicin on β-HEX and histamine release from HMC-1 cells
Granules, such as those containing histamine, derived from mast cells are important mediators of itching and vasodilation. Therefore, the effect of rifampicin on the degranulation of mast cells using the HMC-1 cell line was investigated. An MTT assay was performed to examine the effect of rifampicin on cell viability. HMC-1 cells were treated with various concentrations of rifampicin. Rifampicin did not alter cell viability even at 50 μM, a concentration greater than that routinely used in this study, which showed its inhibitory effect on pro-inflammatory cytokine production (data not shown).
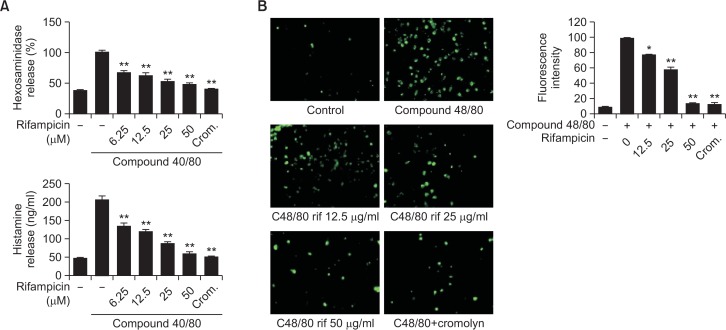
β-HEX has been used as a marker of degranulation of mast cells (Schwartz et al., 1979; Wang et al., 2012). We evaluated the effect of rifampicin on compound 48/80-induced β-HEX release from HMC-1 cells. Although compound 48/80 stimulated HMC-1 cells to release β-HEX, rifampicin significantly inhibited the secretion of β-HEX induced by compound 48/80 in a concentration-dependent manner (Fig. 3A). We next assessed the ability of rifampicin to affect compound 48/80-induced histamine release from HMC-1 cells. Cromolyn was used as a positive control because it is a mast cell stabilizer. The HMC-1 cells released increased levels of histamine when stimulated with compound 48/80. As we expected, rifampicin inhibited compound 48/80-induced histamine release in a concentration-dependent manner (Fig. 3A). Histamine release from mast cells is correlated with increased intracellular calcium concentrations (Pearce, 1985). Therefore, we assessed the effect of rifampicin on intracellular calcium levels in HMC-1 cells activated with compound 48/80. The results show that, while intracellular calcium was increased in compound 48/80-stimulated HMC-1, calcium levels were decreased in HMC-1 cells pretreated with rifampicin (Fig. 3B). These results demonstrate that rifampicin has anti-histamine activity via an inhibitory effect on mast cell degranulation.
Fig. 3.
Effect of rifampicin on degranulation of mast cells. The HMC-1 cells were stimulated with compound 48/80 (10 μg/ml) and then treated with rifampicin (6.25 μM to 50 μM). Cromolyn (100 μM) was used as a positive control. (A) β-hexosaminidase and histamine release, (B) intracellular calcium levels were assessed in the HMC-1 cells. *p<0.05, **p<0.01, compared with the C48/80-treated group.
Effect of rifampicin on the secretion of inflammatory mediators in HMC-1 cells
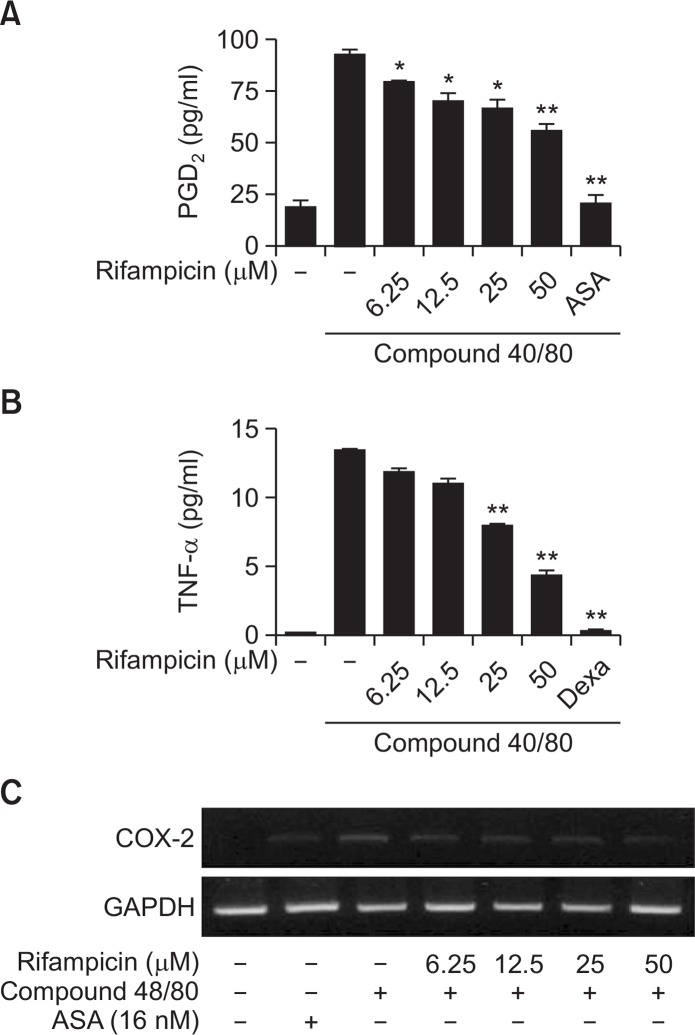
PGD2 and TNF-α are important mediators in allergic inflammation (Gordon et al., 1990). COX-2-derived PGD2 is known to mediate AD (Fournier et al., 1997). To investigate the anti-inflammatory effects of rifampicin, cell culture media were used for PGD2 and TNF-α ELISAs. Treatment of rifampicin attenuated the secretion of PGD2 and TNF-α in HMC-1 stimulated with compound 48/80 (Fig. 4A, 4B). COX-2 is known to be associated with inflammatory reactions. The mRNA expression of COX-2 was determined by RT-PCR (Fig. 4C). The results show that rifampicin inhibited COX-2 expression in compound 48/80-activated HMC-1 cells, suggesting that rifampicin inhibits compound 48/80-induced PGD2 secretion via suppression of COX-2 expression in mast cells.
Fig. 4.
Effect of rifampicin on anti-inflammatory mediator expression in mast cells. The HMC-1 cells were stimulated with compound 48/80 (10 μg/ml) and then treated with rifampicin (6.25 to 50 μM). Acetylsalicylic acid (ASA: 16 nM) or dexamethasone (Dex; 2 nM) were used as positive controls. The production of (A) PGD2 and (B) TNF-α was assessed in the cells. (C) The expression of COX-2 mRNA was also measured in the cells. *p<0.05, **p<0.01, compared with the C48/80-treated group.
DISCUSSION
Our study showed a novel anti-AD activity of the existing drug, rifampicin. During our study for novel anti-AD agents, we found that rifampicin possessed anti-AD activity in a mouse model. Our results showed that rifampicin effectively reduced the characteristic features of AD, such as elevated serum IgE and IL-4 levels (Fig. 1, 2). The mice treated with rifampicin did not show evidence of skin disease 7 days after oral administration of rifampicin, which was comparable to the outcome in the dexamethasone-treated group (positive control group). The reduction in the severity of symptoms and IgE production suggest that rifampicin is effective for the treatment of AD in a dose-dependent manner.
Furthermore, we assessed whether rifampicin has anti-allergic activity using an in vitro model in this study. In the 1970s, researchers discovered that rifampicin suppressed both humoral and cellular immunity in vitro and in vivo (Paunescu, 1970; Nilsson, 1971). Recent studies have again brought the immunomodulating ability of rifampicin into the spotlight (Mlambo and Sigola, 2003; Tsankov and Angelova, 2003; Ziglam et al., 2004). Our study showed that rifampicin has anti-allergic activity. β-HEX is stored in secretory granules of mast cells and basophils, and is released along with histamine when mast cells are activated (Wang et al., 2012). Rifampicin reduced both β-HEX and histamine secretion, which are biomarker of allergic reactions (Fig. 3). Intracellular calcium, as well as IgE, activates mast cells, and histamine release is affected by intracellular calcium levels (Pearce, 1985; Eisenhut and Wallace, 2011). Stimulation with compound 48/80 increased the intracellular calcium levels in HMC-1 cells. When the cells were pretreated with rifampicin, however, the intracellular calcium levels decreased (Fig. 3). Thus, it seems that rifampicin suppresses the degranulation of mast cells by controlling intracellular calcium concentrations, suggesting that rifampicin can be used as a new anti-allergic agent.
The early-phase of an allergic reaction occurs within minutes after allergen exposure, whereas the late-phase reaction occurs hours later and involves secretion of cytokines, such as TNF-α and IL-4. Proinflammatory cytokines have been shown to play a critical role in late-phase hypersensitivity reactions (Wang et al., 2012). The present study showed that rifampicin also decreased the production of TNF-α in mast cells (Fig. 4).
In a recent study, it was demonstrated that COX-2 plays important roles in mast cell-mediated inflammation (Kim et al., 2005). PGD2 is a marker of mast cell activation by allergens in bronchial asthma, and it is a major cyclooxygenase metabolite generated by mast cells (Bochenek et al., 2004). The expression of COX-2 and PGD2 is related to mast cell-mediated chronic inflammation. Our study showed that their expressions were also decreased by rifampicin pretreatment (Fig. 4). Decreasing the release of these cytokines and proinflammatory mediators is a good therapeutic approach.
In conclusion, we demonstrated that rifampicin suppressed the release of allergic mediators, including β-HEX, histamine, PGD2, and the proinflammatory cytokines, TNF-α and COX-2. And it enables cell membrane to become stable. Rifampicin could be used as a treatment to alleviate allergic symptoms.
Drug repositioning is the application of known drugs and compounds to treat new diseases, and is the process of identifying new uses for drugs outside the scope of their original medical indication. Traditional drug discovery approaches have largely failed to deliver on promises of improved productivity, despite large increases in funding (Ashburn and Thor, 2004; Sleigh and Barton, 2010). By utilizing existing drugs, drug repositioning can provide a faster and cheaper approach than traditional drug development because the repositioned drug has already passed many regulatory requirements, its safety is known, and the risk of failure due to unacceptable side effects is reduced.
Rifampicin has been used for the treatment of mycobacterial infection for many years. Our study revealed a novel anti-AD activity of rifampicin. The effect of rifampicin on AD was comparable to that of dexamethasone, which is a well-known anti-inflammatory agent, suggesting that rifampicin is an alternative medication for the management of AD. Understanding the relationships between each step involved in the anti-AD activities and the compounds targeting them is pivotal to study the molecular mechanisms underlying the cellular responses. Our future studies will establish the biochemical and molecular mechanism of rifampicin’s anti-AD activity. Nevertheless, our current findings suggest that rifampicin it effective, and it could also be used to develop future, potent therapeutic reagents for the treatment of AD.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1A02937476).
REFERENCES
- Ashburn TT, Thor KB. Drug repositioning: identifying and developing new uses for existing drugs. Nat Rev Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Bellahsene A, Forsgren A. Effect of rifampin on the immune response in mice. Infect Immun. 1980;27:15–20. doi: 10.1128/iai.27.1.15-20.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann RL, Edenharter G, Bergmann KE, Forster J, Bauer CP, Wahn V, Zepp F, Wahn U. Atopic dermatitis in early infancy predicts allergic airway disease at 5 years. Clin. Exp. Allergy. 1998;28:965–970. doi: 10.1046/j.1365-2222.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- Bochenek GE, Nizankowska E, Gielicz A, Swierczynska M, Szczeklik A. Plasma 9α,11β-PGF2, a PGD2 metabolite, as a sensitive marker of mast cell activation by allergen in bronchial asthma. Thorax. 2004;59:459–464. doi: 10.1136/thx.2003.013573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TS, Shiung YY. Anti-IgE as a mast cell-stabilizing therapeutic agent. J Allergy Clin Immunol. 2006;117:1203–1212. doi: 10.1016/j.jaci.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Chung TH, Kang TJ, Cho WK, Im GY, Lee GS, Yang MC, Cho CW, Ma JY. Effectiveness of the novel herbal medicine, KIOM-MA, and its bioconversion product, KIOM-MA128, on the treatment of atopic dermatitis. Evid Based Complement Alternat Med. 2012;2012:762918. doi: 10.1155/2012/762918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E, Synder L, Marino P, Lamberti A, Coppo A, Tocchini-Valentini GP. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969;222:533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]
- Eisenhut M, Wallace H. Ion channels in inflammation. Pflugers Arch. 2011;461:401–421. doi: 10.1007/s00424-010-0917-y. [DOI] [PubMed] [Google Scholar]
- Eule H, Werner E, Winsel K, Iwainsky H. Intermittent chemotherapy of pulmonary tuberculosis using rifampicin and isoniazid for primary treatment: the influence of various factors on the frequency of side-effects. Tubercle. 1974;55:81–89. doi: 10.1016/0041-3879(74)90069-5. [DOI] [PubMed] [Google Scholar]
- Fournier T, Fadok V, Henson PM. Tumor necrosis factor-α inversely regulates prostaglandin D2 and prostaglandin E2 production in murine macrophages. Synergistic action of cyclic AMP on cyclooxygenase-2 expression and prostaglandin E2 synthesis. J Biol Chem. 1997;272:31065–31072. doi: 10.1074/jbc.272.49.31065. [DOI] [PubMed] [Google Scholar]
- Furue M, Terao H, Rikihisa W, Urabe K, Kinukawa N, Nose Y, Koga T. Clinical dose and adverse effects of topical steroids in daily management of atopic dermatitis. Br J Dermatol. 2003;148:128–133. doi: 10.1046/j.1365-2133.2003.04934.x. [DOI] [PubMed] [Google Scholar]
- Gordon JR, Burd PR, Galli SJ. Mast cells as a source of multifunctional cytokines. Immunol. Today. 1990;11:458–464. doi: 10.1016/0167-5699(90)90176-A. [DOI] [PubMed] [Google Scholar]
- Jung JW, Kim SJ, Ahn EM, Oh SR, Lee HJ, Jeong JA, Lee JY. Ribes fasciculatum var. chinense attenuated allergic inflammation in vivo and in vitro. Biomol. Ther. (Seoul) 2014;22:547–552. doi: 10.4062/biomolther.2014.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalesnikoff J, Galli SJ. New developments in mast cell biology. Nat Immunol. 2008;9:1215–1223. doi: 10.1038/ni.f.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Jeong HJ, Choi IY, Lee KM, Park RK, Hong SH, Kim HM. Cyclooxygenase-2 inhibitor SC-236 [4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1-pyrazol-1-l] benzenesulfonamide] suppresses nuclear factor-kappaB activation and phosphorylation of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and c-Jun N-terminal kinase in human mast cell line cells. J Pharmacol Exp Ther. 2005;314:27–34. doi: 10.1124/jpet.104.082792. [DOI] [PubMed] [Google Scholar]
- Kim SK, Kim YM, Yeum CE, Jin SH, Chae GT, Lee SB. Rifampicin inhibits the LPS-induced expression of Toll-like receptor 2 via the suppression of NF-κB DNA-binding activity in RAW 264.7 Cells. Korean J Physiol Pharmacol. 2009;13:475–482. doi: 10.4196/kjpp.2009.13.6.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SH, Ma SX, Ko YH, Seo JY, Lee BR, Lee TH, Kim SY, Lee SY, Jang CG. Vaccinium bracteatum Thunb. exerts anti-inflammatory activity by inhibiting NF-κB activation in BV-2 microglial cells. Biomol.Ther. (Seoul) 2016;24:543–551. doi: 10.4062/biomolther.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung DY, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–657. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SC, Lee KM, Kang TJ. Chitin from cuttlebone activates inflammatory cells to enhance the cell migration. Biomol. Ther. (Seoul) 2015;23:333–338. doi: 10.4062/biomolther.2015.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeffler AM. Uses of rifampin for infections other than tuberculosis. Pediatr Infect Dis J. 1999;18:631–632. doi: 10.1097/00006454-199907000-00012. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Watanabe N, Geba GP, Sperl J, Tsudzuki M, Hiroi J, Matsumoto M, Ushio H, Saito S, Askenase PW, Ra C. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol. 1997;9:461–466. doi: 10.1093/intimm/9.3.461. [DOI] [PubMed] [Google Scholar]
- Mlambo G, Sigola LB. Rifampicin and dexamethasone have similar effects on macrophage phagocytosis of zymosan, but differ in their effects on nitrite and TNF-α production. Int Immunopharmacol. 2003;3:513–522. doi: 10.1016/S1567-5769(03)00022-5. [DOI] [PubMed] [Google Scholar]
- Nilsson BS. Rifampicin: an immunosuppressant? Lancet. 1971;2:374. doi: 10.1016/S0140-6736(71)90087-0. [DOI] [PubMed] [Google Scholar]
- Park JY, Lim MS, Kim SI, Lee HJ, Kim SS, Kwon YS, Chun W. Quercetin-3-O-β-D-glucuronide suppresses lipopolysaccharide-induced JNK and ERK phosphorylation in LPS-challenged RAW264.7 cells. Biomol. Ther. (Seoul) 2016;24:610–615. doi: 10.4062/biomolther.2016.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu E. In vivo and in vitro suppression of humoral and cellular immunological response by rifampicin. Nature. 1970;228:1188–1190. doi: 10.1038/2281188a0. [DOI] [PubMed] [Google Scholar]
- Pearce FL. Calcium and mast cell activation. Br J Clin Pharmacol. 1985;20:267S–274S. doi: 10.1111/j.1365-2125.1985.tb02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LJ, Mosbech H, Skov PS. Allergen-induced histamine release in intact human skin in vivo assessed by skin microdialysis technique: characterization of factors influencing histamine releasability. J Allergy Clin Immunol. 1996;97:672–679. doi: 10.1016/S0091-6749(96)70313-5. [DOI] [PubMed] [Google Scholar]
- Schwartz LB, Austen KF. Enzymes of the mast cell granule. J Invest Dermatol. 1980;74:349–353. doi: 10.1111/1523-1747.ep12543620. [DOI] [PubMed] [Google Scholar]
- Schwartz LB, Austen KF, Wasserman SI. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J Immunol. 1979;123:1445–1450. [PubMed] [Google Scholar]
- Sidbury R, Hanifin JM. Systemic therapy of atopic dermatitis. Clin Exp Dermatol. 2000;25:559–566. doi: 10.1046/j.1365-2230.2000.00697.x. [DOI] [PubMed] [Google Scholar]
- Sleigh SH, Barton CL. Repurposing strategies for therapeutics. Pharmaceut Med. 2010;24:151–159. [Google Scholar]
- Suto H, Matsuda H, Mitsuishi K, Hira K, Uchida T, Unno T, Ogawa H, Ra C. NC/Nga mice: a mouse model for atopic dermatitis. Int. Arch. Allergy Immunol. 1999;120(Suppl 1):70–75. doi: 10.1159/000053599. [DOI] [PubMed] [Google Scholar]
- Tsai MJ, Saunders GF. Action of rifamycin derivatives on RNA polymerase of human leukemic lymphocytes. Proc Natl Acad Sci USA. 1973;70:2072–2076. doi: 10.1073/pnas.70.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankov N, Angelova I. Rifampin in dermatology. Clin Dermatol. 2003;21:50–55. doi: 10.1016/S0738-081X(02)00328-0. [DOI] [PubMed] [Google Scholar]
- Tsudzuki M, Watanabe N, Wada A, Nakane Y, Hiroi J, Matsuda H. Genetic analyses for dermatitis and IgE hyperproduction in the NC/Nga mouse. Immunogenetics. 1997;47:88–90. doi: 10.1007/s002510050330. [DOI] [PubMed] [Google Scholar]
- Umeda K, Noro Y, Murakami T, Tokime K, Sugisaki H, Yamanaka K, Kurokawa I, Kuno K, Tsutsui H, Nakanishi K, Mizutani H. A novel acoustic evaluation system of scratching in mouse dermatitis: rapid and specific detection of invisibly rapid scratch in an atopic dermatitis model mouse. Life Sci. 2006;79:2144–2150. doi: 10.1016/j.lfs.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhou Q, Liu L, Zou K. Anti-allergic activity of emodin on IgE-mediated activation in RBL-2H3 cells. Pharmacol Rep. 2012;64:1216–1222. doi: 10.1016/S1734-1140(12)70917-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Grace PM, Pham MN, Cheng K, Strand KA, Smith C, Li J, Watkins LR, Yin H. Rifampin inhibits Toll-like receptor 4 signaling by targeting myeloid differentiation protein 2 and attenuates neuropathic pain. FASEB J. 2013;27:2713–2722. doi: 10.1096/fj.12-222992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehrli W, Knusel F, Schmid K, Staehelin M. Interaction of rifamycin with bacterial RNA polymerase. Proc Natl Acad Sci USA. 1968;61:667–673. doi: 10.1073/pnas.61.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziglam HM, Daniels I, Finch RG. Immunomodulating activity of rifampicin. J Chemother. 2004;16:357–361. doi: 10.1179/joc.2004.16.4.357. [DOI] [PubMed] [Google Scholar]